*Corresponding Author:
Tsuneo Ishida,
2-3-6, Saido, Midori-Ku, Saitama-Shi, Saitama-Ken, 336-0907, Japan
E-mail: ts-ishida@ac.auone-net.jp
Abstract
Zinc(II) homeostatic status has antiviral effects, improves immune responses, and suppresses viral replication. Zn2+ ions can prevent SARS-CoV-2 infection by antiviral zinc homeostatic immunity and have important roles for respirarory and pulmonary process of COVID-19 disease. Zn2+ ions-induced prevention and antibody against SARS-CoV-2 infection are required with Zn homeostatic immune concentration 50 mg/day,Zn supplementation with CQ/HCQ,and transient receptor potential vanilloid 1(TRPV1) prevention.
Zn2+ ions-induced virucidal defenses from COVID-19 severe bronchitis and acute pneumonia are required that the zinc ions have important roles for respirarory and pulmonary process of COVID-19 disease. Zinc serves for thrombus prevention and anti-thrombus formation that zinc controls blood clot formation on fibrin (ogen) expression and function, in which demonstrate its central role in clot formation during hemostasis and thrombosis. Zinc can prevent respiratory thrombosis and pulmonary thromboembolism by inhibition of thrombus formation growth in COVID-19 infection.
Zinc-finger Antiviral Protein (ZAP) controls viral entry, DNA/RNA replication, and spreading against viral infection. ZAP specifi-cally inhibits the replication of certain viruses and promotes viral RNA degradation. The mutations of both protein and RNA at the RNA-ZAP interacting surface reduce the binding affinity and antiviral activity, in which ZAP coordination promotes downstream RNA degradation. The ZAP could be found to restrict SARS-CoV-2 RNA virus replication, subsequently; ZAP inhibits viral replication and mediates viral genome degradation. Zinc induced ROS generation promotes platelet activation that functional association between zinc ion concentration [Zn2+] and ROS generation could influence thrombus formation. ZAP-mediated ROS generation may promote an effect that is conceivable that chronic inflammation-induced production of ROS in the lung may predispose individuals to lung diseases.
Finally, Zinc(II) ions molecular binding mechanism is involved in respiratory and pulmonary organ, anti-thrombus formation, and ZAP-mediated RNA replication that zinc ions may be bound with respiratory and pulmonary proteins, Zn2+-RNA gene reaction, and thrombosis proteins by Zn2+ ions-coordinated tetrahedrally binding pattern.
Keywords
SARS-CoV-2 RNA virus and RNA mutants; Zinc homeostatic immunity; COVID-19 prevention; Respiratory and pulmatory disease; Anti-thrombus formation; ZAP-mediated RNA replication; Zinc ions-coordinated binding
Abbreviations
ADAR: Adenosine Deaminases Acting on RNA
ACE2: Angiotensin-Converting Enzyme 2
APN: Amino-Peptidase N protein
ARDS: Acute Respiratory Distress Syndrome
CoVs: Corona Viruses
COVID-19: Coronavirus Disease 2019
CPGs: 3’-O-(2’-Deoxy-5’-guanylyl)-2’-deoxy-5’-cytidylic acid
CQ/HCQ: ChloroQuine/HydroxyChloroQuine
CVB3: Human Coxsackievirus Strain B3
CVDs: CardioVascular Diseases
EAV: Equine Arteritis Virus
EPDTC: N-ethyl-N-phenyldithio-carbamate
ER: Endoplasmic Reticulum
FDA: Food and Drug Administration
HCoV: Human Corona Virus
HR1: Heptad Repeat 1
IAV: Influenza A virus
IFITMs: Inter Feron Induced TransMembrane Proteins
INFs: Interferons
ISGs: Interferon-Stimulated Genes
MERS-CoV: Middle East Respiratory Syndrome Coronavirus
MLV: Murine Leukemia Virus
NAC: N-acetyl-cysteine
ORFs: Open Reading Frames
2019-nCoV: Novel Corona Virus 2019
PCR: Polymerase Chain Reaction
PPI: Protein-Protein Interaction
RCT: Randomized Controlled Trial
RdRp: RNA-dependent RNA-polymerase
ROS: Reactive Oxygen Species
RSV: Respiratory Syncytial Virus
SARS-CoV: Severe Acute Respiratory Syndrome Corona Virus
SARS-CoV-2: Severe Acute Respiratory Syndrome Corona Virus 2
SIN: Sindbis Virus
SNP: Single Nucleotide Polymor-phisms
6-HB: Six-Helical Bundle
TMPRSS2: TransMembrane Protease, Serine2
TPEN: N,N,N’,N’ -tetrakis(2-pyridinyl-methyl)-1,2-ethanedi- amine
TPEN: Transient Zinc Chelation
TRPV1: Transient Receptor Potential Vanilloid 1
ZAPs: Zinc Finger Antivirus Proteins
ZNFs: Zinc-Finger Proteins
UTR: Un Translated Region
Introduction
Zinc(Ⅱ) play important role for these infectious diseases that zinc is essential for highly growth and development of all organisms in the human body, especially the immune system that zinc has antiviral effects; it improves immune responses and suppresses viral replication, in which zinc homeostatic status is a key factor in maintaining a healthy immune system. Zinc ions are involved in regulating intracellular signaling pathways in innate and adaptive immune cells that the influences of zinc status on the overall immune function are present in zinc defficiency as overproduction of pro-inflammatory cytokines with zinc homeostasis as balanced immune cell functions [1].
In a concentration of 100 μmol/L, zinc suppresses natural killer cell killing and T-cell function whereas monocytes are activated direcly, and in a concentration of 500 μmol/L, zinc evokes a direct chemotactic activation of neutrophil granulocytes [2]. Zn2+ ions have an important role for RNA viral destruction that the zinc-finger antiviral protein could regulate RNA virus degradation of SARS-CoV’s and MERS-CoV’s RNA virus. Zinc ions can lead to RNA virus degradation by a receptor-destroying enzyme [3]. Zinc ions become used as Zn2+-coordinated inhibitors for viral regulation of virucidal activities [4].
Zinc(Ⅱ) is essential to preserve zinc homeostasis and natural tissue barriers such as the respiratory epithelium, preventing pathogen entry for a balanced function of the immune system and the redox system that Zn2+ ions can protect human body from entering of the virus and inhibit inflammatory stimulation, viral replication, and respiratory and premonary infections [5]. Zinc can modulate Acute Respiratory Distress Syndrome (ARDS) and severe pneumonia, in which 20 mg zinc, was supplemented daily for 7 days showed a reduction in the recovery time and improvement in symptoms in patients with severe pneumonia [6].
As zinc therapy for the global COVID-19 pandemic, anti-viral effect of zinc is high as immune effects and association with pneumonia such as fusion, replication, viral protein translation, viral particle entry, especially those involving respiratory system pathology, in which zinc supplementation in preventing and managing COVID-19 participates in protecting the body from viral and bacterial infections, improving immunity, antiviral and immunity potency [7]. This COVID-19 has single-stranded positive-sense RNA virus with 5’-cap and 3’-poly-A tail that caused a major outbreak of coronavirus disease 2019 COVID-19 has threatened global health seculity [8]. Factor of present outbreak of disease 19 COVID-19 may be considered to be due to RNA virus mutation [9].
In this review, firstly COVID-19 molecular structure with RNA virus mutation pandemic and zinc (Ⅱ) induced immune enhance-ment are described. Secondly, zinc ions-induced homeostatic anti-immune infectious activities for preventative and antibody SARS-CoV-2, defending of severe respiratory and acute pulmonary COVID-19 patient, anti-thrombus formation, and Zinc-finger Antiviral Protein (ZAP)-mediated RNA replication are discussed under the concept that Reactive Oxygen Species (ROS) generate COVID-19 infection, subsquently, in which the molecular mechanism of zinc ions-centered coordinated binding is clarified against COVID-19 infection.
SARS-CoV-2 structure and COVID-19 RNA mutation
Coronaviruses (CoVs) are viruses whose genome structure is best known among all RNA viruses that typical CoV genome contains at least six Open Reading Frames (ORFs) which the first ORF (ORF1a/b) is about two-thirds of the whole genome length encodes 16 non-structural proteins, in which ORFs near 3’ end of the genome encodes at least four main structural proteins including Spike (S), Membrane (M), Envelope (E), and Nucleocapsid (N) proteins [10]. Most of the non-structural proteins have RNA-dependent-RNA-polymerase (RdRp). Spike is cleaved into S1 and S2 by the host cell protease that the main function of S1 is to bind ewith the host cell surface receptors, and the S2 submit mediates virus-cell and cell-cell membrane fusion, Hence, the therapeutic stragies to block coronavirus from entering host cells by spike proteins or specific receptors on the host surface may be valuable for the antiviral development [11].
SARS-CoV-2 has a unique four amino acid insertion between S1 and S2 domains of the spike protein, which created a potential furin or TMPRSS2 cleavage site. 2019-nCoV may increase its infectivity through the receptor binding domain recombination and a cleavage site insertion [12]. The SARS-CoV-2 E protein is a small, integral membrane protein involved in several aspects of the virus’ life cycle that the most progress has been made on SARS-CoV E, highlighting specific structural requirements for its functions in the CoV life cycle as well as mechanism behind its pathogenesis [13].
The othe, zinc induced COVID-19 is involved that zinc could enhance ACE2 activity and consequently increase the activity and four structural proteins such as (S), (E), (N), and (M) ptoteins which the S protein is responsible to attach virus to host cell of COVID-19. This outcome needs further investigation while treating patients [14].
On the other hand, mutations and adaptation in the S and N genes could affect virus stability and pathogenicity. As more genomes are made publicly available, analysis of the genome sequence diversity across samples has revealed the highest diversity occurring in the structural genes, especially the S protein, ORF3a, and ORF8 [15]. This nove Yinl coronavirus (SARS-CoV-2) outbreak has caused a global pandemic resulting many infected persons and deaths worldwide that the RdRp catalyzed the synthesis of viral RNA, is a key component of coronaviral replication/transcription as a primary targeted antiviral drug [16].
High-affinity complex of the Receptor-Binding Domain(RBD) RBD-62 and ACE2, including all rapidly spreading mutations, provides a structural basis for future drug and vaccine development and for in silico evaluation of known antibodies that high-affinity variant RBD-62 can be used as a drug to inhibit infection with SARS-CoV-2 and variants Alpha, Beta and Gamma in vitro that Evolved RBD mutants include prominently the amino acid substitutions found in the RBDs of B.1.620, B.1.1.7 (Alpha), B1.351 (Beta) and P.1 (Gamma) variants [17].
However, COVID-19 RNA mutants are most important problem in recently up-date that there are four types of coronavirus such as α, β, γ, and δ that the current virus classified in β group. Thus, the major mutations are in the critical proteins, including the S protein, RNA polymerase, RNA primase, and nucleoprotein [18]. It is unclear whether zinc ions can suppress RNA mutation and outbreak by RNA mutation. The other, Zn2+ ions could inhibit virus entry and membrane fusion of S1 and S2 domains of spike protein with zinc ion-binding interaction. This process is triggered when the S1 submit binds to a host cell receptor, and receptor binding destabilizes the prefusion trimer, resulting in shedding of the S1 subunit and transition of the S2 subunit to a stable post fusion conformation [19].
Zinc (Ⅱ) induced human immune function enhancement against COVID-19 infection
Zinc binding to proteins in high zinc concentration can activate or inactivate thir activity, whiles, zinc homeostasis is primarily con- trolled via the expression, but also the transport of zinc into one of those organelles that zinc homeostasis during acute phase response is the temporal transfer of serum zinc to the tissues, causing tran- sient serum hypozincemia, which is rebalanced during resolution of the inflammatory response that intracellularly increased zinc can in- toxicate engulfed pathogens and acts cytoprotective by promotion of neutralizing Reactive Oxygen Species (ROS) and Nitrogen Species (RNS) [20].
Zinc has a regulation of immunity that zinc homeostasis in im- mune system pathways is complex, since it participates both in pro-inflammatory and regulatory pathways, and it seems clear that deficient or excessive zinc levels can lead to malfunction of the adap- tive and innate immune systems. Inflammation is a natural process required to protect the host from tissue damage and infections, which leads to the resolution of the inflammatory response and the resto- ration of homeostasis. Zinc regulates the proliferation, maturation and functioning of lymphocytes [21]. Zinc is involved in inflamma- tion, elevating inflammatory responses and inducing cell-mediated immunity, and is a key component of pathogen-eliminating transduc- tion pathways that contribute to neutrophil extracellular traps. Many organs are affected by zinc deficiency, especially the immune system that is markedly susceptible to changes of zinc levels which the im- mune response involves in the regulation of the innate and adaptive immunity, and this zinc homeostasis is critical for sustaining proper immune function [22].
Reference Intakes recommended intake for the adult of 11 mg/ day for males and 8 mg/day for females. However, besides of reduced zinc dietary intake, some age-related factors including intestinal ab- sorption, drug interactions, subcellular processes, among others, may jeopardize his activity. Zinc supplementation must be assessed indi- vidually, considering cases of zinc deficiency, low dietary intake, and related diseases. Evaluated zinc supplementation with different doses and duration, 20~40 mg/day appears to be a safe and effective dosage [23].
In COVID-19 infection, human being must have human immune system consisting of the immune homeostasis and protective functions that zinc has important role in immune homeostasis and functions and zinc is associated with modulating signalling pathways of adap- tive immune systems, in which Upper limits of zinc intake has been obtained that Estimated Average Requirement (EAR) is 1-3 year; 0.0025 g -Daily and 4-8 year; 0.003g -Daily, 9-13yr; 0.005 g-Daily, 14-18yr; 0.011 g-Daily for boys and 14-18yr; 0.011 g-Daily for girls, and19-30yr;, 31-50yr;, 51-70yr; 0.012 g -Daily,>70yr; 0.012 g -Daily for man, and 19-30yr; 0.0065 g -Daily, 31-50yr; 0.0065 g -Daily, 51- 70yr; 0.0065 g -Daily,>70yr; 0.0065 g -Daily for woman [24].
Thus, zinc has exert homeostasis, immune-boosting, and antiviral responses that amount of zinc intake is likely to be beneficial 40-50 mg/day against the COVID-19 severe infection to have about 50 mg of zinc that would need to take 220 mg of zinc sulfate [25].
Zn2+ ions-induced immune prevention and antibody against SARS-CoV-2 infection
Zn2+ ions-induced prevention and antibody against SARS-CoV-2 infection are required Zn homeostatic immune concentration 50 mg/ day, Zn supplementation in combination with ChloroQuine/Hydroxy- ChloroQuine (CQ/HCQ), zinc supplement (15~30 mg/d) preventing pneumonia in children, and Transient Receptor Potential Vanilloid 1 (TRPV1) prevention [26]. Zinc ions inhibit the RNA-dependent RNA polymerase, which crucially replicates copies of viral RNA in the host cells. Zinc may possess protective effect as preventive and adjuvant therapy of COVID-19 through reducing inflammation, improvement of mucociliary clearance, prevention of ventilator-induced lung inju- ry, modulation of antiviral immunity [27].
Higher intracellular zinc concentration has shown to increase monocyte resistance to apoptosis via suppressing the activation of caspase; zinc 50 mg/day might provide an additional shield against the COVID-19 pandemic. The potential beneficial role of zinc in COVID-19 infection needs further clinical validation, however, in this pandemic situation, using zinc to reduce disease burden would be a well-intentioned trial [25]. In order to prevent that an outbreak of respiratory sickness caused by a novel coronavirus (Covid-19) has become a serious public threat and disrupted many lives, assessing the efficacy of FDA-approved Zn-ejector drugs such as disulfiram combined with interferon to treat COVID-19 infected patients has been proposed [28]. Zinc ions anti-inflammatory Transient Receptor Potential Vanilloid 1 (TRPV1) prevention against COVID-19 would lead one to look for therapeutic agents to down regulate the inflamma- tory response due to TRPV1 activation [29].
Evidence for vitamins C, D and zinc and their roles in preventing pneumonia and respiratory infections (vitamins C and D) and rein- forcing immunity (zinc) appears to look particularly promising. Tol- erable upper intake levels (ULs) are intake levels which should not be surpassed as toxicity problems could appear. For vitamin D a UL of 50 μg/day is advised and for zinc a UL of 25 mg/day is recommended, supplemental daily doses of up to about 1 g, in addition to normal dietary intake, are not associated with adverse gastrointestinal effects [30].
Enhancement of zinc immunity for preventing infection with the SARS-CoV-2 is needed to cause COVID-19. Clinical trials are being set up at a rapid rate to test various approaches to preventing COVID-19 that such impaired antibody‐mediated responses could be restored by zinc supplementation [31]. Zinc induced preventative an- tibody will be useful for development of antigen detection tests and serological assays targeting SARS-CoV-2. Neutralizing antibodies can alter the course of infection in the infected host supporting virus clearance or protect an uninfected host. Hence, this antibody offers the potential to prevent and/or treat COVID-19, and possibly also oth- er future emerging diseases in humans caused by viruses from the Sarbecovirus subgenus [32].
Zn2+ ions-induced immune virucidal activities for COVID-19 severe bronchitis and acute pneumonia
Zn2+ inhibits coronavirus and anterivirus RNA polymelase activi- ty, and zinc ionophores block the virus replication. Zn2+ and pyrithi- one at low concentrations inhibit the replication of SARS-CoV and arterivirus RNA which high zinc ion concentration and the addition of compounds that stimulate cellular zinc ions inhibit the replication of various RNA virus, influenza viruses, respiratory syncytial virus and coronaviruses [33]. The defense on the severe bronchitis patients infected with SARS-CoV, MERS-CoV and SARS-CoV-2 has clinical features range from mild respiratory illness to severe acute respiratory disease.
The pneumonia appears to be the most frequent manifestation of SARS-CoV-2 infection, characterized primarily by fever, cough, dys- pnea, and bilateral infiltrates on chest imaging that the period from infection to appearance of symptoms varies [34]. SARS-CoV-2 en- ters the target cells through the Angiotensin-Converting Enzyme 2 (ACE2) receptor and the TransMembrane Protease, Serine 2 (TM- PRSS2). The TMPRSS2 inhibitors block the cellular entry of the SARS-CoV-2 virus through the downregulated priming of the SARS- CoV-2 spike protein [35].
The other, zinc used as anti-inflammatory agent inhibits Transient Receptor Potential Vanilloid 1 (TRPV1) to alleviate neuropathic pain [36]. That TRPV1 might decrease the severity of the acute respiratory distress syndrome present in COVID-19 patients [37]. For defenses from severe acute COVID-19 disease, parenteral zinc + ChloroQuine/ HydroxyChloroQuine (CQ/HCQ) in the treatment of hospitalized COVID-19 patients may help to improve clinical outcomes and to limit the COVID-19 fatality rates. Therefore, whether zinc supple- mentation in combination with CQ/HCQ should be recommended for high risk or also younger patients outside of clinical trials as a pre- vention or treatment approach during SARS-CoV-2 pandemic, should be considered only on a case-by-case basis [38,39]. SARS coronavi- rus envelope protein ion channel activity promotes virus fitness and pathogenesis that inflammasome-activated IL-1β levels were reduced in the lung airways of the animals infected with viruses lacking E protein ion channel activity and Acute Respiratory Distress Syndrome (ARDS) leading to death, in which E protein ion channel activity rep- resents a new determinant for SARS-CoV virulence [40].
On the case of preventing lung and pulmonia, drug treatment; (1) at present, there is no evidence from Randomized Controlled Trial (RCT) to support specific drug treatment against the new coronavi- rus in suspected or confirmed cases. (2) The α-interferon atomization inhalation can be considered 5 million U per time for adults in sterile injection water, twice a day [41]. The first strategy is avoiding ex- posures that could result in widespread damages to lungs and taking post exposure mitigating measures that would reduce disease severity. The second strategy is reducing death rate and disability rate from the current levels to one tenth for infected patients by using multiple fac- tors health optimization method. The double reduction strategies are expected to generate a series of chain reactions that favor mitigating or ending the pandemic [42].
Improve lung to prevent damages to the lungs, vitamins and es- sential nutrients for the immune system may shorten the phase lag by one to two days and thus make a difference; deep breaching can improve energy metabolism by as much as 30% (for experienced, it may improve more); and avoiding exercise may save MET values by up to 70%; relaxation exercise can reduce blood circulation by 10% to 30%; avoiding a secondary infection can reduce burden on the immune system, reduce viral burden on lungs, kidneys and heart, and help maintain the waste balance in the lungs [43]. However, Zn supplementation did not yield a statistically significant reduction in symptoms in children with severe pneumonia. Zinc supplements giv- en during an acute episode are not beneficial in short-term clinical recovery from severe pneumonia in hospitalized children [44,45].
Transient zinc chelation N, N, N’, N’-tetrakis (2-pyridinylmeth- yl)-1,2-ethanediamine (TPEN) induces Endoplasmic Reticulum (ER) stress and antiviral response by activating NF-kappaB leading to induction of interferon signaling and zinc plays divergent roles in rotavirus and dengue virus infections in epithelial cells [46]. The an- tiviral compounds including zinc N-ethyl-N-phenyldithio-carbamate (EPDTC) inhibit the viral protease, thus preventing humancoxsack- ievirus strain B3 (CVB3) genome replication. The interactions had been found on the binding specificity by Zn2+ ions-centered tetrahe- dral geometric coordination (Zinc-Coordination Pattern) of the inhib- itors against 3C and 3C-like proteases coordinated to such as catalytic triad of Serine, Histidine and Aspartate hydrogen residues of CVB3 3Cpro [47]. Thus, zinc ions can prevent in the early stage of COVID-19 coronavirus outbreak, and in the final stage could defend COVID-19 infection from severe respiratory and acute pulmonary disease.
Zinc promotes anti-thrombus formation
Zinc serves for thrombus prevention as a ubiquitous modulator of haemostasis and thrombosis, aggregation, anti-coagulation and fibrinolysis that zinc controls blood clot formation on fibrin (ogen) expression and function, in which demonstrate its central role in clot formation during hemostasis and thrombosis [48]. For prevention of SARS-CoV-2 and most importantly for general health, given that zinc supplements are readily available, they may be indicated for people with low or borderline low results, low dietary intake and/or increased needs. To optimise safety, a daily dose lower than the tolerable upper limits (<7 mg for children aged 1-3 years up to 22 mg for those aged 15-17 years) should be used along with dietary modifications when- ever possible. In adults, doses up to the No Observed Adverse Effect Level (NOAEL) of 50 mg/ day should be considered [49]. Intracel- lular zinc homeostasis is beneficial for prevention of Cardio Vascular Diseases (CVDs) that zinc tranporters can prevrent endothelial CVDs and Vascular Smooth Muscle Cells (VSMCs) functions [50]. Thus, zinc can prevent respiratory thrombosis and pulmonary thromboem- bolism by inhibition of thrombus formation growth in COVID-19 infection [51].
ZAP mediated RNA replication and spreading
Zinc-finger Antiviral Protein (ZAP) has high immune effect with COVID-19 therapy that ZAP restricts this pandemic viral pathogen. ZAP could control virus entry, DNA/RNA replication, and spreading against viral infection. The ZAP in first steps of HCV infection may be used as entry inhibitor [52]. That ZAP inhibits alphavirus repli- cation that elucidation of the antiviral mechanism by which ZAP in- hibits Sindbis virus (SINV) translation may lead to the development of agents with broad activity against alphaviruses [53]. The ZAP in- hibits virus entry, RNA replication and spread and promotes RNA degradation [54]. The ZAP inhibits Influenza A virus (IAV) protein expression, in which suggests an important role of ZAP in the host effort to control IAV infection and the importance of the threat of ZAP to the virus [55].
ZAP also may regulate DNA and RNA virus replication. ZAP in- hibits Retroviral RNA production [56]. That ZAP specifically inhibits the replication of certain viruses and promotes viral RNA degrada- tion [57]. The four zinc fingers of ZAP form extensive interactions with RNA, but mutations of both protein and RNA at the RNA-ZAP interacting surface reduce the in vitro binding affinity and antiviral activity, in which ZAP coordination promotes downstream RNA degradation [58]. That the ZAP could inhibit SARS-CoV-2 RNA vi- rus replication that SARS-CoV-2 is highly susceptible to interferons (IFNs), specifically targets of 3’-O-(2’-Deoxy-5’-guanylyl)-2’-de- oxy-5’-cytidylic acid (CpG) dinucleotides in viral RNA sequences re- stricts SARS-CoV-2, and might motivate assessment of combination therapies including IFN for treatment of COVID-19, in which ZAP restricts SARS-CoV-2 and contributes to its inhibition by IFNs [59].
Furthermore, ZAP is a host antiviral factor that specifically inhib- its the replication of Moloney Murine Leukemia Virus (MLV) and Sindbis virus (SIN) by preventing accumulation of the viral mRNA in the cytoplasm, ZAP inhibits HIV-1 infection by promoting the deg- radation of specific viral mRNAs that the mRNA decay is largely de- termined by the cis-acting elements [60]. Depletion of the exosome component with small interfering RNA significantly reduced ZAP’s destabilizing activity which ZAP is a trans-acting factor that mod- ulates mRNA stability [61]. Thus, ZAP could inhibit SARS-CoV-2 RNA virus replication. In addition, ZAP regulates spread that ZAP’ stress with antiviral activity and induced virus replication are regulat- ed upon virus infection to inhibit virus spread [62].
Zinc Binding Domain (ZBD) also could regulate 2019-nCoV RNA spike that zinc-binding status having Zn2+ ions-centered coordination structure could serve as the development of potential drugs for SARS therapies. A complex zinc finger ZBD modulates the enzymztic activities of coronaviridae-Nidovirus helicases, leading that the ZBD is critically involved in nidovirus replication and transcription [63]. Furthermore, zinc-finger proteins (ZNFs) for health and disease have play an important role with DNA, RNA, PAR (poly-ADP-ribose) and ZFNs are involved in the regulation of several cellular processes such as transcriptional regulation, ubiquitin-mediated protein degradation, signal transduction, DNA repair, and cell migration [64]. Increasing the intracellular Zn2+ dose could capably damage the replication of a wide range of RNA viruses, such as poliovirus, influenza virus and SARS-CoV, and Equine Arteritis Virus (EAV) [65].
Zinc induced ROS generation in respiratory and pulmo- nary COVID-19 infected cells, thrombosis, and ZAP-mediated RNA replication
Zinc induced ROS generation in respiratory and pulmonary COVID-19 infected cells is that the univalent reduction of oxygen generates superoxide (•O −), hydrogen peroxide (H O ), and hydroxyl radicals (•OH), all of which are Reactive Oxygen Species (ROS). The production of ROS and its elimination by the anti-oxidant defense system in cells is a highly modulated process for maintaining nor- mal physiological function in the body, in which the Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidases are a group of plasma membrane-associated enzymes which catalyze the production of superoxide •O − from oxygen by using NADPH as the electron do- nor [66].
Zinc induced ROS generation in pulmonary COVID-19 infected cells is that alterations of ROS-producing and scavenging pathways that are caused by respiratory viral infections are implicated in in- flammation, lung epithelial disruption, and tissue damage, and, in some cases, even pulmonary fibrosis. The observation that ROS are implicated in the pathology of these viruses is mainly based on ex- perimental infection models. Such inflammatory processes, especially sustained chronic conditions of inflammation, along with inflamma- tion-induced oxidative stress from dead or injured cells, could lead to irreversible cell ularortissue damage with the passage oftime,which further contributes to the development of chronic degenerative dis- eases [67].
The role of excessive immune activation as the cause of lung de- struction by SARS-CoV-2 supposes the causative virus of the pan- demic COVID-19. The oxidative stress by virally induced ROS pro- duction spirals cytokine release and immune cell infiltration in the lung as a result [68]. ROS generation in pulmonary inflammation is caused that zinc deficiency not only directly leads to oxidative stress through increased presence of ROS, causing it to generate superoxide to further compound ROS elevation [69]. Zinc induced ROS genera- tion promotes platelet activation that functional association between zinc ion concentration [Zn2+] and ROS generation could influence thrombus formation and ROS generation in pulmonary disease lead- ing to the induction of lung diseases [70].
ZAP-mediated ROS generation also may promote an effect that is conceivable that chronic inflammation-induced production of ROS in the lung may predispose individuals to lung diseases [71]. As men- tioned above, Zn2+ ions-induced immune antiviral activities for pre- vention and antibody of SARS-CoV-2, defenses from severe respira- tory ailment and acute pulmonary disease, anti-thrombus formation, and ZAP-mediated RNA replication against COVID-19 infection are represented in Table 1. However, COVID-19 degradation or destruc- tion by zinc-finger antiviral proteins remains yet unclear and further COVID-19 pulmonary care by zinc ion solutions may become of im- portance. Finally, accordingly, zinc ions induced immune molecular antiviral mechanism is caused that Zn2+ ions may be bound with re- spiratory and pulmonary proteins, thrombosis protein, and RNA rep- lication of Zn2+-RNA gene reaction by Zn2+ ion-centered coordinated tetrahedrally or Zn2+ triad binding structure formation [51,72].
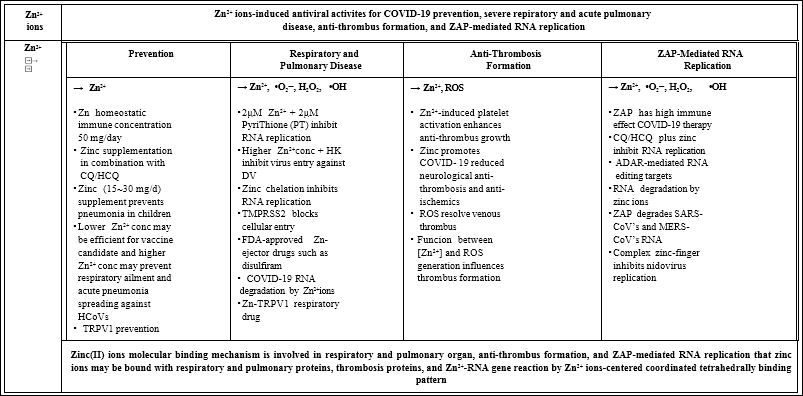
Table 1: Zn2+ ions-induced immunological virucidal activities for COVID-19 prevention, severe respiratory and acute pulmonary disease, anti-thrombus formation, and ZAP-Mediated RNA replication.
Conclusion
The zinc-homeostatic immune concentration may provide a pro- tective role against the COVID-19 pandemic, likely by improving the host’s resistance against viral infection. Zinc has exert homeostasis, immune-boosting, and antiviral responses that amount of zinc intake is likely to be beneficial 40-50 mg/day against the COVID-19 severe infection to have about 50 mg of zinc that would need to take 220 mg of zinc sulfate. Zinc ions-induced immune prevention is involved against COVID-19 infection that Zn may possess protective effect as preventive and adjuvant therapy of COVID-19 through reducing inflammation, improvement of mucociliary clearance, prevention of ventilator-induced lung injury, and modulation of antiviral immunity.
Zn2+ ions-induced prevention and antibody against SARS-CoV-2 infection are required Zn homeostatic immune concentration 50 mg/day, Zn supplementation in combination with CQ/HCQ, Zinc supplement (15 ~ 30 mg/d) preventing pneumonia in children, and Transient Receptor Potential Vanilloid 1 (TRPV1) prevention. Zinc induced immune antibody offers the potential to prevent and/or treat COVID-19, and possibly also other future emerging diseases in hu- mans caused by viruses from the Sarbecovirus subgenus for zinc a UL of 25 mg/day is recommended, supplemental daily doses of up to about 1 g, in addition to normal dietary intake, are not associated with adverse gastrointestinal effects.
In order to prevent that an outbreak of respiratory sickness caused by COVID-19 has become a serious public threat and disrupted many lives, assessing the efficacy of Zn-ejector drugs such as disulfiram combined with interferon to treat COVID-19 infected patients has been proposed. Stimulate cellular zinc ions were found to inhibit the replication of various RNA virus, influenza viruses, respiratory syncy- tial virus and coronaviruses that the defense on the severe respiratory patients infected with SARS-CoV, MERS-CoV and SARS-CoV-2 has clinical features range from mild respiratory illness to severe acute respiratory disease. Both MERS and SARS patients in later stages develop respiratory distress and renal failure.
The other, zinc chelation abrogated dengue virus RNA replication and zinc chelation abrogated dengue virus RNA replication. Transient zinc chelation induces Endoplasmic Reticulum (ER) stress and anti- viral response by activating NF-kappaB leading to induction of inter- feron signaling and zinc plays divergent roles in rotavirus and dengue virus infections in epithelial cells. Thus, zinc ions can prevent in the early stage of COVID-19 coronavirus outbreak and in the final stage could defend COVID-19 infection from severe respiratory and acute pulmonary disease. Lower Zn2+ concentration may be efficient for vaccine candidate and higher Zn2+ concentration may prevent respi- ratory ailment and acute pneumonia spreading against HCoVs. Thus, Zn2+ ions-induced virucidal defenses from COVID-19 severe bron- chitis and acute pneumonia are required that zinc ions can prevent in the early stage of 2019-nCoV infected patient, and the zinc ions have important roles for respirarory and pulmonary process of COVID-19 disease. Zn2+ inhibits coronavirus and anterivirus RNA polymelase activity, and zinc ionophores block the virus replication that Zn2+ ions + pyrithione at low concentrations inhibits the replication of SARS- CoV and arterivirus RNA.
Thus, the interaction had been found on the binding specificity by Zn2+ ions-centered tetrahedral geometric coordination of the in- hibitors against 3C proteases. Zinc ions complexes as SARS-CoV-2 3C-like protease inhibitors may play important role for this Zn2+-centered coordination pattern that the zinc-coordinating inhibitor of tet- rahedral zinc sites is tetrahedrally coordinated binding to such as the catalytic triad (Serine, Histidine and Aspartate Hydrogen Residues) of CVB3 3Cpro. Zinc serves for thrombus prevention as a ubiquitous modulator of haemostasis and thrombosis, aggregation, anti-coagula- tion and fibrinolysis that zinc controls blood clot formation on fibrin (ogen) expression and function, in which demonstrate its central role in clot formation during hemostasis and thrombosis. Zinc can prevent respiratory thrombosis and pulmonary thromboembolism by inhibi- tion of thrombus formation growth in COVID-19 infection. ZAP has high immune effect with COVID-19 therapy and ZAP restricts this pandemic viral pathogen that ZAP inhibits virus entry, RNA replication and spread, and promotes RNA degradation. ZAP also may regulate DNA and RNA virus replication. Inhibition of bacterial DNA replication during nitrosative stress is accompanied by zinc mobilization. ZAP inhibits Retroviral RNA production that ZAP specifically inhibits the replication of certain viruses and promotes viral RNA degradation.
Zinc induced ROS generation promotes platelet activation that functional association between zinc ion concentration [Zn2+] and ROS generation could influence thrombus formation. ROS generation in pulmonary disease leading to the induction of lung diseases, Finally, Zinc(Ⅱ) ions molecular binding mechanism is involved in respiratory and pulmonary organ, anti-thrombus formation, and ZAP-mediated RNA replication that zinc ions may be bound with respiratory and pulmonary proteins, thrombosis proteins, and Zn2+-RNA gene reaction by Zn2+ ions coordinated tetrahedrally binding pattern.
Conflicts of Interest
The author declares there are no conflicts of interest.
Sources of Funding
None.
References
- Wessels I, Maywald M, Rink L (2017) Zinc as a gatekeeper of immune Nutrients 9: 1-44.
- Read SA, Obeid S, Ahlenatiel C, Ahlenstiel G (2019) The role of zinc in antiviral immunity. Adv Nutr 0:1-15.
- Ishida T (2020) Zinc Immune Anti-Infectious Activities of Bacteriolysis by Zn2+-induced Bacterial PGN Autolysins and ZAP’s Viral Destruction by Cell Surface Receptors. Biomedical Research and Reviews 3: 1-8.
- Ishida T (2020) Virucidal Activities of Zinc-Finger Antiviral Proteins and Zinc-Binding Domains for Virus Entry, DNA/RNA Replication, and Edelweiss Journal of Biomedical Research and Review 2: 9-13.
- Wessels I, Rolles B, Rink L (2020) The Potential Impact of Zinc Supple- mentation on COVID-19 Frontiers in Immunology 11: 1-11.
- Chinni V, El-Khoury J, Perera M, Bellomo R, Jones D, et al. (2021) Zinc supplementation as an adjunct therapy for COVID-19: Challenges and op- Br J Clin Pharmacol 87: 3737-3746.
- Samad N, Sodunke TE, Abubakar AR Jahan I, Sharma P, et (2021) The Implications of Zinc Therapy in Combating the COVID-19 Global Pan- demic. Journal of Inflammation Research 14: 527-550.
- Mirza MU, Froeyen M (2020) Structural elucidation of SARS-CoV-2 vital proteins: Computational methods reveal potential drug candidates against Main protease, Nsp12 RNA-dependent RNA polymerase and Nsp13 heli- Preprint 5: 2-23.
- Duffy S (2018) Why are virus mutation rates so damn PLOS Biology 16: 1-6.
- Belouzard S, Millet JK, Licitra BN, Whittaker GR (2012) Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 4: 1011-1033.
- Wu C, Liu Y, Yang Y, Zhang P, Zhong W, et (2020) Analysis of thera- peutic targets for SARS-CoV-2 and discovery of potential drugs by com- putational methods. Acta Pharmaceutica Sinica B 10: 766-788.
- Wu A, Niu P, Wang L, Zhou H, Zhao X, et (2020) Mutation, recombi- nation and insertion in the evolution of 2019-nCoV. bioRxiv preprint 29: 1-38.
- Schoeman D, Fielding BC (2019) Coronavirus envelope protein: Current Viology Journal 69: 1-22.
- Mohseni M, Mansouri MRM, Gowder SJT (2020) Critical Analysis of the Role of Micronutrient-Zinc on Covid-19 Activity. Biomed J Sci & Tech Res 30: 1-5.
- Johns Hopkins Bloomberg School Of Public Health (2020) SARS-CoV-2 16: 1-2.
- Gao Y, Yan L, Huang Y, Liu F (2020) Structure of RNA-dependent RNA polymerase from 2019-nCoV, major antiviral drug BioRxiv preprint 6: 1-24.
- Zahradník J , Marciano S, Shemesh M, Zoler E, Harari D, et al. (2021) SARS-CoV-2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. Nature Microbiology 6: 1188-1198.
- Yin C (2020) Genotyping coronavirus SARS-CoV-2: Methods and impli- ArXiv preprint 25: 1-12.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, et al. (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion Science 367: 1260-1263.
- Maywald M, Wessels I, Rink L (2017) Zinc signals and J Mol Sci 18: 1-34.
- Mayor-Ibarguren A, Busca-Arenzana C, Robles-Marhuenda Á (2020) A hypothesis for the possible role of zinc in the immunological pathways related to the COVID-19 infection. Frontiers in Immunology 1736: 1-8.
- Gammoh NZ, Rink L (2017) Zinc in infection and Nutrient 9: 1-25.
- de Almeida Brasiel PG (2020) The key role of zinc in elderly immunity: A possible approach in the COVID-19 crisis. Clinical Nutrition ESPEN 38: 65-66.
- Mustafa KS, Aljohani MMH, Alomrani NA, Oyouni AAA, Alzahrani O, et (2020) COVID-19 and Immune Function–“A Significant” Zinc. Orien- tal Journal Of Chemistry 36: 1026-1036.
- Razzaque MS (2020) COVID-19 Pandemic: Can Maintaining Optimal Zinc Balance Enhance Host Resistance? Tohoku J. Exp. Med 251: 175-
- Derwand R, Scholz M (2020) Does zinc supplementation enhance the clinical efficacy of chloroquine/ hydroxychloroquine to win today’s battle against COVID-19?. Medical Hypotheses 142: 1-3.
- Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, et al. (2020) Zinc and respiratory tract infections: Perspectives for COVID-19 (Review). International Journal of Molecular Medicine 13: 1-10.
- Sargsyan K, Chen T, Grauffel C, Lim C (2020) Identifying COVID-19 Drug-Sites Susceptible To Clinically Safe Zn-ejector Drugs Using Evolu- tionary /Physical Principles. Print: 1-10.
- Janda KD, Iadarola MJ (2020) Standing out from the crowd in treating COVID-19. Medicine in Drug Discovery 6: 1.
- Derbyshire E, Delange J (2020) COVID-19: Is there a role for immunonu- trition, particularly in the over 65s?. BMJ Nutritioh Prevention & Health 0: 1- 6.
- Lipsitch M, Kahn R, Mina MJ (2020) Antibody testing will enhance the power and accuracy of COVID-19-prevention Nature Medicine 27: 1-2.
- Wang C, Li W, Drabek D, Okba NMA, van Haperen R, et al. (2020) A human monoclonal antibody blocking SARS-CoV-2 infection. BioRxiv 12: 1-24.
- te Velthuis AJW, van den Worm SHE, Sims AC, Baric RS, Snijder EJ, et (2010) Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLOS Pathogen 11: 1-10.
- Gupta MK, Vemula S, Donde R, Gouda G, Behera L, et (2020) In-silico approaches to detect inhibitors of the human severe acute respiratory syn- drome coronavirus envelope protein ion channel. Journal of Biomolecular Structure and Dynamics 739-1102.
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, et al. (2020) SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181: 271-280.
- Luo J, Bavencoffe A, Yang P, Feng J, Yin S, et al. (2018) Zinc Inhibits TRPV1 to Alleviate Chemotherapy-Induced Neuropathic Pain. The Jour- nal of Neuroscience 38: 474-483.
- Nahama A, Ramachandran R, Cisternas AF, Ji H (2020) The role of af- ferent pulmonary innervation in ARDS associated with COVID-19 and potential use of resiniferatoxin to improve prognosis: A Medicine in Drug Discovery 5: 1-5.
- Derwand R, Scholz M (2020) Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win todays battle against COVID-19?. Preprint 8: 1-10.
- Noeparast A, Verschelden G (2020) Can Zinc correction in SARS-CoV-2 patients improve treatment outcomes? Preprint 7: 1-14.
- Nieto-Torres JL, DeDiego ML, Verdiá-Báguena C, Jimenez-Guardeño JM, Regla-Nava JA, et (2014) Severe acute respiratory syndrome coronavi- rus envelope protein ion channel activity promotes virus fitness and patho- genesis. PLOS pathogens 5: 1-19.
- Jin Y, Cai L, Cheng ZH, Deng T, Fan Y, et al. (2019) A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (COVID-19) infected pneumonia (standard version). Military Medical Research 7: 1-23.
- Wu JQ, Zha P (2020) Preventive, Mitigating and Treatment Strategies for Containing or Ending The COVID-19 Pandemic. Preprint for comments 1-38.
- Wu JQ, Zha P (2020) Treatment Strategies for Reducing Damages to Lungs In Patients with Coronavirus and Other Infections. Preprint 1-31.
- Laghari GS, Hussain Z, Taimur M, Jamil N (2019) Therapeutic Role of Zinc Supplementation in Children Hospitalized with Pneumonia. Cureus 11: 1-6.
- Cuevas UE, Koyanagi A (2005) Zinc and infection: A Annals of Tropical Paediatrics 25: 149-160.
- Kar M, Khan NA, Panwar A, Bais SS, Basak S, et al. (2019) Zinc Che- lation Specifically Inhibits Early Stages of Dengue Virus Replication by Activation of NF-κB and Induction of Antiviral Response in Epithelial Frontiers in Immunity 10: 1-15.
- Lee C, Kuo C, Ko T, Hsu M, Tsui Y, et al. (2009) Structural Basis of In- hibition Specificities of 3C and 3C-like Proteases by Zinc-Coordinating and Peptidomimetic Compounds. Journal of Biological Chemistry 284: 7646-7655.
- Kattula S, Byrnes JR, Wolberg AS (2017) Fibrinogen and Fibrin in Hemo- stasis and Thrombosis. Arterioscler Thromb Vasc Biol 37: e13-e21.
- Arentza S, Huntera J, Yanga G, Goldenbergb J, Beardsley J, et al. (2017) Zinc for the prevention and treatment of SARS-CoV-2 and other acute viral respiratory infections: A rapid review. Advances in Integrative Med- icine 7: 252-260.
- Tamura Y (2021) The role of zinc homreostasis in the prevention of dia- betes mellitus and cardiovascular diseases. Journal Atheroscler Thrombo 28: 1-14.
- Ishida T (2021) Zinc (Ⅱ)-induced neurological anti-thrombus formation against severe COVID-19 infection. Aditum Journal of Clinical and Bio- medical Research 2: 1-8.
- The Rockefeller University (2021) Virus entry and virus-host Laboratory of Virology and Infectious Disease.15: 1.
- Bick MJ, Carroll JWN, Gao G, Goff SP, Rice CM , et al, (2003) Expression the zinc-finger antiviral protein inhibits alphavirus replication. Journal of Virology 77: 11555-11562.
- Nchioua R, Kmiec D, Müller JA, Conzelmann C, Groß R, et al. (2020) SARS-CoV-2 Is Restricted by Zinc Finger Antiviral Protein despite Pread- aptation to the Low-CpG Environment in mBIO American Soci- ety for Microbiology mBIO 11: 1-19.
- Tang Q, Wang X, Gao G (2017) The short form of the zinc finger antiviral protein inhibits influenza A virus porotein expression and is antagonized by the virus-encoded NS1. Journal of Virology 91: 909-916.
- Gao G, Guo X, Goff SP (2002) Inhibition of Retroviral RNA production by ZAP, a CCCH-type zinc finger protein. Science 297: 1703-1706.
- Wang X, Lv F, Gao G (2010) Mutagenesis analysis of the zinc-finger anti- viral protein. Retrovirology 7: 1-9.
- Luo X, Wang X, Gao Y, Zhu J, Liu S, et (2019) Molecular mechanism of RNA recognition ny zinc-finger antiviral protein. Cell Reports 30: 46- 52.
- Nchioua R, Kmiec D, Müller J, Conzelmann C, Groß R, et al. (2020) The Zinc Finger Antiviral Protein restricts SARS-CoV-2. BioRxiv preprint 1-40.
- Sapiro AL, Freund EC, Restrepo L, Qiao H, Bhate A, et al (2019) Zinc fin- ger RNA binding protein Zn72D regulates ADAR-mediated RNA editing in neurons. BioRxiv reprint 1-43.
- Guo X, Ma J, Sun J, Gao G (2007) The zinc-finger antiviral protein recruits the RNA processing exosome to degrade the target mRNA. PNAS 104: 151-156
- Law LMJ, Razooky BS, Li MMH, You S, Jurado A, et al. (2019) ZAP’s stress granule localization is correlated with its antiviral activity and in- duced by virus replication. PLOS Pathogens 15: 1-22.
- Seybert A, Posthuma CC, Dinten LCV, Snijder EJ, Gorbalenya AE, et al. (2005) A complex zinc finger controls the enzymatic activities of nidovirus Journal of Virology 79: 696-704.
- Cassandri M, Smirnov A, Novelli F, Pitolli C, Agostini M , et al. (2017) Zinc-finger protein in health and disease. Cell Death Discovery 3: 1-12.
- Velthuis AJW, Worm SHE, Sims AC, Baric RS, Snijder EJ, et al. (2010) Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLOS Pathogen 6: 1-10.
- Prasad AS,Bao B (2019) Molecular Mechanisms of Zinc as a Pro-Anti- oxidant Mediator: Clinical Therapeutic Antioxidants 8:1-22.
- Khomich OA, Kochetkov SN, Bartosch B, Ivanov AV (2018) Redox Biol- ogy of Respiratory Viral Infections. Viruses 10: 1-27.
- Nasi A, McArdle S, Gaudernack G (2020) Proteasome and reactive oxy- gen species dysfunction as risk factors for SARS-CoV-2 infection; consid- er N-acetylcystein as therapeutic intervention 4: 1-5.
- Sethuram R, Bai D, Abu-Soud HM (2021) Potential Role of Zinc in the COVID-19 Disease Process and its Probable Impact on Re- productive Sciences 7: 1-6.
- Pires MEL, Ahmed NS, Vara D, Gibbins JM, Pula G, et al. (2021) Zinc regulates reactive oxygen species generation in Platelets 32: 368- 377.
- Rosanna DP, Salvatore C (2012) Reactive Oxygen Species, Inflammation, and Lung Diseases. Current Pharmaceutical Design 18: 3889-3900.
- T Ishida (2021) Zinc-Induced Neurological Anti-Thrombosis and Throm- bolysis in Children Under Severe Covid-19 Infective Journal of Current Trends in Nursing & Health Care 2: 1-9.
Citation: Ishida T (2021) Zinc (Ⅱ)-Induced Immunological Antiviral Activities for COVID-19 Prevention, Respiratory and Pulmonary Infection, Anti-Thrombus Forma- tion, and ZAP-Mediated RNA Replication. J Immuno Immunothe 4: 010.
Copyright: © 2021 Ishida T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.


