*Corresponding Author:
Ping Chung Leung,
Department of Chemical Pathology, The Chinese University of Hong Kong, Hong Kong
Tel: +852 22528868
E-mail: pingcleung@cuhk.edu.hk
Abstract
This Protocol of Research Procedures is designed for the urgent justification of a herbal supplement for the prevention of the CoVID-19 infection among workers exposed to the virus. The study includes a pilot clinical trial for the effects of the supplement on the quality of life of consumers including clinical ‘Cold’ symptoms and their immunological changes responding to the supplement. The major investigation will be concentrated on the in-vitro and in-vivo studies on the immune responses to the herbal extracts with special emphasis on Macrophage reactions.
The herbal formula aimed at the boostering of one’s innate immunological defense and had been clinically tested in the 2003 SARS crisis when over 2,000 at risk hospital attendants took it as a preventive supplement for 2 weeks after which none was infected compared with a 0.4% infection rate among over 10,000 other attendants who served as ‘Controls’. Subsequent studies in the laboratory further supported its immunological effects. It is expected this extension study could further justify the herbal formula as an evidence-based self-protective prevention agent against respiratory tract viral infection like Vitamin D which has undergone decades of investigation eventually endorsed.
Keywords
CoVID-19; SARS-CoV-2 infection
Introduction
An outbreak of pneumonia caused by Novel Corona virus (CoVID-19) in Wuhan, Hubei province in China, started in December 2019, and spread to more than 40 countries worldwide. This outbreak brought back memories of the severe acute respiratory syndrome (SARS) in China and Hong Kong in 2003, caused by a SARS-CoV2-coronavirus [1-5]. SARS-CoV-2 rapidly spread from southern China in 2003 and infected more than 3000 people, killing 774 by 2004. In 2012, the Middle East Respiratory Syndrome (MERS) Corona-virus (MERS-CoV), a lethal zoonotic pathogen that was first identified in humans in the Kingdom of Saudi Arabia. It continued to emerge and re-emerge through intermittent sporadic cases, community clusters and nosocomial outbreaks [6]. Severe pneumonia is associated with the coronavirus infections [7-10]. There was significant correlation between the levels of interleukin (IL)-6, IL-10 and IL-15 and disease severity [2,11,12]. From clinical studies of patients who died of SARS and other related animal studies, extensive lung damage was is associated with high initial viral loads, increased inflammatory monocyte-macrophages accumulation in the lungs, and elevated serum proinflammatory cytokines [13,14]. Therefore, inflammation is the most important pathological process in the airway of patients with viral pneumonia. Treatment of corona virus associated pneumonia up to now, is mainly supportive due to the lack of specific anti-viral medication and vaccination.
The present CoVID-19 virus greatly resembles the SARS virus in structure, although its infectivicity greatly exceeds that of SARS while the mortality appears lower. In spite of the differences, the principle of treatment for both coronal viruses up to now, follows closely, while vaccination is to be waited.
Pathological changes in the immunological system in response to viral attack
Innate immune cells are the frontline cells in respond to viral infections. Virus replication-induced production of cytokines from airway epithelium recruits innate immune cells to the site of infection. These leukocytes, including neutrophils, monocytes, macrophages, dendritic cells, eosinophils, natural killer cells, innate lymphoid cells and Gamma delta (γδ) T cells, become activated in response to virus, to contain the virus and protect the airway epithelium while triggering the adaptive immunity. However, excessively activated immune response can lead to the increasing influenza pneumonia severity [15,16]. Anti-influenza virus activities of innate immune cells play the central regulatory roles for the balance between immune protection and immunopathology during virus infection [17].
Corona virus is an enveloped positive-sense RNA virus, which is characterized by club-like spikes projecting from its surface [18]. Macrophages, the major effector cells in the innate immune system, recognizes viral infection through Pattern Recognition Receptors (PRRs) such as Toll like receptors (TLRs) and RIG-I-like receptor (RLRs) which detect the conserved microbial components called pathogen-associated molecular patterns (PAMPs). During infection, TLR and RLR are essential for the recognition of microbial pathogens to activate intracellular signaling pathways for distinct pattern of gene expression that result in innate immune response against microbial infections and the development of antigen-specific acquired immunity. Among various know PRRs,TLR3 responses to double stranded RNA, a replication intermediate for many viruses [19]. TLR3 is therefore involved in antiviral responses by triggering the production of antiviral cytokines such as interferon (IFN) and other Th1 cytokines. RIG-1-like receptors (RLRs) constitute a family of cytoplasmic RNA helicases which are important to initiate the host antiviral responses. For example, RIG-I/retinoic-acid-inducible gene 1 has been shown to sense viral RNA, leading to production of type I interferons/IFNs [20]. In our previous studies on adults hospitalized with viral infection, we confirmed that TLRs played an important role for innate viral inhibition in naturally occurring influenza [21].
What can we do against getting infected?
We all yearn for the vaccine which directly challenges the CoVID-19 virus. Before the vaccine becomes a reality, we could only concentrate on Self-Protection.
Apart from physically guarding against the coronal virus like avoiding personal contacts and safety apparels, face masks etc. susceptible people in the epidemic areas could be enlightened on how to protect themselves via normal physiological channels. Immunological defense has been mentioned in the last paragraph. A state of immunological harmony exists in the normal individual when his/her nutritional state is perfect and physical activities well balanced. In addition, specific immunological boostering supplements could be considered under special circumstances. Good choices of immunological boostering supplements are available. They do not target against the pathogen; neither do they claim specific respiratory relieves. They help the individual to improve his/her immunological defense against infection.
Traditional Chinese Medicine has long been used for flu-like syndrome prevention with reported good results. In the SARS outbreak in 2003, we have developed an innovative herbal formula Kwan Du Bu Fei Dang (抗毒補肺湯) [KDBFD] with the aim of helping frontline healthcare workers to prevent contracting the disease. It was based on two classical, popularly used herbal formulae for treating influenza-like syndrome known as Wan Bin (溫病) [8,22]. The formula was a combination of Sang Ju Yin (桑菊飲) and Yu Ping Feng San (玉屏風散) plus two other herbs with well-known antiviral properties. The front-line workers were spread in 11 hospitals which were accommodating over 900 infected patients under special care. Serving attendants were obviously under high risks although they were physically well protected. The aim of the supplement was to initiate better innate immunological function among them.
Details of the components of the formula KDBFD and the dosages are given in the table 1 as below.
2061 Frontline workers took the formula for 2 weeks, none of them was infected, in contrast to a 0.4% infection rate among those not taking KDBFD. Laboratory studies done subsequently, provided more evidence of the immuno-boostering effects of the innovative formula [8,22].
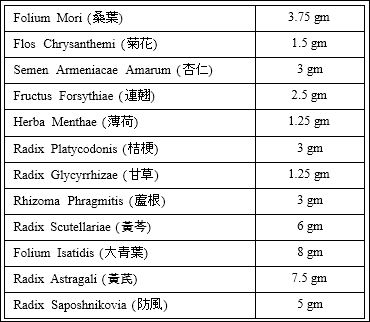
Table 1: KDBFD Formula.
Protocol for the Development of a Preventive Agent against CoVID-19 infection for personal protection
Aims and Hypotheses
To cope with the current life threatening coronavirus outbreak, we herein hypotheses that KDBFD could be a specific preventive agent for the personal protection against the CoVID-19 infection.
Historically, KDBFD has been used to prevent flu-like syndrome. During the SARS crisis 17 years ago, it has helped at risk front-line hospital workers successfully resisting the infection. Since there are similarities between SARS and the present CoVID-19 pandemic, there is good chance that KDBFD could work equally well. Apart from investigating the underlying mechanisms of action which will provide more evidence for its use in the prevention of viral infections, a proper clinical study would give additional evidence of its preventive value.
A. Clinical Study
Since the crisis of CoVID-19 epidemic in Hong Kong is not yet over, a clinical trial on the preventive value of KDBFD could be done on volunteers facing extra risks of infection like certain hospital workers, people under quarantine and perhaps discharged patients, many of whom were reported to get ‘re-infected’. The design of the study follows the standard requirements of a single arm, self-control, shift over pilot study arrangements. A total of 30 volunteers would be the target inclusion.
(As a matter of fact, a single arm, self-control, cross-over pilot study focusing on prevention using only KDBFD already started in March 2020).
Inclusion: a) All ages who fulfil the “high-risk” criteria. b) All subjects undergoing quarantine. c) Patients recovered from the infection.
Exclusion: a) Acute infections, chronic diseases experiencing exacerbations. b) Children below 12 Sensitivity to Herbal Medicine.
Medication: One standard dose of KDBFT daily for 2 weeks.
Outcome measures: a) Quality of Life. b) Symptoms of influenza. c) Immunological studies.
Subjects will be required to take one sachet of KDBFD (4 g) or pla- cebo once per day for 14 days, then the two groups would shift over. Blood samples will be collected from them, and checked for the ex vivo production of cytokines, and the percentage and absolute num- bers of CD4+ and CD8+ T-lymphocytes, the CD4/CD8 ratio, CD56+ NK cells, and CD19+ B-lymphocytes will be evaluated. The plasma concentrations of inflammatory cytokines/chemokines (e.g. IL-1β, IL- 6, TNF-α, CXCL8, CCL2 and IL-17) of participants will be measured by enzyme-linked immunosorbent assay (ELISA) kits following the manufacturer’s instructions.
B. Statistical Analysis
Statistical analysis will be performed using SPSS ver. 22 (IBM SPSS Inc., Chicago IL.).
For the sample size consideration, we think 30 volunteers is sufficient for the self-control pilot study.
Continuous variables will be compared by Student’s T test or Mann-Whitney U-test.
Paired t-test will be utilized to evaluate the difference between pretreatment mean and post-treatment mean.
Categorical variables will be compared by Chi-square test or Fisher’s Exact Test.
The percentage changes from baseline to 2 weeks of treatment with KDBFD and Vitamin D will be analyzed by using Chi-Square test.
A two-tailed P value of less than 0.05 is considered statistically significant.
C. Immunological Studies
Macrophage Studies
a. To investigate the direct in vitro effects of KDBFD extract on coronavirus-related CoVID-19 spike protein, TLR3 ligand polyinosinic-polycytidylic acid (polyIC) and RIG-like receptor ligand polyIC/ LyoVec complexes-activated the co-culture of human peripheral macrophages and alveolar basal epithelial cells A549 cells in term of (i) production of essential inflammatory cytokine IL-1β and IL-6, and chemokine CXCL8 for neutrophils, CCL2 for monocytes and Th17 cytokine IL-17 (ii) cell surface expression of adhesion molecule CD11b/Mac-1 on macrophages and alveolar basal epithelial cells and (iii) adhesion of macrophages onto alveolar basal epithelial cells.
b. To focus on the relationship between the KDBFD extract-mediated activation of crucial intracellular signaling molecules, including ERK, c-Jun amino-terminal kinase (JNK), p38 MAPK, and inflammatory transcription factor NF-kB, and the expression of cytokines, chemokines and adhesion molecules, as well as macrophage adhesion.
c. Using viral derivative dsRNA PolyIC-mediated pneumonia BALB/c mice to evaluate the in vivo immunoregulatory and anti-inflammatory activity of the intraperitoneal administration of murine KDBFDextract on the concentration of pneumonia-related cytokines/ chemokines, the accumulation of neutrophils, macrophages and regulatory T cells (Treg), Th17 and Th1/2 cells in circulation and bronchoalveolar lavage (BAL) fluid, and histopathological changes of lung.
d. Human Monocyte-derived Macrophage from fresh human buffy coat will be obtained from healthy volunteers of the Hong Kong Red Cross Blood Transfusion Service for the purification of primary human macrophages. Peripheral blood mononuclear cells (PBMC) will be isolated by Ficoll density (1.082 g/ml) centrifugation for 25 min at 1800 rpm. After RBC lysis, CD14 specific MACS beads (Miltenyi Biotec) will be used for the enrichment of CD14+ Monocytes. For the induction of macrophage differentiation, CD14+ monocytes will be cultured in tissue-culture plates for 6-7 days in RPMI 1640 medium with L-glutamine, 10% FCS, 1% Penicillin-Streptomycin, 1% Sodium pyruvate and 1% Glutamax (GIBCO) and GM-CSF (25 ng/mL) at a density of 1.5 x 105/cm2 [28].
e. Assay of cytokines and chemokines.
The concentrations of pneumonia-related cytokines/chemokines (IL- 1β, IL-6, TNF- α, CXCL8, CCL2 and IL-17) in culture supernatant or mouse serum will be quantitated by Bio-plex human cytokine/ chemokines multiplex assay by using the Bio-plex 200 System (Bio- Rad) [24,29-31].
f. Assessment of adhesion.
Alveolar basal epithelial A549 cells will be cultured to confluence in a 24-well culture plate, and then rinsed with PBS at 37oC to prevent cell detachment. Macrophage suspensions (5 x 105/ml) with different treatment will then be applied onto A549 cells in the culture plate. The epithelial cells-macrophages co-cultures will be incubated, with or without KDBFD extract, for a further 2 hours and 18 hours. After incubation, the plate will be rinsed twice with warm PBS, dried and stained with Giemsa staining solutions. The numbers of macrophages attached onto the A549 cells will be counted in three randomized low-power fields (100X) per well under an inverted microscope, in accordance with our established method [32].
g. Luciferase reporter assay for NF-kB activity.
Macrophages will be cultured in 96-well plate (4 x 104 cells/well) with RPMI 1640 medium supplemented with 10% heat inactivated FBS for 4 hours, and transfected with 200 ng of NF-kB reporter plas- mid (Promega) using FuGENE® HD transfection reagent together with a DNA-transfection reagent (ratio of 1:3). After 36-hours incubation, the cells will be treated with KDBFD extract with or without other stimulators and Luciferase activity will be measured using established Luciferase Assay System [24].
h. Flow cytometry of adhesion molecules on the cell surface
Direct immunofluorescent staining will be used to determine the cell surface expression of CD11b/Mac-1 on macrophages and epithelial A549 cells [24-27]. The cells will be washed and blocked with human pooled serum and incubated either with FITC-conjugated primary mouse anti-human IgG antibody against the specific cell surface antigens, or with mouse IgG isotype antibody. After washing, the cells will be resuspended in PBS with 0.1% BSA. Surface adhesion molecules will be analyzed by flow cytometry (BD FACSVia flow cytometer). The expression of the adhesion molecules on these two cell types will be analyzed separately by flow cytometry gated by their distinct pattern of forward and side light scatter [25,27-30].
i. Intracellular staining of activated (phosphorylated) signalling molecules
Established quantitative flow cytometry will be used to investigate the activation of signalling molecules in macrophages and epithelial A549 cells. Macrophages or epithelial A549 cells with different treatments will be fixed with 4% formaldehyde for 10 minutes at 37oC. The cells will be washed once and permeabilized in ice-cold absolute methanol for 30 minutes, then stained with FITC-conjugated mouse anti-hu- man monoclonal antibodies against phosphorylated ERK1,2, JNK, p38 MAPK and inhibitor kappaB (IKB) or isotypic control mouse IgG1 for 30 minutes at 4oC in the dark. The cells will then be washed, re-suspended and subjected to analysis. Expression as mean fluores- cence intensity (MFI) of intracellular signaling molecules of 10,000 macrophages or epithelial A549 cells, will be separately analyzed by flow cytometry gated by their distinct pattern of forward and side light scatter by using flow cytometer [25,28,31].
Note: All in-vitro studies would need two times repetitions
Animal Study
Mice with CoVID-19 spike protein, Poly(IC) and polyIC/Ly- oVec-induced pneumonia [32]
Inbred female BALB/c mice (6-8 weeks old) will be bred under specific pathogen-free conditions and maintained at our Laboratory Animal Services Centre, CUHK. Mice will be anesthetized by inhaled diethyl ether, and 50 mg of CoVID-19 spike protein with poly (IC) or polyIC/LyoVec (Inivivogen) will be administered intranasally (i.n.). Optimized dose of KDBFD extract will be given by intraperitoneal (i.p.) injection just before the sensitization and challenge. Mice will be given CoVID-19 spike protein and poly (I:C), polyIC/LyoVec or PBS on day 0 and day 3 and will be sacrificed at day 1, 4 and 7. Broncho- alveolar lavage fluid (BALF) will be collected for the flow cytometric analysis of immune cell infiltration. Lung tissue will be fixed and sec- tions will be stained with hematoxylin and eosin (H&E) and toluidine blue/Giemsa staining solutions for examining morphological changes, and pulmonary infiltration of neutrophils, macrophages and T cells. Serum will be obtained for the assay of pneumonia-related inflamma- tory cytokines/chemokines (e.g. IL-1β, IL-6, TNF-α, CXCL8, CCL2 and IL-17). Splenic CD4+CD25+Foxp3+ Treg and Th17 cells will be analysed by flow cytometric analysis [29,33]. Results will then be compared among Poly(IC)/polyIC/LyoVec-induced pneumonia mice with or without Kwan Du Bu Fei Dang extract and normal mice. Two groups of mice with 6 in each group would be the rule of study.
Discussion
While we ae still actively living and working within the present most insidious environment of the CoVID-19 epidemic, the fear and uncertainties have reached an ever-incomparable altitude. Since no effective vaccine is yet available, people living either in the seriously affected zone or vicinities are all longing for some preventive agent with sufficient reliability, to protect themselves against getting infected.
We come across the Harvard Gazette of February, 2017 [34] which quoted two most informative publications concerned with the use of Vitamin D to protect against Colds and Flu. Scientific evaluations are concentrated on immunological defence while clinical reports are concerned with epidemiological efficacy data. The authors gave good evidences of Vitamin D providing anti-inflammatory effects via innate and adaptive immune responses [35]. On the clinical side, controversial reports did not allow definite conclusions on the preventive efficacy [36]. In spite of the failure to get an unequivocal one-word answer of “yes” or “no” to the question of whether Vitamin D should be endorsed as a preventive, or adjuvant agent for prevention, in view of the lack of effective preventive agent, authors still advise that Vitamin D should be considered together with other essential nutritives as favorable in the overall support and fight against the infection [34].
Cold and Flu affections most probably have been common for all human beings ever since the pre-historical era. Traditional Chinese Medicine which served the Chinese people since over 3,000 years ago, has valuable records on the use of herbs for the treatment and prevention of Cold and Flu. In the past decades, many of these herbs and herbal formulae have been put on bioactivity platforms and shown to be anti-inflammatory and immunologically boostering [37,38]. Clinical trials with different levels of reliability have also been done, resulting in an over-all conclusion of unconfirmed efficacy, yet showing positive complex immunological responses favouring prevention in situations of viral attacks [39]. The conclusion looks similar to that of Vitamin D research [40].
During an epidemic, clinical trial arrangement is difficult if at all possible. In the 2003 SARS outbreak in Hong Kong, over 900 infected patients were hospitalized in eleven hospitals. A total of over 12,000 workers were involved in the services involved. We managed to arrange within a short time of 10 days a clinical trial of self-control nature for the at-risk workers using the herbal formula KDBFD as a self-protection measure. 3,160 workers volunteered to take the prescription for two weeks, followed by a quality of life assessment and infection rate check. The result gave a zero infection for the herbal group compared with a 0.4% infection rate of other hospital workers serving as controls. 37 donated blood samples before and after herbal consumption for immunological assessments which demonstrated boostering effects on the innate protective ability [23]. We have confidence therefore choosing KDBFD as a potential preventive agent against the CoVID-19 coronal viral infection this present time.
The research protocol being presented has a major emphasis on the herbal medication’s immunological effects in the laboratory while the clinical trial could only serve as a preliminary investigation on its real preventive effects. However, the changes in the immunological state of the volunteers would be helpful as further evidence on the preventive value of KDBFD.
Many medicinal herbs have been described as preventive and treatment choices to combat against Cold and Flu. KDBFD contains herbs that are most frequently used for the same purpose of anti Cold and Flu. None of the constituents is phytochemically toxic and reported adverse effects have all been mild. KDBFD could be considered an up-date representation of the classical wisdom of ancient healers who were ignorant about causes of diseases and pathological changes involved.
Once the complex events which affect the immune responses during the consumption of the herbal formula could be clearly worked out, its endorsement of application as a personal protection agent, like Vitamin D is expected. The platform studies will give evidence-based support to the said formula’s immunological boostering effects, thus giving it solid support for subsequent development into a Preventive Agent against viral respiratory infection at large.
Before the target orientated vaccine is ready, the self-protective immunologically boostering supplement could be advocated as a good personal choice.
References
- Leung PC, Ooi EE (2003). SARS War-Combatting the Disease. World Scientific, Singapore.
- Tang NL, Chan PK, Wong CK, To KF, Wu AK, et al. (2005) Early enhanced expression of interferon-inducible protein-10 (CXCL- 10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin Chem 51: 2333-2340.
- Wong RS, Wu A, To KF, Lee N, Lam CW, et al. (2003) Haema- tological manifestations in patients with severe acute respiratory syndrome: Retrospective analysis. BMJ 326: 1358-1362.
- Chan MH, Wong VW, Wong CK, Chan PK, Chu CM, et (2004) Serum LD1 isoenzyme and blood lymphocyte subsets as prognostic indicators for severe acute respiratory syndrome. J Intern Med 255: 512-518.
- Panesar NS, Lam CW, Chan MH, Wong CK, Sung JJ (2004) Lymphopenia and neutrophilia in SARS are related to the prevailing serum cortisol. Eur J Clin Invest 34: 382-384.
- Chu DK, Poon LL, Gomaa MM, Shehata MM, Perera RA et al. (2014) MERS coronaviruses in dromedary camels, Emerg Infect Dis 20: 1049-1053.
- Lam CW, Chan MH, Wong CK (2004) Severe acute respiratory syndrome: clinical and laboratory manifestations. Clin Biochem Rev 25: 121-132.
- Fung KP, Leung PC, Tsui KW, Wan CC, Wong KB, et al. (2011) Immunomodulatory activities of the herbal formula Kwan Du Bu Fei Dang in healthy subjects: a randomised, double-blind, placebo-controlled study. Hong Kong Med J 17 Suppl 2: 41-43.
- Hui DS, E IA, Madani TA, Ntoumi F, Kock R, et al. (2020) The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wu- han, China. Int J Infect Dis 91: 264-266.
- Zhu N, Zhang D, Wang W, Li X, Yang B, et al. (2020) A Novel Coronavirus from Patients with Pneumonia in China, N Engl J Med.
- Ng PC, Lam CW, Li AM, Wong CK, Leung TF, et (2005) Chemokine response in children with SARS. Arch Dis Child 90: 422-423.
- Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, et (2004) Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 136: 95-103.
- Clay C, Donart N, Fomukong N, Knight JB, Lei W, et al. (2012) Primary severe acute respiratory syndrome coronavirus infection limits replication but not lung inflammation upon homologous re J Virol 86: 4234-4244.
- Liu L, Wei Q, Lin Q, Fang J, Wang H, et (2019) Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight: 4.
- Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, et al. (2013) The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 153: 112-125.
- Uematsu T, Iizasa E, Kobayashi N, Yoshida H, Hara H (2015) Loss of CARD9-mediated innate activation attenuates severe influenza pneumonia without compromising host viral immunity. Sci Rep 5: 17577.
- Lamichhane PP, Samarasinghe AE (2019) The Role of Innate Leukocytes during Influenza Virus J Immunol Res 2019: 8028725.
- Phan T (2020) Novel coronavirus: From discovery to clinical diag Infect Genet Evol 79: 104211.
- Hopkins PA, Sriskandan S (2005) Mammalian Toll-like receptors: to immunity and beyond. Clin Exp Immunol 140: 395-407.
- Loo YM, Gale M Jr (2011) Immune signaling by RIG-I-like recep- Immunity 34: 680-692.
- Lee N, Wong CK, Hui DS, Lee SK, Wong RY, et (2013) Role of human Toll-like receptors in naturally occurring influenza A infections. Influenza Other Respir Viruses 7: 666-675.
- Poon PM, Wong CK, Fung KP, Fong CY, Wong EL, et al. (2006) Immunomodulatory effects of a traditional Chinese medicine with potential antiviral activity: a self-control Am J Chin Med 34: 13-21.
- Lau J, Ko WM, Lam CW, Leung PC, Wong EL, et (2005) Using Herbal Medicine as a means of prevention experience during the SARS Crisis. Am J of Ch Med 33: 345-356.
- Wong CK, Dong J, Lam CW (2014) Molecular mechanisms regu- lating the synergism between IL-32gamma and NOD for the acti- vation of eosinophils. J Leukoc Biol 95: 631-642.
- Sun X, Hou T, Cheung E, Iu TN, Tam VW, et (2019) Anti-inflam- matory mechanisms of the novel cytokine interleukin-38 in allergic asthma. Cell Mol Immunol.
- Cheung PF, Wong CK, Ip WK, Lam CW (2008) FAK-mediated ac- tivation of ERK for eosinophil migration: a novel mechanism for infection-induced allergic inflammation. Int Immunol 20: 353-363.
- Wong CK, Cheung PF, Ip WK, Lam CW (2007) Intracellular signal- ing mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol 37: 85-96.
- Zhu J, Dong J, Ji L, Jiang P, Leung TF, et al. (2018) Anti-Allergic Inflammatory Activity of Interleukin-37 Is Mediated by Novel Sig- naling Cascades in Human Eosinophils. Front Immunol 9: 1445.
- Wong CK, Hu S, Leung KM, Dong J, He L, et (2013) NOD-like receptors mediated activation of eosinophils interacting with bron- chial epithelial cells: a link between innate immunity and allergic asthma. Cell Mol Immunol 10: 317-329.
- Qiu HN, Wong CK, Chu IM, Hu S, Lam CW (2013) Muramyl di- peptide mediated activation of human bronchial epithelial cells interacting with basophils: a novel mechanism of airway inflam- Clin Exp Immunol 172: 81-94.
- Wong CK, Leung KM, Qiu HN, Chow JY, Choi AO, et al. (2012) Activation of eosinophils interacting with dermal fibroblasts by pru- ritogenic cytokine IL-31 and alarmin IL-33: implications in atopic PLoS One 7: e29815.
- Lee CS, Yi EH, Lee JK, Won C, Lee YJ, et (2013) Simvastatin suppresses RANTES-mediated neutrophilia in polyinosinic-poly- cytidylic acid-induced pneumonia. Eur Respir J 41: 1147-1156.
- Loebbermann J, Thornton H, Durant L, Sparwasser T, Webster KE, et al. (2012) Regulatory T cells expressing granzyme B play a critical role in controlling lung inflammation during acute viral Mucosal Immunol 5: 161-172.
- Mc Greevey S, Morrison M (2017) Stuty Confiems Vitamin D pro- tects against Cold and Flu. The Harvard Grazeet.
- Gruber-Bzura BM (2018) Vitamin D and Influenza - Prevention or Int J Mol Sci 19: E2419.
- Martireau AR, Jolliffe DA, Greenberg L, Aloia JF, Camargo CA, et (2017) Vitamin D supplementation to prevent acute respiratory tract infections: Systemic review and meta-analysis of individual participant data. BMJ 356: i6583.
- Lau KM, Lee KM, Koon CM, Cheung CS, Lau CP, et (2008) Im- munomodulatory and anti-SARS activities of Houttuynia cordata. J Ethnopharmacol 118: 79-85.
- Chen CN, Lin PC, Huang KK, Hsu TA (2005) Inhibition of SARS- COV 3C-Like protease activity by Theaflavin-3, 3’-diagallate. eCAM 2: 209-215.
- Lin L, YJ Xu, He DP, Lin ZX (2003) A retrospective study on clinical features of and treatment metholds of or SARS Am J Clin Med. 31: 821-839.
- Leung PC (2007) The Efficacy of Chinee Medicine for SARS: A review of Chinese Publications after the Crisis. Am J Chin Med 35: 575-581.
Citation: Chan B, Wong C, Leung PC (2020) What can we do for the Personal Protec- tion against the CoVID-19 Infection? Immuno-boostering Specific Supplement could be the Answer. J Emerg Med Trauma Surg Care 2: 007.
Copyright: © 2020 Chan B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.


