*Corresponding Author:
Mohammad Kamil,
TCAM Research, Zayed Complex For Herbal Research & Traditional Medicine, DHLME, DOH, Abu Dhabi, UAE
Email: drkamil55@hotmail.com
Abstract
According to WHO projections, there will be over 2.3 billion overweight adults and 700 million obese by 2019, a survey of around 150 herbal weight loss drugs and dietary supplements in UAE with special reference to Abu Dhabi market, as well as from samples received from DOH, and other government organizations, VIP’s and various other sources revealed the presence of adulteration in more than one fifth herbal medicinal products. These findings of Zayed Herbal Research laboratory will help consumers, health care practitioners, and the general public understands ZCHRTM’s action regarding weight loss products contaminated with various prescription drugs and chemicals.
FDA has identified an emerging trend where over-the-counter products, frequently represented as dietary supplements, contain hidden active ingredients that could be harmful. Consumers may unknowingly take products laced with varying quantities of approved prescription drug ingredients, controlled substances, and untested and unstudied pharmaceutically active ingredients. These deceptive products can harm. Hidden ingredients are increasingly becoming a problem in products promoted for weight loss [1].
There is a growing trend where herbal medicines, dietary supplements and conventional foods are adulterated with hiddendrugsandchemicals.Theseproductsaretypicallypromotedforweightloss, sexual enhancement, and bodybuilding and are often represented as being “Natural.” Consumers should exercise caution before purchasing any product in the above categories.
We have identified an emerging trend where over-the-counter weight loss herbal products, frequently representedasdietarysupplements,containhiddenactiveingredientsthatcouldbeharmful.Consumers may unknowingly take products laced with varying quantities of approved prescription drug ingredients, controlled substances, and untested and unstudied pharmaceutically active ingredients.
In continuation of our earlier studies [2,3], the main objective of the present study is to check pharmaceutical medicine adulteration of nonprescription and even prescription slimming medicines in the laboratory using chromatographic and spectrometric techniques and to discuss its side effects in the best interest of consumers and public health safety (Chart -1). This paper also gives an overview of health-related risks after consuming such spurious products and challenges for future perspectives to control such type of malpractices.
Introduction
Traditional herbal medicines are gaining popularity worldwide as an alternative approach to prescription drugs for many reasons including a general perception that they are safe. Recently there have been number of reported studies that reveal adulteration of herbal medicines with undeclared synthetic drugs, which may potentially cause serious toxic adverse effects. This paper reviews the various classes of synthetic drugs that were found to be adulterated in herbal slimming medicines. The focus is to highlight newer analytical tools used to detect adulteration. Due to the advanced chromatographic and spectrometric techniques and other conventional tools, it has become possible to detect synthetic drugs and their analogues as adulterants even if they are present in small quantities.
The Food and Drug Administration (FDA) is advising consumers to stop using multiple weight-loss products that contain the undeclared drug ingredients e.g. sibutramine, which was removed from the market in 2010 for safety Reasons and may present significant risks for those with coronary artery disease and other heart issues increasing global interest in herbal medicines creates a serious need for truly objective data, on not only their efficacy, but also their side effects and interactions. In the interests of public health safety and looking into severity of this epidemic, an emphasis for the regular laboratory checking of herbal medicines for their safety & quality is a need of the day. Since intentional adulteration of “natural herbal medicines” with unknown synthetic drugs or chemicals is a common and dangerous phenomenon of alternative medicine, it is important to modify and validate analytical tools to monitor and evaluate these herbal drugs.
Method
Chemicals
Alverine (A) Fluoxetine (B) Tripropyl amine (C) Sibutramine hydrochloride (D) Atenolol (E) Ducolax (F) Hydroxyzine (G) were received from the Sigma Aldrich and other recognized agencies. HPLC grade methanol was obtained from Merck, USA. High purity water was prepared by a Waters Milli Q plus purification system.
Equipment
Gas Chromatography-Mass Spectrometer QP-2010 (GC-MS) analysis for all samples and standards were performed.
Sample preparation
Approx. 1.0 mg of sample was weighed and mixed with 5.0 mL of methanol. The samples were ultra-sonicated for 30.0 minutes and filtered using a 0.45 μm membrane. The volume of the filtered supernatant was completed to 10.0 mL with methanol.
Gas Chromatographic Mass Spectrometric (GC-MS) Studies
Different mobile phases were used to develop the thin layer chromatograms using petrol /acetone, methanol extracts. Compounds are identified in the sample taken in above solvents and on comparison with reference standards on Chromatographic studies and Mass spectrometric analysis:
Results and Discussion
Research analyses on 5 Herbal Medicinal Products (HMPs) purchased from Thailand, it has been found that the undeclared active pharmaceutical ingredients are present in all five of these products and a couple of them contained more than one including either sibutramine (a controlled substance), alverine (adrug for functional gastrointestinal disorders), tripropyl amine (a laboratory chemical not advised for food, drug), atenolol (used with or without other medications to treat high blood pressure (hypertension).), Ducolax (a stimulant laxative drug), besides other ingredients. The tainted products are listed here (1-5 Unknown capsules/tablets), along with the undeclared drugs and/or chemical ingredients along with their side effects, analyzed using chromatographic& mass spectrometric technique. Final recommendation is given in each case for not using these medicines for public health safety.
Starting with five (5) unknown herbal medicines, purchased from Thailand for weight reduction. These unknown medicines were subjected to chromatographic and spectrometric analysis (GC-MS) to find out their ingredients and chemical compounds.

Figure a: Photographs of five (5) Herbal medicines, purchased from Thailand.
Unknown white blue capsules:
The white Blue Capsules purchased for weight reduction contains:
- Alverine
- Fluoxetine
- Tripropylamine
- Ethylphthalate
- Propenyl propylether
- Pyridine-2, 6-dioldiacetate
- 2-Tertiarybutyl-3-methyl-2-cyclopentendione
- Valeraldehyde 2-methylene, isopropyl hydrazine
- Androsta-2, 4-dien-3-ol, heptafluorobutanoat
Fluoxetine: (An anti-depressant medicine) present in a major concentration,
Alverine: (A drug for functional gastrointestinal disorders),
Tripropyl amine: (A laboratory chemical not advised for food, drug, pesticide or biocidal product use (The 2012 OSHAD Hazard Communication Standard considers this chemical hazardous).
Based on above lab. studies ‘Unknown white Blue Capsules’ are not at all safe to use.
Unknown white tablet:
The following compounds are identified in the powder content of ‘Unknown white Tablets on Chromatographic studies and Mass spectrometric analysis:
- Pregn-5-en-20-3, 16-bis[(TMS)oxy]-o-methyloxime
- Triacontane
- Nonanoic acid
- Atenolol (Very very high concentration)
Atenolol is present in a very very high concentration in the tested white tablets for weight reduction. Due to the presence of Atenolol (Tenormin) in a very high concentration, the ‘Unknown white Tablets’ does not at all safe to use.
Unknown white and green capsules:
The following compounds are identified in the powder content of ‘Unknown white Green Capsules ‘on Chromatographic studies and Mass spectrometric analysis:
- Triacontane
- Nonanoic acid
- Sibutramine HCl
- Ceanothamine A /Frangulanine
- Tripropyl amine/1-PropanamineN-N-dipropyl (Very high concentration)
- Phthiodiolone-A
- Alpha L-Galactopyranoside, methyl6-deoxy
Due to the presence of Sibutramine HCl and Tripropyl amine the ‘Unknown white and green Capsules’ does not at all safe to use.
Unknown pink and blue capsules
The following compounds are identified in the powder content of ‘Unknown pink Blue Capsules ‘on comparison with reference standards on Chromatographic studies and Mass spectrometric analysis:
- Caprylic ether
- Ducolax (Bisacodyl)
- 2-Bromooctane
- Bis (2-ethyl hexyl) fumarate
- 2-Butyl isopentylidine amine
- Acrylic acid, 2-ethyl hexylester
- Bis (2-amino,5-pyridyl phenyl methane)
- 2-(alpha-(4-hydroxy phenyl)-4-hydroxy benzyl)pyridine
- Benzothiazole,2-(4-butyl-3,5-dimethyl-1Hpyrazol-1-yl)-6-chloro
The Unknown pink and blue capsules contain Ducolax (Bisacodyl), a stimulant laxative drug in a high concentration besides other unsafe compounds.
Looking into above studies ‘Unknown Pink and Blue capsules’ does not seem safe to use.
Unknown blue tablets
The following compounds are identified in the powder content of ‘Unknown blue tablets ‘on Chromatographic studies and Mass spectrometric analysis:
- Oleic acid
- Hydroxyzine
- Trimethyl silyl derivative of1-monolinolein
- 9,12-Octadecadienoicacid
- Triacontanoic acid, methylester
- n-pentadecanoicacid
- Hydroxyzine (Atarox; Vistaril, Fenorol)
The Unknown Blue Tablets contain an undeclared compound Hydroxyzine (Atarox; Vistaril, Fenorol) in a major concentration Based on above studies ‘Unknown blue Tablet ‘seems not safe to use.
Besides the above-unknown medicines, quite a large number of herbal medicines have been tested in laboratory and were found adulterated with pharmaceutical medicines (Chart-1).
Some of the above herbal medicines from the above-mentioned chart are described here:
Miaomiao slimming capsules:
Manufacturer : Hong Kong Tian Lun pharmacytech.co.Ltd.
Description : Light green/dark green colored capsules having light brown powder.
The following compounds were identified in the content of the capsule taken in above solvents and on comparison with reference standards on TLC studies and GCMS analysis:
2Aminopropanol Propylene diamine
Sibutramine HCl Isoamyl acetate
Momeinositol 1,3-Propane diamineN-(2-aminoethyl)
L-Alanine ethylester 2,3-Dimethylhexahydro-6H-pyrazolo-[1,2,a][1,2,4,5] tetraazine
Discussion : Since Miaomiao Slimming Capsules contain Sibutramine Hydrochloride as an undeclared ingredient which is a banned chemical medicine.
|
S.No |
Herbal Medicine |
Laboratory Test Results- Undeclared drugs and/or chemical ingredients (other than herbal ingredients) |
|
1 |
Fashion Slimming Coffee |
Sibutramine Hydrochloride |
|
2 |
Majestic Slimming Capsules |
Sibutramine Hydrochloride |
|
3 |
Slimming Bomb |
Sibutramine hydrochloride & Phenolphthalein |
|
4 |
Hoodia gordonii capsules |
Sibutramine hydrochloride |
|
5 |
Slim Body Capsules |
Sibutramine hydrochloride |
|
6 |
AlMalaka Capsules |
Sibutramine hydrochloride |
|
7 |
Blue Tablets |
Hydroxyzine (Atarox) |
|
8 |
Pink Blue Capsules |
Ducolax (Bisacodyl) |
|
9 |
White green capsules |
Sibutramine hydrochloride & Tripropyl Amine |
|
10 |
White tablets |
Atenolol |
|
11 |
White blue capsules |
Fluoxetine, Alverine & Tripropyl Amine |
|
12 |
Novac capsules |
Raspberry ketone |
|
14 |
Smart Diet |
Chromium picotanate |
|
15 |
Acai Power Dietary Sup- plement |
|
|
16 |
Makkah Shape Dietary Supplement |
Chromium picotanate |
|
17 |
Hoodia Gordonii Gold Capsules |
Phenolphthalein Sibutramine hydrochloride |
|
18 |
Green Cofee Bean Extract |
|
|
19 |
Miaomiao Slimming Capsule |
Sibutramine Hydrochloride |
|
20 |
Unknown Weight Gain Herbal Product |
Cyproheptadiene (Periactin) |
|
21 |
Natural Max Slimming Capsules |
Fluoxetine & Phenolphthalein Sibutramine hydrochloride |
|
22 |
Super Power Fatting 007 |
Cyproheptadiene |
|
23 |
Diet Capsules1-Day |
Phenolphthalein Sibutramine hydrochloride |
|
24 |
Magrim Capsule |
Sibutramine hydrochloride |
|
25 |
Natural Unknown Sample for increasing weight |
Cyproheptadiene |
|
26 |
Super Slim Capsule |
Phenolphthale Sibutramine hydrochloride |
|
27 |
Fat Burner Tablets |
Sibutramine Hydrochloride |
|
28 |
Unknown Weight Gain Herbal Product |
Cyproheptadiene |
|
29 |
Mega T Green Tea Caplets (Fat Burner Supplement) |
Pyrogallol & Caffeine (V.H.Conc.) |
|
30 |
Sabr Plus Capsules |
Phenolphthalein Sibutramine hydrochloride |
|
31 |
Quick Acting Slimming Capsule Zein |
Sibutramine hydrochloride Phenolphthalein |
|
32 |
Seven Slim Capsules |
Sibutramine hydrochloride |
|
33 |
Phyto Shape |
Sibutramine hydrochloride |
|
34 |
Slimming Capsules |
Sibutramine hydrochloride Phenolphthalein |
|
35 |
Super Slim |
Sibutramine hydrochloride Phenolphthalein |
|
36 |
AB Slim |
Sibutramine hydrochloride |
|
37 |
Fat Burning Capsules |
Sibutramine hydrochloride Phenolphthalein |
Chart 1: Intentional adulteration found in Slimming Herbal Medicines.
It is, therefore, recommended that this product should be taken off from the market.
Hoodia gordonii gold capsules:
Batch Number : CF9550TE671
Description : Pink /Red capsules
Chromatographic Studies: Different mobile phases were used to develop the thin layer chromatograms using petrol /acetone, methanol extracts. The following plants & compounds are identified in the
‘Hoodia Gordonii Gold Capsules ‘taken in above solvents and on comprison with reference standards on TLC studies and GCMS analysis.
Caffeine Cyclo octacoasane
Phenolphthalein Sibutramine Hydrochloride
DisalylAldehyde Cyclo hexane(1-hexa decyl heptadecyl)
Glyceryl trilaureate Cis-2,3-dihydroxy diphenyl methyl bicycle [2.2.1] hepta-5-ene
Transcrotylalcohol p-Phenyl azobenzoicacid
Discussion : Phenolphthalein and Sibutramine hydrochloride are found as an undeclared and banned compounds.
Recommendation : On the basis of above two undeclared and banned chemicals ‘Hoodia Gordonii Gold Capsules ‘is not at all safe to use.
Nature max slimming capsule
Chemical compounds: Isopentyl acetate; Trans-2-Dodecan-1ol; Momeinositol; Benzoic acid; Sibutramine HCl; Phenolphthalein; 4-Octen-3-one ; 4,7,9-Decatrien-2-amineN-butyl (Bottle2) X anthosine; Cytosine riboside; Sibutramine HCl; Phenolphthalein; Fluoxetine; Farnesol; Caffeine ; Alpha,alpha,alpha-Trifluorop-cresol
Super power fatting 007
Chemical compounds isolated: Honey; Oleic acid; Behenic acid; Cypproheptadine; DL-Arabinose; 4-Methyl-1, 3-dioxane; 3-Acetoxy dodecane; Sugar; Hexanoic acid, 3-OH-methyl ester; Zingiberene; Levo Alpha Cedrene; Cuparene; Caryophyllene oxide; Alpha Bisabolol; Tumerone; Alpha Atlantone; 4-Ethylguaicol; Elaidic acid, methyl ester; Beta Himachalene; Longifolene-(V4) Alpha & beta –D-glucopyranose; Hydroxymethylfurfural 3Deoxy-d-mannoic acid.
Mega T green tea caplets:
Product Type : Fat Burner Supplement
Manufacturer : USA
Lot Number : #0176-03-1
Description : Orange colour capsule with yellow powder
The following plants & compounds are identified in the ‘Mega T Green Tea Caplets:
Caffeine (Very high Conc.)
Pyrogallol (High Conc.)
Quinic acid
Capric acid
Myristic acid
Propylene glycol
Nitrosomethane
3-Methoxy pyrocatechin
Pentadecanoic acid
Propyl hexacosanoate
Octamethyl cyclotetrasiloxane
Discussion : It won’t contain sibutramine or phenolphthalein but Mega T Green Tea contains high level of Caffeine, which may cause anxiety and insomnia. Pyrogallolis also present in high concentration and does not seems from a plant origin. The expiry date of the dietary supplement is 3/14, hence expired 4 years before.
Recommendation: Based on above facts ‘Mega T Green Tea Caplets ‘is not advisable to use.

Structures of the Detected Compounds (Adulterated)
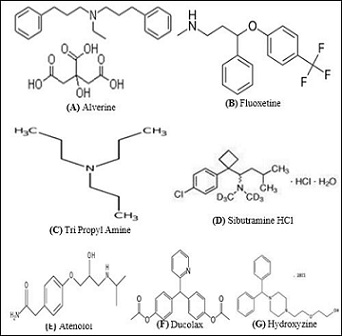
Associated Risk And Side Effects Of These Adulterated Pharmaceuticals
Sibutramine and associated risk:
Sibutramine is in a class of medications called appetite suppressants and is used to help people lose weight. It is a controlled substance and the active pharmaceutical ingredient in Meridia / Reductil, previously an approved prescription drug to treat obesity.
Populations who would be at increased risk of serious adverse health effects from consuming a standard dose of sibutramine include:
Patients with a history of hypertension, coronary artery disease, congestive heart failure, arrhythmias, or stroke, narrow angle glaucoma, history of seizure, predisposed to bleeding events and those taking concomitant medications known to affect hemostasis or platelet function, severe hepatic dysfunction. and those concurrently taking the following medications: Sumatriptan, Dihydroergotamine, Dextromethorphan, Meperidine, Pentazocine, Fentanyl, Lithium, Tryptophan, MAO inhibitors.
Looking into its risk many countries have banned Sibutramine HCl. In Jan’2010 Health Authority Abu Dhabi, decided to suspend Sibutramine from Abu Dhabi Market. The UAE Health Ministry asked hospitals and pharmacies to recall all products containing the substance sibutramine in accordance with the recommendations of the US Drug and Food Administration and European Association for Medicine. Clinical trials have found that the substance may cause heart attacks and cardiac arrests.
Phenolphthalein and associated risk:
Phenolphthalein was an ingredient in some Over-the-Counter laxative products until 1999 when the FDA reclassified the drug as “not generally recognized as safe and effective” after studies indicated that phenolphthalein presented a potential carcinogenic risk. Phenolphthalein has also been found to be genotoxic in that it can damage or cause mutations to DNA.
Hydroxyzine (Atarox; Vistaril, Fenorol):
SIDE EFFECTS: Hydroxyzine reduces activity in the central nervous system. A new study shows that the antihistamine hydroxyzine is an effective treatment for restoring normal sleep patterns when liver damage causes chemical pathways in the brain to be disrupted. “In patients with cirrhosis of the liver, histamine levels in the brain can be altered,” says study author Dr. Laurent Spahr. Hydroxyzine should be used with caution (if at all) in persons with narrow-angle glaucoma, prostatic hypertrophy (enlarged prostategland), hyperthyroidism, cardiovascular disease, hypertension, and asthma.
Drugs or supplements interact with hydroxyzine:
- diazepam (Valium),
- lorazepam(Ativan),
- clonazepam (Klonopin), and
- alprazolam(Xanax).
Tripropyl amine and associated risks:
Tripropyl amine, which is a laboratory chemical, not advised for food and drug. It is toxic if swallowed; toxic in contact with skin; causes severe skin burns and eye damage; moreover, may cause respiratory irritation.
Atenolol and associated risks:
Atenolol is available under the brand name Tenormin. Atenolol is used with or without other medications to treat high blood pressure (hypertension). Lowering high blood pressure helps prevent strokes, heart attacks, and kidney problems. This medication is also used to treat chest pain (angina) and to improve survival after a heart attack. Atenolol belongs to a class of drugs known as beta-blockers. It works by blocking the action of certain natural chemicals in your body, such as epinephrine, on the heart and blood vessels. This effect lowers the heart rate, blood pressure, and strain on the heart. Atenolol may also be used to treat irregular heartbeat, heart failure, alcohol withdrawal symptoms, and to prevent migraine headaches.
Side effects of atenolol include:
Low blood pressure (hypotension); Slow heart rate; Cold extremities; Dizziness upon standing; Depression; Nausea; Fatigue; Leg pain ;Lethargy; Lightheadedness; Spinning sensation (vertigo); Shortness of breath; 2°/3° atrioventricular (AV) block. Other side effects of atenolol include severe congestive heart failure (CHF); Sick sinus syndrome; Catatonia.
References
- FDA (2019) Tainted Weight Loss
- Kamil M (2010) PDIC, HAAD, D. Newsletter 4 : 16.
- Kamil M (2011) Determination of Undeclared Chemicals in Herbal Slimming Medicines using HPTLC. V International Journal of Re- search 2: 1-7.
Citation: Kamil M (2019) Undeclared Chemicals in Slimming Herbal Medicines, Di- etary Supplements and their Associated Risks. J Diab Meta Syndro 2: 006.
Copyright: © 2019 Kamil M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


