*Corresponding Author:
Andrey N Belousov,
Laboratory of Applied Nanotechnology of Belousov, Kharkov Medical Academy of Postgrad- uate Education, Department Anesthesiology, Intensive Care, Transfusi- ology and Hematology, pr. Nauky, 31-v, fl 32, Kharkov, 61072, Ukraine
Tel: +380 509151889
E-mail: an.belousov2012@yandex.ua
Abstract
In order to determine ultrastructural reconstructions in hepatic cells under effect of magnetite, an experiment was conducted on 45 rabbits. In ear vein of the rabbit, from a calculation 6-8 ml/ kg, 0.0225% was injected magnetite nanoparticles (preparation of ICNB) for 24 hours before the investigation. Analysis of the state of submicroscopic architectonics of hepatic cells in rabbits after injection ICNB reveals a significant activation of metabolic intracellular processes in these organs. Ultrastructural organization of the liver testifies about intensification of synthetic intracellular processes, it being structurally manifested by enlargement of cisterns in the rough endoplasmic reticulum, an increased number of ribosomes, a moderate hypertrophy of the laminar cytoplasmic Goldi’s complex. For the first time in the study was the evidence of the possibility of using intravenous forms of magnetite nanoparticles (preparation of ICNB) as an effective means of non specific activation of metabolic processes in the liver at the ultrastructural level.
Keywords
Activation; Hepatic cells; Magnetite nanoparticles ICNB; Reparative; Ultrastructure
Introduction
Regenerative medicine is a pioneering field aimed at restoring and regenerating the function of damaged cells, organs and tissues in order to establish normal function. Toxicity issues are a major concern and are important factors in the context of regenerative medicine. Nanotechnology has been instrumental in the development and translation of basic research to the clinically relevant therapies. Safety is an issue of constant concern and emphasizes on the importance of investigating the issue of toxicity. Any indication of toxicity can ultimately limit the therapeutic efficiency of the therapy. Toxicity is highly dependent on the physical, chemical and structural properties of the Magnetite Nanoparticles (MNPs) itself as well as dose and intended use. Few in vitro studies have reported adverse effects of MNPs on cells at in vitro in therapeutic doses. However, in vivo studies have not been studied [1].
Recently, Fe3O4 Magnetite Nanoparticles (MNPs) have been widely used for biological applications, such as magnetic resonance imaging therapy [2], in hyperthermia tumor therapy [3], in drug delivery systems [4] and molecular detection of biomarkers in biological cells [5]. It is crucial to widely study the biological toxicity of MNPs to ensure their safety for biological and medical applications. In a literature, after 3 weeks of intravenous administration of MNPs (193 nm), serum iron levels gradually increased for up to 1 week but levels slowly declined thereafter. Also, MNPs were localized in the liver and spleen parenchyma more than other tissues and cleared gradually after 3 weeks of treatment [6]. In another study, the biodistribution of intravenously injected MNPs (5 nm) showed 75% of injected dose was found in the spleen and liver at 15 min post injection. Moreover, 24% of the MNPs remain in liver after 48 hrs post injection [2]. Consequently, as reported by Briley-Saebo et al., (2004) MNPs were distributed equally in both liver endothelial and Kuepfer cells but not liver parenchyma following intravenous injection in rats.
After 2 days of intraperitoneal injection of MNPs (10 nm), the liver showed nuclei atrophy and vacuolar degeneration in hepatocytes. Contradictory, after intravenous injection, MNPs (193 nm) did not show any histological abnormalities in the liver after 7 days of treatment [6,7]. The dose and size of injected MNPs can affect the cellular and tissue toxicity in vivo and in vitro. After 2 weeks of intravenous injection of higher dose of titanium dioxide NPs (40 nm), the liver showed hepatocyte degeneration, multifocal lesions, spotty necrosis of liver hepatocytes and portal lymphocyte infiltrations [8,9].
In spite of the fact that application of magnetite of nanoparticles looks simple, it is not necessary to forget about a high danger of the origins of complications as a result of their intravessel injection. At least it is necessary to take into account such indexes as a concentration, doze, rate of entered solution of nanoparticles, time of allocation nanoparticles in blood circulation after injection. The enumerated parameters for reliable have influence on haemorreology and state of microcirculation on the whole. The high local concentration of magnetite in vessels is caused by disturbances of blood circulation, microcirculation and hypoxia of tissues [10,11]. It is dangerous in main vital organs: brain, heart, lungs, liver and kidneys. Direct cross correlation dependence between concentration of nanoparticles and level hypoxia is physiopathology obvious. Consequently, before injecting intravessel magnetite of nanoparticles, it is necessary to have standardized water solution magnetite of nanoparticles with early studied and well proven noninvasive physical and chemical properties.
Unfortunately, to date the advanced studies that would take into account it are absent. In the published advanced studies, we met not a single reference to the use of the early studied standardized noninvasive forms magnetite of nanoparticles and methodologies of their application. Information about the mechanism of influence magnetite of nanoparticles on main biological systems of living organism including respiratory, cardiovascular, secretory, immune systems, cellular exchange is absent. Also, we did not discover among the scientific publications of reliable dates about quantitative distribution magnetite of nanoparticles in organs and tissues after intravenous injection. Information about a mechanism of eliminate magnetite of nanoparticles from an organism is absent.
On the whole, aforesaid does not allow properly estimating the scientific and practicing significance early advanced studies which were published on theme to use magnetite of nanoparticles in medicine. It was found in the choice of theme of the present investigation. The task was set in an experiment on animals to check possibility of the use of the before worked out and studied methodology of intravenous injection of the standardized form water solution magnetite of nanoparticles (ICNB) [11-21]. The main purpose is to investigate the ultrastructural reconstructions in hepatic cells after single intravenous injection of standardized form water solution magnetite of nanoparticles (preparation of ICNB).
Material and Methods of Research
In order to determine ultrastructural reconstructions in hepatic cells under effect of magnetite, an experiment was conducted on 45 rabbits. In ear vein of the rabbit, from a calculation 6-8 ml/kg, 0.0225% was injected ICNB for 24 hours before the investigation. Physical and chemical properties of ICNB (Figures 1 & 2; Tables 1 & 2):
- Osmolality theoretical of colloid solution is 500 mosm/l
- 0225% colloidal solution of magnetite nanoparticles
- Size of magnetite of nanoparticles is 6-12 nm
- Total area of surface magnetite of nanoparticles Ss = 800-1200 m2/g
- Magnetized of saturation is = 15 кА/m
- ζ Potential = - 19 mV

Figure 1: Study of magnetite nanoparticles (ICNB) with use microscope ion-electronic raster-type Quanta 200 3 D.
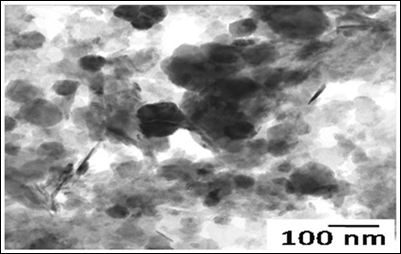
Figure 2: Study of magnetite Nanoparticles (ICNB) with use microscope electronic translucent JEM-2100.
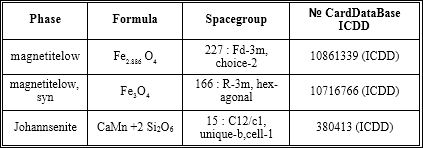
Table 1: X-ray analysis of ICNB in X-ray diffractometer RigakuUltima IV (CuKα, Kβ filter - Ni), one-coordinate DTeX semiconductor detector.

Table 2: The phases of ICNB (RIR - method; error 8± 3%).
Pieces of hepatic tissue were put for preliminary fixation into a buffered (3-4%) solution of glutar aldehyde cooled down to +4°C for 2-4 hours. After the preliminary fixation, the tissue was washed in several mixtures of the buffer at the same temperature and for final fixation it was put into 1% buffered solution of osmium tetraoxide for 2-3 hours at the temperature of +4°C. After the end of fixation the pieces of the tissue were washed in the buffer solution, dehydrated in alcohols with rising concentrations and acetone and then embedded into a mixture of epoxy resins (epon-araldite) following conventional techniques. After polymerization in a thermostat at 60°C, the obtained blocks were used for making ultrathin sections by UMTP-6 ultramicrotom. These ultrathin sections were contrasted by lead citrate and uranyl acetate.
The preparations were studied under EMB-100 BR electron microscope with accelerating voltage of 75 kilovolts. Magnification was selected to be adequate for purposes of the investigation and ranged within 15.000-45.000 times. Necessary areas of sections were photographed on photographic plates which served for subsequent taking of microphotographs. Electron microscopic preparations made out of organs of intact rabbits served as controls.
Result of Researches
Ultrastructural changes in hepatocyte organelles manifested pronounced signs of activation of reparative intracellular processes. Hepatocyte nuclei held their rounded shape (Figure 3). Nuclear membranes had well-defined contours. Chromatin, in the form of small clods, was evenly distributed throughout the section. Condensation of chromatin on the nuclear membrane was observed only in single hepatocytes. Perinuclear space was not enlarged. Single ribosomes were found on the outer membrane of the nucleus.

Figure 3: Ultrastructure of hepatocytes in rabbits after injection of nanoparticles ICNB х 39.000.
Mitochondria were evenly distributed in all parts of the cytoplasm of the hepatic cells (Figure 4).

Figure 4: Ultrastructure of hepatocytes in rabbits after injection of nanoparticles ICNB х 30.000.
Mitochondrial matrix had a moderate electron density and a fine grain structure. Shape of the mitochondria varied from rounded to stick-like. Many cristae were revealed; they had a pronounced typical orientation. The outer membrane remained integral, without any foci of destruction. In single cells, there were mitochondria having the shape of dumbbells and with septa. The rough endoplasmic reticulum underwent the most characteristic reconstructions (Figure 5).
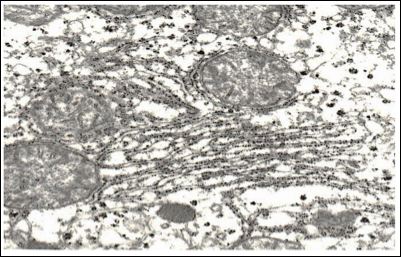
Figure 5: Ultrastructure of hepatocytes in rabbits after injection of nanoparticles ICNB х 42.000.
In the majority of hepatocytes, their rough endoplasmic reticulum was an extensive network of membranes with numerous ribosomes localized on their surfaces. Cisterns of the endoplasmic reticulum were slightly enlarged and their shape resembled flattened vesicles. The substance which filled them was electron transparent. The smooth endoplasmic reticulum was well developed; its vacuoles were mostly localized in basal parts of the cytoplasm. It should be noted that there were great numbers of free ribosomes and granules of glycogen which were evenly distributed throughout the cytoplasm. The laminated cytoplasmic Golgi’s complex (Figure 6) was moderately hypertrophic; its membrane part consisted of parallel smooth membranes.
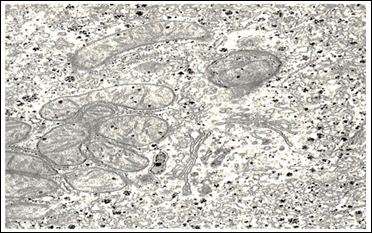
Figure 6: Ultrastructure of hepatocytes in rabbits after injection of nanoparticles ICNB х 40.000.
Packs of these membranes were surrounded with a great number of large and small vesicles. Single vesicles were filled with a rough fibrous osmiophil substance. There was rather a great number of primary lysosomes in the area of localization of the laminar cytoplasmic Golgi’s complex, autophagosomes and small inclusions of lipids being observed in single cells. Bile capillaries were filled with prolonged crimped microvilli and were moderately dilated.
Sinus capillaries and Disse’s spaces were dilated rather extensively. Disse’s spaces were filled with numerous microvilli. Changes in the ultrastructure of Kuepfer cells testified about their functional activity. Nuclei (Figure 7) of Kuepfer cells were of irregular shape with deep invaginations of the nuclear membrane.

Figure 7: Ultrastructure of hepatocytes in rabbits after injection of nanoparticles ICNB х 39.000.
Nuclear matrix had a significant electron density. Karyolemma had no destructive changes. The cytoplasm of Kuepfer cells revealed single and slightly swollen mitochondria which contained a small number of cristae and single cisterns of the rough endoplasmic reticulum. The cytoplasmic membrane did not undergo any changes and held its well-defined bilaminated structure. It should be noted that there was a great number of small electron-transparent micropinocyticvesicles.
Conclusion
Analysis of the state of submicroscopic architectonics of hepatic cells in rabbits after Injection Of Magnetite Nanoparticles (ICNB) reveals a significant activation of metabolic intracellular processes in liver. Ultrastructural organization of the liver testifies about intensification of synthetic intracellular processes, it being structurally manifested by enlargement of cisterns in the rough endoplasmic reticulum, an increased number of ribosomes and a moderate hypertrophy of the laminar cytoplasmic Golgi’s complex. Activation of reparative intracellular processes is another aspect of these reconstructions. It is confirmed by a revealed hyperplasia of the rough endoplasmic reticulum, this hyperplasia testifying about intensive processes of self-renewal in submicroscopic structures. Presence of mitochondria having the shape of dumb bells and with constrictions in the cytoplasm of hepatic cells enables a statement that a process of an intensive increase in the number of these organelles tares place.
Availability of a great number of mitochondria with unchanged structure and numerous cristae in the cytoplasm of hepatocytes indicated a high activity of redox processes and those of oxidative phosphorylation which satisfy needs of synthetic intracellular reactions taking place on the level of membranes and macromolecules.
Submicroscopic structure of endotheliocytes in sinus capillaries indicates activation of processes of transcellular transportation of substances and electrolytes by endocytosis, it being confirmed by presence of numerous micropinocytic vesicles in the cytoplasm of these cells.
Thus, for the first time in the study was the evidence of the possi- bility of using intravenous forms of magnetite nanoparticles (prepara- tion of ICNB) as an effective means of non specific activation of met- abolic processes in the liver at the ultrastructural level.
References
- Alarifi S, Ali D, Al-Doaiss AA, Ali BA, Ahmed M, et al. (2013) Histologic and apoptotic changes induced by titanium dioxide nanoparticles in the livers of Int J Nanomedicine 8: 3937-3943.
- Ansari C, Tikhomirov GA, Hong SH, Falconer RA, Loadman PM, et (2014) Development of novel tumor-targeted theranostic nanoparticles activated by membrane-type matrix metalloproteinases for combined cancer magnetic resonance imaging and therapy. Small 10: 566-575.
- Patent of Ukraine ?14817? UA A61N2/00 Method of production of a magnetic liquid for transport and retention of medicines in organism / N. Belousov ? 96062463. Decl. 21.06.96; Publ. 18.02.97.
- Patent of Ukraine ?42123? UA A61N2/00 Method of treatment diseases which connected with endocrine disturbance / AN Belousov ? 98084233. 04.08.98; Publ. 15.10.01.
- Patent of Ukraine 42132? UA A61N2/00 Method of treatment digestion system diseases / A.N. Belousov ? 98084234. Decl. 04.08.98; Publ. 15.10.01.
- Belousov AN, Nevzorov VP Ulrastructure of hepatic cells in rabbits after injection of / Eighth International Conference on magnetic fluids.Timisoara, Romania, pp. 482-483, 1998.
- Belousov AN, Rykov VG Revealing of mechanisms of the influence exerted by magnetic fluid preparations on biological systems and the whole living / 8-th IPCMF. Plyos. p. 90, 1998.
- Belousov AN Experimental study of effects of Belousov’s magnet-controlled sorbent on parameters of acid-base equilibrium in blood and processes of glycolysis in erythrocytes. / Adsorption technologies and blood purification - Germany. p. 45, 2000.
- Belousov AN Opening the mechanisms of cell regulation by nanotechnology preparations. /7th International Conference on the Scientific and Clinical Applications of Magnetic Carriers - Vancouver, Canada, 234-235, 2008.
- Belousov AN Effect magnetite nanoparticles MCS-B on functional activity of erythrocytes / ICEEP 2012: Electrical Power & Energy Systems. Hohhot, China,June 23-24,2012.
- Belousov AN The influence of magnetite nanoparticles (MCS-B) on the hemolysis of erythrocytes / World Conference and Expo 2011. TechConnect World is the host of the Nanotech, BioNanotech, Microtech, Clean Technology and TechConnect conferences. June 13-16, 2011, in Boston, Massachusetts, S.A Manuscript number: 155.
- Belousov AN Application of products nanotechnology at creation of devices “An Artificial Liver”. / 12-th World Congress of Anesthesiologists, Monreal, Canada, 307, 2000.
- Patent of Ukraine ?31309? UA A61N2/00 Method of treatment diseases which connected with blood circulation disturbance / AN Belousov ? Decl. 04.08.98; Publ. 15.12.00.
- nanolab.com.ua
- Haun JB, Yoon TJ, Lee H, Weissleder R (2011) Molecular detection of biomarkers and cells using magnetic nanoparticles and diagnostic magnetic Methods Mol Biol 726: 33-49.
- Hayashi K, Nakamura M, Sakamoto W, Yogo T, Miki H, et al. (2013) Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment. Theranostics 3: 366-376.
- Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V (2008) Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in Mol Pharm 5: 316-327.
- Kim JS, Yoon TJ, Yu KN, Kim BG, Park SJ, et al. (2006) Toxicity and tissue distribution of magnetic nanoparticles in Toxicol Sci 89: 338-347.
- Markides H,Rotherham M, and El Haj AJ (2012) Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. Journal of Nanomaterials 2012:614094.
- Shanehsazzadeh S, Oghabian MA, Daha FJ, Amanlou M, Allen BJ (2013) Biodistribution of ultra small superparamagnetic iron oxide nanoparticles in BALB J Radioanal Nucl Chem 295: 1517-1523.
- Xu J, Shi H, Ruth M, Yu H, Lazar L, et (2013) Acute toxicity of intravenously administered titanium dioxide nanoparticles in mice. PLoS One 8: 70618.
- Yallapu MM, Othman SF, Curtis ET, Gupta BK, Jaggi M, et al. (2011) Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer Biomaterials 32: 1890-1905.
Citation: Belousov AN (2017) New Prospects Application of Magnetite Nanoparticles for the Diagnosis of Malignant Tumors in MRI Investigation. J Cell Mol Biol 2: 003.
Copyright: © 2017 Belousov AN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.