*Corresponding Author:
Lorenzo Alibardi,
Department of Bigea, Comparative Histolab and University of Bologna, via Selmi 3, 40126, Bologna, Italy
E-mail: lorenzo.alibardi@unibo.it
Abstract
The corneous layer of reptilian epidermis contains bacteria of Gram+ and Gramtypes. The ultrastructural study indicates that both intact and damaged bacterial cells are present on the surface of the corneous layer but only damaged bacterial cells are observed among corneocytes present 2-3 layers underneath the surface. The immunolabeling for three beta-defensins and 1 cathelicidin of lizard or turtle origin shows that these antimicrobial peptides are present among superficial corneocytes and on the surface of the stratum corneum. Moreover, the labeling is also concentrated on bacterial cells found on the epidermal surface or among superficial corneocytes. Some bacterial cells appear damaged in their membranes, cytoplasm and nucleoid region. The present observations suggest that keratinocytes during their migration and differentiation into corneocytes release small amounts of peptides within the epidermis creating an antimicrobial epidermal barrier in pre-corneous and corneous layers, especially in regenerating epidermis. It is likely that bacteria that penetrate inside the corneous layer encounter an efficient antibiotic barrier and are eventually destroyed. The presence of a diffuse anti-microbial barrier in the epidermis is important for survival of these ectothermic vertebrates in the dirty and decay environments where they naturally live. The study suggests that reptilian anti-microbial peptides should be tested for their anti-microbial activity and potential pharmacological applications.
Keywords
Antimicrobial peptides; Bacteria; Epidermis; Reptiles; Ultrastructure
Introduction
The epidermis constitutes a physical and chemical barrier against water transpiration, mechanical lesion, and biochemical insults, including a resistant anti-microbial barrier against proteolytic enzymes used by bacteria to penetrate into the body [1]. Aside the formation of a continuous physical barrier, the epidermis also utilizes epidermal/dermal-derived Antimicrobial Peptides (AMPs) that in mammals are mainly stored within the lamellar granules of the granular layer [2-5]. Among AMPs two groups, the defensins [5,6] and the cathelicidins [7] have been particularly studied in mammals and reptiles [8].
The resistance of reptiles to bacterial invasion is high and these amniotes live in variably polluted or decay environments where they usually are poorly affected by infections. Reptiles also efficiently resist to infections after wounding or toe clipping without any pharmacological treatment. One of the key factors for their anti-microbial resistance is doubtless their scaled epidermis that undergoes to an intense cornification [9-12]. In addition to mechanical factors and tissue structure, recent researches have characterized a number of AMPs present in reptilian tissues, in particular in white blood cells, epidermis, and oral cavity in normal and regenerating conditions [8,13-24]. These studies have also indicated the formation of an efficient granulocyte barrier localized underneath the scab or lizard wound and in the scab itself.
The above studies have suggested the present electron microscopic survey over the epidermis of representative of all reptilian groups, lepidosaurians, chelonians and crocodilians, in order to determine whether the presence of an antimicrobial barrier in the epidermis in reptiles can justify their high microbial resistance. Electron microscopy represents a potent tool for determining the cell alterations leading to the death in bacteria [25]. During past studies on the skin of reptiles it was frequently observed the presence of a number of bacterial cells on the external corneous layer or even within the 2-3 external layers of the epidermis, and their localization was recorded to form the present survey. The present ultrastructural study has been integrated with the immunodetection of specific beta-defensins and cathelicidins previously characterized in reptiles [17-19].
Materials and Methods
The present observations derive from previous studies carried on the skin structure of different species of reptiles and immunolabeling for AMPs [12,21,22,26,27]. The study utilized 2 by 3 mm pieces of limb or tail skin (normal or regenerating) from the lizards Anolis carolinensis, Podarcis muralis, and Hemidactylus turcicus. Also skin biopsies from the trunk of the snake Natrix natrix, from normal and regenerating tail of tuatara Sphenodon punctatus [26], and from the ventral scales of the crocodile Crocodylus niloticus and Crocodylus porosus, were also collected as indicated above. Finally, also skin/scales pieces from the carapace, limb and neck regions of the turtles Chrysemys picta, Pseudemys nelsoni and Apalona spinifera previously utilized were re-utilized for the present survey. Some skin samples were fixed in 2.5 % glutaraldehyde in 0.1 M Phosphate buffer at pH 7.2 for 5-8 hours osmicated and embedded in Epon resin for the study of the plain ultrastructure of the skin. Other skin fragments were instead fixed at 0-4°C in fresh 4% paraformaldehyde in 0.1 M Phosphate buffer at pH 7.4 for 7-8 hours, rinsed in buffer, dehydrated in alcohol, and embedded in the acrylic resin Bioacryl under UV light [28], that allow ultrastructural immunolabeling.
The embedded tissues were sectioned using an ultramicrotome, and semithin sections (2-3 mm thick) were collected, stained with 1% toluidine blue for the histological examination or were utilized for the immunohistochemical study. Sections obtained from Bioacryl, Lowcryl or LR-White resins were utilized for the immunocytochemical study at the light or electron microscope. The antibodies against beta-defensins from lizard (AcBD15, AcBD27) or turtle (TuBD1) or against a lizard cathelicidin (AcCATH1) were raised in rabbits as previously specified [21,22].
Light microscope immunocytochemistry was performed incubating the sections overnight at 0-4°C with the rabbit antibodies diluted 1: 200 in Buffer (Tris 0.05 M at pH 7.6 containing 1% BSA). In control sections, the primary antibodies were omitted. After rinsing in buffer, the sections were incubated for 60 min at room temperature in a fluorescein-conjugated (green) or rhodamine conjugated (red) anti-rabbit antibodies, rinsed in buffer, mounted in 10% glycerol in PBS, and observed under a fluorescence microscope. Ultrastructural immunolabeling was carried out on thin sections of 50-90 nm in thickness that were collected on Nickel grids, and the sections were incubated for 10 min in the Tris buffer containing 1% Cold Water Fish Gelatin to block non-specific binding sites, then the grids were incubated overnight at 0-4°C in the primary antibodies (dilution 1: 200). After rinsing in the Buffer, a 5 or 10 nm gold conjugated anti-rabbit secondary antibodies were applied for one hour at room temperature, the grids were rinsed in buffer and stained for 4 min in 2% aqueous uranyl acetate, rinsed and observed with a Zeiss 10C/10 CR electron microscope. In control sections, the primary antibodies were omitted.
Other samples for the ultrastructural and immunogold labeling derived from bacteria sampled from microbiological cultures after treatment with beta-defensins or cathelicidins, as it is detailed elsewhere [29]. The analysis of the bacteria present in the culture samples (in vitro) was utilized to compare the study in vivo (skin samples) in order to detect possible alterations in bacterial cells. Briefly, spots of the bacterial culture of Escherichia coli or Streptococcus aureus exposed to different concentrations of TuBD1 (a beta-defensin from the soft-shelled turtle Apalona spinifera, see [17]) or to AcChat1 (a cathelicidin from the lizard Anolis carolinensis, see [19]) that produced visible decrement in the culture on Petri dish were selected [29].
From bacterial colonies of E. coli or S. aureus that showed inhibition of growth, some samples of 1-2 mm were collected with their agar support, and they were fixed in 4% paraformaldehyde and processed for embedding in the Resin Bioacryl as specified above. The sections of the damaged bacterial cultures were sectioned, they were immunostained as indicated above for the two peptides, and were observed under the electron microscope Zeiss 10C/10 CR operating at 60kV.
Results
General Epidermal histology
The epidermis of normal scales in lizards consists in a thin but thought and inflexible corneous layer indicated as beta-layer, under which a softer alpha-layer and a viable epidermis are present (Figure 1A). In the dermis, particularly in pigmented scales, numerous melanophores (brown-stained pigment cells) are seen. After injury of the skin or amputation of the tail that lead to regeneration, the epidermis becomes more stratified (wound epidermis) and scales are regenerated (Figure 1B). Initially beneath the scab, consolidated during the first 2-4 day after injury, a connective tissues rich in granulocytes, erythrocytes and numerous bacteria is present, but in the following 8-12 days post-injury a new wound epidermis is formed underneath the scab (Figure 1C). In lizards and snakes the normal epidermis is periodically shed and this process follows a renewal phase in which the outer epidermal layers, destined to be sloughed, are replaced by a new epidermis [10,11]. This process in both lizards and snakes occurs with the formation of an inner beta-layer made of spindle-shaped cells, generated underneath the corneous alpha-layer and the external corneous beta-layer (Figure 1D).
In the sphenodontidae lepidosaur Sphenodon punctatus the epidermis is also covered by a resistant but thin beta-layer, followed by an alpha-layer and living epidermal layers (Figure 1E), After scale injury the epidermis forms a stratified wound epidermis [26]. In crocodilians, a thick stratum corneum covers most of the scale outer (dorsal) surface while it appears thinner with desquamating cells in the hinge regions (Figure 1F). In the shell of turtles a thick corneous layer is generally present and, in some species, its external part can be shed in some periods along a shedding layer (Figure 1G). This is not the case for the shell epidermis of soft-shelled turtles where instead a thinner corneous layer is more or less continuously exfoliated in its superficial part, like in the other regions of the skin (Figure 1H).
Ultrastructure of the corneous layer
The ultrastructural survey over the corneous layers of normal or regenerating scales of different reptilian species shows the presence of numerous bacterial cells, clustered or isolated, that can also be identified among the mores superficial 2-6 layers of corneocytes (Figure 2). Most bacteria are not capsulated and resembles bacilli surrounded by a cell wall (Figures 2B,C,E) while others show a round shape (coccus) and can be single cells or associated in pairs (diplococcus, Figures 2A,D) or tetrads (sarcines), the latter generally surrounded by an electron-paler capsule (Figures 2F). Bacteria cells generally remain extracellular, but while the more superficial bacterial cells are integral with a continuous cell membrane or capsula, numerous of those present among corneocytes appear damaged, with cytoplasmic vacuoles and rupture of the cell membrane, or containing denser cytoplasmic clumps (Figures 2C,D). In the corneous layer of the soft-shelled turtle (Apalona spinifera) also intracellular bacteria are commonly present in the first 2-3 extermal layers, but not in the deeper corneocytes (Figure 2G).
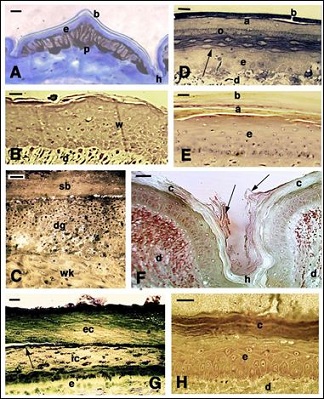
Figure 1: Histology of the epidermis of representative reptiles A: normal dorsal scale in the lizard Anolis carolinensis with intensely pigmented dermis. Bar 20 µm. B: regenerating stratified epidermis of the lizard Podarcis muralis, bar 10 µm. C: regenerating wound epidermis in a limb wound of P. muralis. A degenerating tissue composed of granulocytes mixed to bacteria is present underneath the scab. Bar 10 µm. D: epidermis in renewal phase showing formation of a new beta-layer (arrow) in a trunk scale of the snake Natrix natrix. Bar 10 µm. E: regenerating tail scale of Sphenodon punctatus, showing a multilayered epidermis beneath the corneous layers. Bar 10 µm. F: detail of the hinge region between two ventral scales of the Nile crocodile Crocodylus niloticus showing the thick stratum corneum and desquamating corneocytes (arrows). Bar 20 µm. G: thick corneous layer of the carapace in the turtle Pseudemys nelsoni. The arrow indicates the splitting lane where shedding will take place. Bar 10 µm. H: multilayered epidermis of the carapace of the soft-shelled turtle Apalone spinifera. Bar 10 µm. Legends: a: alpha-layer (corneous); b: beta-layer (corneous); c: corneous layer; d: dermis; dg: degenerating granulocyte/ bacterial layer. e: epidermis; ec: external part of the corneous layer; h: hinge region; ic: internal part of the corneous layer; o: oberhautchen layer; p: pigment cell (melanophore); sb: scab; w: wound (regenerating) epidermis; wk: wound keratinocytes.
The detailed observations on superficial, apparently intact bacteria shows the integrity of the cell membrane, cell wall and capsula when this is present (Figures 3A,B,C). Intracellular bacteria do not have a capsule and appear as Gram-, but their cell wall seems to be directly in contact with the corneous cytoplasm of the corneocyte (Figures 2G,3D). Based on the general ultrastructural characteristics of the different bacterial cells they appear to belong to Escherichia or Salmonella sp while the oval or roundish bacteria surrounded by a capsula include Staphylococcus sp (the more common), Streptococcus, Sarcina or even Mycobacterium (with thick cell walls). However the ultrastructural characteristics are not sufficient to identify genus or species that are, however, beyond the scope of the present and general survey.
Immunodetection of AMPs in the epidermis and bacterial cells
As indicated above, the antibodies here utilized tag specific epitopes present in the studied beta-defensins and cathelicidins that we previously characterized in lizard and turtles. No or a weak immunoreactivity is observed in the normal epidermis of lizard while in the wound and regenerating epidermis of lizards and in normal epidermis of turtles some positive immunostaining for both beta-defensins and cathelicidin-1 are present (Figure 4). After skin injury in the tail or in tail/limb scales of lizard, a clot is formed over the damaged tissue separating the inner tissues from the external environment and its microbial populations. One to two days after skin injury in the tail of the lizard P. muralis or A. carolinensis, a scab is formed, mainly composed by degenerating erythrocytes and few trapped leucocytes, coagulated within a fibrin matrix. Immunofluorescence controls indicate that while erythrocytes are autofluorescent, the surrounding fibrin matrix shows some specific labeling for both Ac-beta-defensin-15 and -27, and for Ac-cathelicidin-1 (Figure 4A-C).
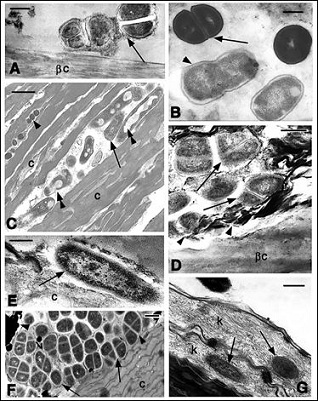
Figure 2: Ultrastructural details of the superficial area of the corneous layer in different reptilian species. A: diplococcus-like bacteria resting on the beta-layer of a digit scale in the gecko Hemidactylus turcicus. The arrow indicates possible capsular material. Bar 500 nm; B: Staphylococcus like cells in division (arrow) and Bacillus/ Salmonella-like cells (arrowhead) on a scale surface of the snake Natrix natrix. Bar 250 nm. C: bacilli (arrow) and coccus (arrowheads) present among corneocytes of the tail wound epidermis of Sphenodon punctatus. Double arrowheads indicate bacilli with internal degeneration. Bar 500 nm; D: other Diplococcus-like bacteria on the surface of the beta-layer in tail scale of S. punctatus. The arrows show membrane loss while the arrowheads point to possible degenerated bacterial bodies. Bar 250 nm. E: apparently viable bacterium (arrow), possible a Samonella or Escherichia sp., detected on the surface of the corneous layer in Crocodylus porosus. Bar 200 nm; F: aggregates of capsulated (arrowheads) bacteria, resembling Mycobacterium or Sarcina sp. on the surface of the corneous layer in the carapace of the turtle Chrysemys picta. Bar 500 nm; G: intracellular Salmonella-like bacteria (arrows) in the external corneocytes of the limb epidermis of the soft-shelled turtle Apalone spinifera. Bar 250 nm. Legends: βc: beta-corneous layer; c: corneous layer; k: keratin filaments.
The normal epidermis of the shell, neck and limbs in the hard-shelled turtles Chrysemys picta or Pseudemys nelsoni, and of the soft-shelled turtle A. spinifera shows small roundish granules, better identified under the electron microscope as bacterial cells, on the surface of the corneous layer or even infiltrated among the superficial layers of the corneous layer (Figure 4D). In the stratum corneum of the carapace and in the softer limb epidermis in the soft-shelled turtle Apalone spinifera, some bacteria are also seen externally to the superficial corneocytes, as later confirmed using the electron microscope. The epidermis shows immunofluorescence, especially in the external stratum corneum for a turtle beta-defensin (TuBD1; Figure 4E), a location where also bacterial cells are seen (see later for its ultrastructure). Controls sections show absence of fluorescence over most of the corneous layer (Figure 4F).
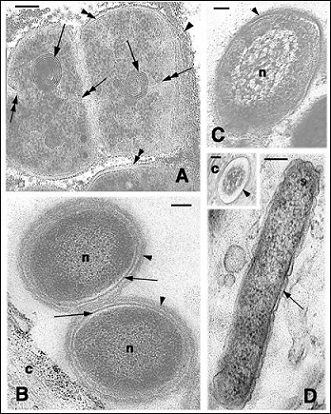
Figure 3: Ultrastructural details of viable cells on the surface of the corneous layer. A: Possible Mycobacterium cells showing active cell division (double arrows indicate the forming septa), mesosomes (arrows), cell wall (double arrowheads), and capsular material (arrowheads) C. picta epidermis. Bar 200 nm. B: two Gram+ bacteria (n, nucleoid) surrounded by an integral membrane (arrows) and cell wall (arrowheads) close to a corneocyte (c) of the turtle A. spinifera. Bar 200 nm. C: Grambacterium resembling Pseudomonas sp (n, nucleoid) on the epidermal surface of a normal scale of S. punctatus. Bar 200 nm. D: intracellular bacterium (Escherichia like, the arrow points the Gramcell wall) in A. spinifera corneocytes. Bar 200 nm. In the inset the membrane of a cross-sectioned bacterium (arrowhead) is directly in contact with the cytoplasm of the corneocyte (c). Bar 100 nm.
Ultrastructural observations using immunogold particles of 5 or 10 nm in diameter show a diffuse immunolabeling for AcCath1, AcBD27 and AcBD15, especially visible along the membranes and in the inter-cellular spaces between corneocytes of the regenerating wound epidermis of the lizards Podarcis muralis and Anolis carolinensis (Figures 5A,B), and in the soft-shelled turtle Apalona spinifera (Figure 5C). Sparse 0.3-0.5 µm large granules present in the cytoplasm of pre-corneous cells also show a diffuse but specific labeling (inset of Figure 5C). After scale or tail/limb injury a blood clot is formed on the surface of the wound and gives rise to a scab within 1-2 days. At 4-7 days post-injury the scab is progressively separated by the underlying healing connective by the infiltration of migrating keratinocytes that eventually determine the detachment of the scab at 8-12 days post-injury (Figure 1C, see details in [27,30]). The ultrastructural immunolabeling for AcBD15 and AcBD27 of the scab confirms the previous light microscope immunolabeling (Figures 3A-C). The scab is derived from degenerated erythrocytes and granulocytes in addition to dead epidermal cells, coagulated within a fibrin material. The degraded cells in the scab at 4-7 days post-injury results poorly identified and most of the scab appears composed by a mix of granular, amorphous and dense material alternated with paler roundish areas that are diffusely labeled (Figure 6). Control sections where the primary antibody was omitted do not show immunolabeling (Figure 6D).
The observations over the corneous layer of normal and regenerating epidermis in the lizards A. carolinensis and P. muralis show roundish or bacillar cells, resembling GramEscherichia or Salmonella sp that result immunolabeled for AcBD15, AcBD27 and AcCath1 (Figure 7). The gold particles are present in the cytoplasm, nucleoid region, and along the plasma membrane. These bacterial cells however appear altered, featuring membrane ruptures or lacking a continuous membrane. They also show the presence of vacuolated areas and cytoplasmic clarification, and these cells contain few ribosomes in their cytoplasms (Figures 7B-D), all indications of cell degeneration (see comparison with cells isolated from microbiological bacterial culture).
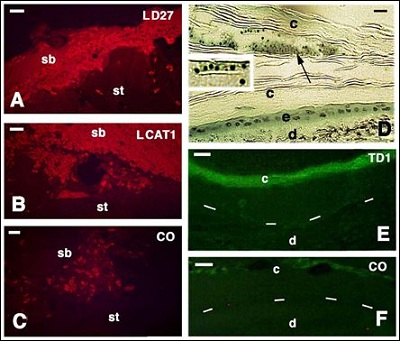
Figure 4: Immunoreactivity for some anti-microbial peptides on the surface of the skin in the lizard P. muralis A-C: histology on hard-shelled turtle P. nelson D: and immunohistochemistry on the soft-shelled turtle A. spinifera E, F: Bars in all figures indicate 10 µm. A: labed scab (sb) for lizard defensin 27 (LD27) on the tail stump (st) of P. muralis at 2 days post-injury. B: labeled scab (sb) for lizard cathelicidin 1 (LCAT1) at 2 days post-injury (st, stump) in P. muralis. C: control section showing loss of immunofluorescenze in the scab P. muralis. aside in sparse autofluorescent red cells. D: localization of clusters of bacteria (arrow, see magnification in the inset) seen within the thick stratum corneum (c) of the carapace of the turtle P. nelsoni (e epidermis; d, dermis). E: immunofluorescence in the stratum corneum (c) of limb epidermis. Dashes underline the basal layer (d, dermis) in A. spinifera. F: immunonegative control (c, stratum cornum; d, dermis).
Also Gram+ bacterial cells, detected on the corneous surface of the regenerating and normal scales of lizard, snake and turtle epidermis, often appear degenerated (Figures 8A-B). Cells identified as Staphylococcus or Bacillus sp, show cytoplasmic vacuolization and formation of clumped cytoplasmic granules, and some of these cells result completely degenerated, empty and they are surrounded by a residual but discontinuous cell wall (Figures 8A). The immunolabeling with TuBD1 shows that these cells contain immunolabeled areas of the cytoplasm, nucleoid and along the cell perimeter, indicating that these regions are targets of this anti-microbial peptide (Figure 8B). Other bacterial cells, immunolabeled for TuBD1, appear as protoplasts, namely cells devoid of a cell wall, and they feature numerous ruptures along the plasma membrane (Figure 8C) or even they do not possess most of the plasma membrane (Figure 8D).
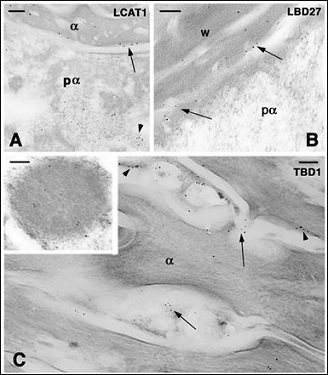
Figure 5: Immunogold labeling in the wound epidermis of the regenerating scales in lizards (A, B) and in the normal epidermis of the soft-shelled turtle (C). Bars indicate 100 nm in all the figures A: diffuse distribution of gold particles detecting AcCath1 (LCAT1) in a pre-corneous alpha-cell cytoplasm (pα, arrowhead) and in the wound epidermis (arrow) of A. carolinensis. Bar 100 nm. B: other immunolabeling (arrows) for AcBD27 (LBD27) in the wound epidermis of P. muralis (pα, pre-corneous alpha-layer). C: TuBD1 immunolabeling of the surface of α-corneocytes (arrowheads) and in the intercellular spaces (arrows) present in the corneous layer of the limb epidermis of the turtle A. spinifera. The inset shows a large granule of a pre-corneous cell that is labeled for TuBD1.
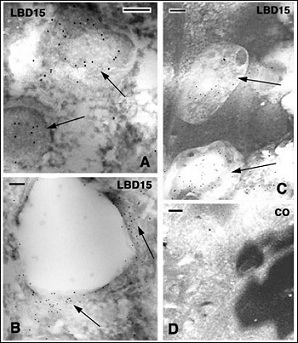
Figure 6: Immunogold labeling for AcBD15 (LBD15) in the scab of lizards at about 4 days post-injury. Bars indicate 100 nm in all the figures. A: immunolabeled reticulate granules (arrows) present among non-structured scab material in A. carolinensis. B: other area of the scab in A. carolinensis, likely derived from a dead granulocyte and containing a diffuse labeling (arrows) around a vesicle. C: labeling in two pale areas (arrows) localized among the scab material in P. muralis. D: immunonegative control section (CO) of an area of the scab.
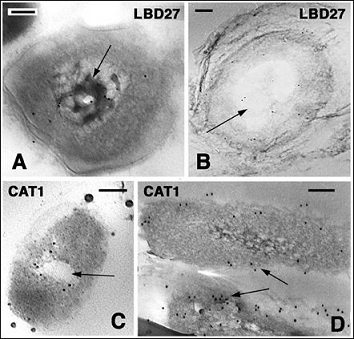
Figure 7: Labeled bacteria found on the corneous layer of the lizard A. carolinensis. Bars indicate 100 nm in all the figures. A: immunolabeled nucleoid for AcBD27 (LBD27, arrow) in a Grambacterium located on the surface of the wound epidermis. B: other labeled bacterial cell (LBD27) with vacuolated area (arrow) and un-distinct membrane (arrowheads) detected on the epidermis of a normal digital scale. C: AcCath1 (CAT1) immunolabeled bacterium with a vacuolated central cytoplasm and undistinct plasma membrane, present on the surface of the wound epidermis. D: two CAT1 immunolabeled, Salmonella or Escherichia-like bacteria located on the surface of the beta-layer of a normal scale.
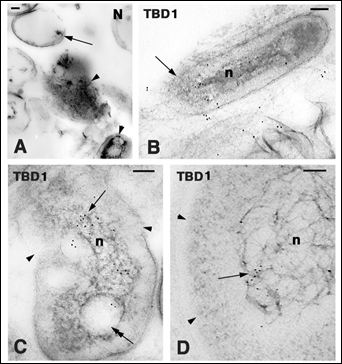
Figure 8: Bacterial cells localized on the limb (A-C) and neck (D) epidermis in the soft-shelled turtle A. spinifera. Bars indicate 100 nm in all the figures. A: damaged coagulated bacterial cells (arrowheads) of Staphylococcus sp. The arrow points to the remnant of a cell wall. B: TuBD1 intracellular labeling in a bacterium (n, nucleoid) with discontinuous plasma membrane (arrow). C: TuBBD1 labeling (arrow) in the nucleoid region (n) of a bacterium with discontinuous plasma membrane (arrowheads) soft epidermis limb. The double arrow points to one vacuolated area. D: TuBD1 labeling (arrow) in the scaffold material of the nucleoid (n). Arrowheads point to the incomplete membrane located along the cell perimeter.
The ultrastructural comparison of the damaged bacterial cells found in the corneus layer of reptiles, with those isolated from bacterial cultures treated with TuBD1 or AcCath1 [29], show similar alterations. These consist in membrane ruptures, cytoplasm clarification with loss of ribosomes, and vacuolization in both the microbiological cultures of Escherichia coli and Staphylococcus aureus (Figure 9). Also the nucleoid region and the protein scaffolding filaments have decreased or appear altered in comparison to the normal euchromatic nucleoid. These damages are associated with the immunolocalization of the peptides within the cells, along their perimeter and in the nucleoid area (Figure 9D,E,H).
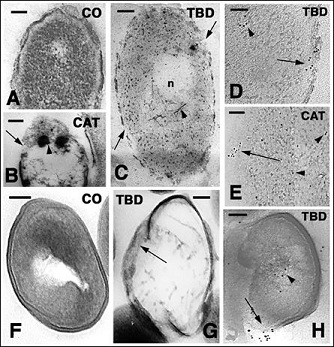
Figure 9: Ultrastructural images of Escherichia coli (A-E) and Staphylococcus aureus (F-H) bacteria in culture in normal conditions and after treatment with antimicrobial peptides. Bars indicate 100 nm in all figures. A: normal control bacterium. B: treated with AcCath1 for 3 hours. Most cytoplasm is empty, large part of the cell membrane is lacking (arrow) and dense, likely coagulated protein granules are seen (arrowhead). C: other damaged bacterial cell after 3h incubation with TuBD1 showing membrane ruptures (arrows), ribosome paucity, disassembling of nucleoid (n) scaffolds filaments (arrowhead). D: detail on TuBD1 immunogold labeling on the discontinuous plasma membrane (arrow) and within the cytoplasm (arrowhead) 3 h after treatment. E: immunolabeling along the interrupted plasma membrane (arrow) and diffusely in the cytoplasm (arrowheads) 3 hours post-incubation with AcCath1. F: untreated control bacterium. G: three hours after exposition to TuBD1 the cytoplasm is empty and the membrane (arrow) is largely disappeared. H: immunolabeling for TuBD1 shows the gold particles along the broken plasma membrane and cell wall (arrow) but also in the core cytoplasm (arrowhead).
Discussion
The present qualitative ultrastructural survey on the epidermis of all reptilian representatives (lepidosaurians, crocodilians and turtles) documents the almost constant presence of Gram+ bacteria, possessing a thick cell wall (Staphylococcus sp or possible Bacillus or Mycobacterium sp), and Grambacteria with a thinner cell wall (especially Salmonella or Escherichia sp). These bacteria are not only observed on the surface of the corneous layer but are also localized in the extracellular space of the more superficial 2-3 or even deeper layers of the reptilian stratum corneum, as also noted in the human epidermis [1]. Various species of bacteria have been found on the skin or oral and anal cavities in turtles, lizards, and snakes in normal or pathological conditions, such as Salmonella [31], Pseudomonas [32], Mycobacterium and Clamydia [33], Staphylococcus and many others [34]. Bacteria have been also observed among corneocytes in shed molts of numerous species of snakes and lizards (Alibardi, unpublished observations).
The study shows some morphological alterations in these bacteria of the stratum corneum, especially in the deeper regions of the corneous layer, where they appear largely degenerated [22]. The progressive cell destruction as the bacteria penetrate inside the stratum corneum is strengthened from the comparison of the alterations observed in vivo and similar alterations occurring in microbial tests (Figures 8,9) [29]. The latter consists in the rupture of small regions of the plasma membrane and cell wall, lowering in ribosome number, increase of the cytoplasmic vesiculation with the clarification of the cytoplasm, the protein clumping and clarification of the nucleoid, and by the complete degradation of the cell wall and plasmalemma. The latter process is followed by the fragmentation of the bacterial cell in both Gramand Gram+ bacteria [29]. Therefore both the membranes and the DNA or scaffolding proteins of the nucleoid region can be directly or indirectly affected from these peptides. Similar ultrastructural alterations or the destruction of bacterial cells of the genus Escherichia, Staphylococcus, Streptococcus, Moraxella, Hemophilus, Salmonella, Lactobacillus, Pseudomonas, Helicobacter, were seen in various studies using traditional antibiotics [35-37] or antimicrobial peptides of mammalian [38-42] or reptilian [20,43] origin. Other antimicrobial test on Pseudomonas aeruginosa have indicated that antimicrobial peptides penetrate into the cell of bacteria, and show a similar membrane or nuclear localization as here reported before the disruption of the bacterial cell [40,44].
The present survey further supports the hypothesis that the high resistance to infection in normal skin integrity and after disruption of the skin barrier following injury in lizards, snakes, and likely in other reptiles, is largely due to the release of AMPs in the corneous layer or within the consolidating scab that form a chemical antimicrobial barriers aside the mechanical barrier role (Figure 10). The epidermis makes likely the main immunological organ of the body in terms of extension [4,6]. While the carapace epidermis of turtles and the beta-layer of lizard epidermis do not show immuno-detectable peptides, their detection occurs in the regenerating epidermis and in the soft-shelled carapace and limb epidermis. The detection of immunolabeling for beta-defensins and cathelicidins observed among corneocytes and inside bacterial cells present in the more superficial corneous layers in turtle epidermis suggests that these peptides are constitutively released among corneocytes at low concentration, reaching the superficial surface of the corneous layer.
When AMPs encounter bacteria, they enter in their cells determining damage and eventually killing those that penetrate some corneous layers below the surface. The intense immunolabeling of scabs and in the regenerating epidermis present at the surface of wounds indicate the formation of a solid antimicrobial barrier, as it is also seen in mammalian wounds [45-47]. The study also confirms that the scab covering wounds incorporates and likely maintains for sometime the AMPs, contributing to the formation of a lasting anti-microbial barrier while the underlying re-epithelization is completed [27,30].
The expression of AMPs is also correlated with the stimulation of epithelial proliferation in addition to their antimicrobial activity. Migrating or stimulated mammalian keratinocytes have been shown to express high levels of cathelicidins and beta-defensins that later decrease at complete re-epithelization [41,45]. Whether, in addition to the AMPs, also the keratins or the corneous beta-proteins (beta-keratins) of reptilian corneocytes may also release antimicrobial peptides after their degradation in the stratum corneum [48] remains to be evaluated. After large amputations that would instead kill many mammals or birds (homeotherms) from excessive bleeding or septicemia, many reptiles heal with no severe infections. Since reptiles in general posses a less efficient adaptive immunity in comparison to homeotherms [49], their high resistance to infections suggests that they possess potent innate immunity in the epidermis and other epithelia. The production of effective anti-microbial proteins or peptides that function at variable and low temperatures in addition to macrophages [50] is likely advantageous for the microbe resistance in reptiles. This is particularly shown after tail amputation in lizards that induces a small inflammatory reaction and is followed by the regeneration of this organ [27,30]. It is likely that the limited inflammation determined by the presence of autotomy planes (anatomical pre-formed planes of amputation) also is coupled with the rapid activation of antimicrobial mechanisms with the production of antimicrobial molecules that act at lower temperatures, below 10°C [50]. In this way reptilian AMPs are active when the bacterial proliferation is relatively low, so that the spreading of infections is diminished under these conditions.
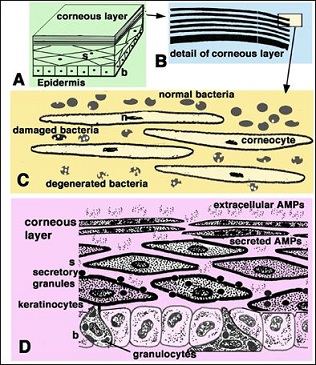
Figure 10: Schematic representation featuring the current interpretation of the antimicrobial barrier in reptilian epidermis. A: section of a generalized epidermis. B: detail of the stratification of the corneous layer. C: further detail showing the progressively more damaged bacterial cells as they penetrate more internally among corneocytes of the stratum cornum. D: summarizing drawing depicting the possible secretion of antimicrobial peptides (AMPs) among suprabasal keratinocytes. Legend: b: basal layer of the epidermis; n: nuclear remnants; s: suprabasal layers.
In conclusion, the presence of anti-microbial peptides in the epidermis of reptiles and in bacterial cells localized on the surface of the corneous layer or among the superficial corneocytes, suggests that an antimicrobial barrier stops microbe penetration (Figure 10). In case of wounding the antimicrobial barrier is initially made by the scab that contains these peptides derived from the degradation of granulocytes, until re-epithelialization with active AMP secretion is re-established underneath the scab. The potential antimicrobial activity of reptilian AMPs for medical application remains to be fully explored [8,29].
Acknowledgments
Comparative Histolab, Padova, Italy, largely supported the present research.
References
- Lange-Asschenfeldt B, Marenbach D, Lang C, Patzelt A, Ulrich M, et al. (2011) Distribution of bacteria in the epidermal layers and hair follicles of the human Skin Pharmacol Physiol 24: 305-311.
- Oren A, Ganz T, Liu L, Meerloo T (2003) In human epidermis, beta-defensin 2 is packaged in lamellar Exp Mol Pathol 74: 180-182.
- Braff MH, Di Nardo A, Gallo RL (2005) Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol 124: 394-400.
- Elias PM, Choi EH (2005) Interactions among stratum corneum defensive Exp Dermatol 14: 719-726.
- Sahl HG, Pag U, Bonness S, Wagner S, Antcheva N, et al. (2005) Mamma- lian defensins: structures and mechanism of antibiotic activity. J Leukoc Biol 77: 466-475.
- Stolzenberg ED, Anderson GM, Ackermann MR, Whitlock RH, Zasloff M (1997) Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci U S A 94: 8686-8690.
- Zanetti M (2004) Cathelicidins, multifunctional peptides of the innate immu- J Leukoc Biol 75: 39-48.
- van Hoek ML (2014) Antimicrobial peptides in Pharmaceuticals (Ba- sel) 7: 723-753.
- Parakkal PF, Alexander NJ (1972) Keratinization: a survey of vertebrate epi- Academic Press New York, USA.
- Maderson PFA (1985) Some developmental problems of the reptilian integu- In: Gans C, Billett F, Maderson PFA (eds.). Biology of the Reptilia. John Wiley & Sons, New York, USA 14: 525-598.
- Landmann L (1986) The skin of Reptiles: epidermis and dermis. In: Bereit- er-Hahn J, Matoltsy AG, Sylvia-Richards K (eds.). Biology of the integument, Springer Verlag. Berlin-Heidelberg-New York, USA 150-187.
- Alibardi L, Toni M (2006) Cytochemical, biochemical and molecular aspects of the process of keratinization in the epidermis of reptilian Prog His- tochem Cytochem 40: 73-134.
- Chattopadhyay S, Sinha NK, Banerjee S, Roy D, Chattopadhyay D, et al. (2006) Small cationic protein from a marine turtle has beta-defensin-like fold and antibacterial and antiviral Proteins 64: 524-531.
- Wang Y, Hong J, Liu X, Yang H, Liu R, et al. (2008) Snake cathelicidin from Bungarus fasciatus is a potent peptide PLoS One 3: 3217.
- Lakshminarayanan R, Vivekanandan S, Samy RP, Banerjee Y, Chi-Jin EO, et al. (2008) Structure, self-assembly, and dual role of a beta-defensin-like peptide from the Chinese soft-shelled turtle eggshell matrix. J Am Chem Soc 130: 4660-4668.
- Stegemann C, Kolobov A Jr, Leonova YF, Knappe D, Shamova O, et al. (2009) Isolation, purification and de novo sequencing of TBD-1, the first be- ta-defensin from leukocytes of Proteomics 9: 1364-1373.
- Dalla Valle L, Benato F, Maistro S, Quinzani S, Alibardi L (2012) Bioinformatic and molecular characterization of beta-defensins-like peptides isolated from the green lizard Anolis Dev Comp Immunol 36: 222-229.
- Valle DL, Benato F, Paccanaro MC, Alibardi L (2013) Bioinformatic and molecular characterization of cathelicidin-like peptides isolated from the green lizard Anolis carolinensis. Ital J Zool 80: 177-186.
- Benato F, Dalla Valle L, Skobo T, Alibardi L (2013) Biomolecular identification of beta-defensin-like peptides from the skin of the soft-shelled turtle Apalone J Exp Zool B Mol Dev Evol 320: 210-217.
- Pata S, Yaraksa N, Daduang S, Temsiripong Y, Svasti J, et (2011) Characterization of the novel antibacterial peptide Leucrocin from crocodile (Crocodylus siamensis) white blood cell extracts. Dev Comp Immunol 35: 545-553.
- Alibardi L (2013) Granulocytes of reptilian sauropsids contain beta-defensin-like peptides: a comparative ultrastructural survey. J Morphol 274: 877-886.
- Alibardi L (2013) Immunolocalization of a beta-defensin (Tu-BD-1) in the skin and subdermal granulocytes of turtles indicate the presence of an antimicrobial skin barrier. Ann Anat 195: 554-561.
- Alibardi L (2014) Ultrastructural immunolocalization of chatelicidin-like peptides in granulocytes of normal and regenerating lizard tissues. Acta Histochem 116: 363-371.
- Alibardi L (2015) Immunocytochemical detection of beta-defensins and cathelicidins in the secretory granules of the tongue in the lizard Anolis caro Acta Histochem 117: 223-227.
- Diaz-Vissurraga J, Cardenas G (2010) Morphological changes induced in bacteria as evaluated by electron In “Microscopy: science, technology, applications and education”, Mendez-Vilas A, J Diaz (eds.). Micros- copy Book Series 4, Formatex Research Center, Budajoz, Spain. 307-315.
- Alibardi L (2004) Formation of the corneous layer in the epidermis of the tuatara (Sphenodon punctatus, Sphenodontida, Lepidosauria, Reptilia). Zoology (Jena) 107: 275-287.
- Alibardi L, Celeghin A, Valle DL (2012) Wounding in lizards results in the release of beta-defensins at the wound site and formation of an antimicrobial Dev Comp Immunol 36: 557-565.
- Scala C, Cenacchi G, Ferrari C, Pasquinelli G, Preda P, et al. (1992) A new acrylic resin formulation: a useful tool for histological, ultrastructural, and immunocytochemical J Histochem Cytochem 40: 1799-1804.
- Holthaus KB, Spisni E, Alibardi L (2016) Microbicide activity of two reptilian antimicrobial peptides on Gram positive and Gram negative bacteria. J Immunobiol 1:104.
- Alibardi L (2010). Ultrastructural features of the process of wound healing after tail and limb amputation in Acta Zool 91: 306-318.
- Bradley T, Angulo F, Mitchell M (2001) Public health education on Salmonella spp and J Am Vet Med Assoc 219: 754-755.
- Aguirre AA, Balazs GH, Zimmerman B, Spraker TR (1994) Evaluation of Hawaiian green turtles (Chelonia mydas) for potential pathogens associated with J Wildl Dis 30: 8-15.
- Soldati G, Lu ZH, Vaughan L, Polkinghorne A, Zimmermann DR, et (2004) Detection of mycobacteria and chlamydiae in granulomatous inflammation of reptiles: a retrospective study. Vet Pathol 41: 388-397.
- Jho YS, Park DH, Lee JH, Cha SY, Han JS (2011) Identification of bacteria from the oral cavity and cloaca of snakes imported from Vietnam. Lab Anim Res 27: 213-217.
- Weerkamp A, Heinen-von Borries UT, Vogels GD (1978) Biochemical and ultrastructural changes in Staphylococcus aureus treated with staphylococcin Antonie Van Leeuwenhoek 44: 35-48.
- Gottfredsson M, Erlendsdóttir H, Kolka R, Gudmundsson A, Gudmundsson S (1993) Ultrastructural alterations of bacteria during the postantibiotic Chemotherapy 39: 153-162.
- Hartmann M, Berditsch M, Hawecker J, Ardakani MF, Gerthsen D, at al. (2010) Damage of the bacterial cell envelope by antimicrobial peptides Gramicidin S and PGLa as revealed by transmission and scanning electron Antimicr Agents Chemiother 54: 3132-3142.
- Shimoda M, Ohki K, Shimamoto Y, Kohashi O (1995) Morphology of defensin-treated Staphylococcus Infect Immun 63: 2886-2891.
- Friederich CL, Moyles D, Beveridge TJ, Hancock RE (2000) Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicr Agents Chemiother 44: 2086-2092.
- Kalfa VC, Jia HP, Kunkle RA, McCray PB Jr, Tack BF, et al. (2001) Congeners of SMAP29 kill ovine pathogens and induce ultrastructural damage in bacterial Antimicrob Agents Chemother 45: 3256-3261.
- Midorikawa K, Ouhara K, Komatsuzawa H, Kawai T, Yamada S, et (2003) Staphylococcus aureus susceptibility to innate antimicrobial peptides, ß-defensis and CAP18, expressed by Human Keratinocytes. Infect Immun 71: 3730-3739.
- Lee HY, Andalibi A, Webster P, Moon SK, Teufert K, et (2004) Antimicrobial activity of innate immune molecules against Streptococcus pneumoniae, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect Dis 4: 12.
- Chen XX, Yu GY, Zhan Y, Zhang Y, Shen JH, et al. (2009) Effects of the antimicrobial peptide OH-CATH on Escherichia Zool Res 30: 171-177.
- Vega LA, Caparon MG (2012) Cationic antimicrobial peptides disrupt the Streptococcus pyogenes Mol Microbiol 85: 1119-1132.
- Heilborn JD, Nilsson MF, Kratz G, Weber G, Sørensen O, et al. (2003) The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol 120: 379-389.
- Borregaard N, Theilgaard-Mönch K, Cowland JB, Ståhle M, Sørensen OE (2005) Neutrophils and keratinocytes in innate immunity--cooperative actions to provide antimicrobial defense at the right time and place. J Leukoc Biol 77: 439-443.
- Sørensen OE, Cowland JB, Theilgaard-Mönch K, Liu L, Ganz T, et (2003) Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol 170: 5583-5589.
- Tam C, Mun JJ, Evans DJ, Fleiszig SM (2012) Cytokeratins mediate epithelial innate defense through their antimicrobial properties. J Clin Invest 122: 3665-3677.
- Zimmerman LM, Vogel LA, Bowden RM (2010) Understanding the vertebrate immune system: insights from the reptilian perspective. J Exp Biol 213: 661-671.
- Johnson JC, Schwiesow T, Ekwall AK, Christiansen JL (1999) Reptilian melanomacrophages function under conditions of hypothermia: observations on phagocytic Pigment Cell Res 12: 376-382.
Citation: Alibardi L (2016) Ultra Structural Immunolocalization of Antimicrobial Peptides Targeting Bacteria in the Corneous Layer Supports the Presence of an Anti-Microbial Barrier in Reptilian Epidermis. J Cytol Hisitol 1: 001.
Copyright: © 2016 Alibardi L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


