*Corresponding Author:
Jerry T Thornthwaite,
Cancer Research Institute of West Tennessee, 114 East Main Street Henderson, TN 38340, United States
E-mail: jtt@criwt.com
Summary
For over 1,000 years, the Chinese have used the Sweet Worm- wood plant to make tea that served as a fever reducer, which cured a variety of diseases including what is now called Malaria. Current treatment options for malaria treatment are associated with significant limitations including widespread drug resistance, severe adverse effects, restricted populations to treat (pregnant women and babies), and complicated drug administration procedures. Artemisinin, Bioflavonoids, and Curcumin are key ingredients of this Sweet Wormwood Tea, which is still being used in the poorer countries to minimize the effects of malaria. There is no evidence that the tea quit working and resistance developed. We have taken the key components in the Sweet Wormwood plant and used our modern-day knowledge of the maturation the immune system to develop an “in vivo immunization “against malaria.
TriAntiMal™ is a treatment that is “once and for all”. Babies, children, and adults are all cured after the low dose 16-day protocol. The treatment schedule is important to allow the “feeding” of the immune system with new Plasmodium falciparum antigens from the blood and liver to occur. Therefore, the full maturation of the humoral immune system in the transition from IgM to IgG production along with any cell mediated and Defensin long-term immunity can take place. With the disappearance of the parasite from the blood within 48 hrs, the processing of antigen for immunization continues to occur in the liver and other organs over the full 16 days. All the components work synergistically to destroy the parasites, protect the artemisinin, and provide antioxidant activity to assure a healthy immune environment during the process of developing long-term immunity against malaria.
Recently, a water-soluble version of TriAntiMal™ was developed using our NutraNanoSphere™ technology to treat babies by encapsulating the three components in fatty acid micelles. This process produces a water-soluble TriAntiMal, which is especially useful for adding drops to milk in a baby bottle for treating babies.
We originally developed our long-term remission in Haiti with the babies, children, and adults over ten years of doing medical missions with seven Christian churches in the Port du Prince area and conducting research in Nigeria over the past four years. These malaria victims do not have adequate protection with nets and spraying. They are bitten by African estimates over 800 times per year. Despite this, we are curing over 90% of the babies, children, and adults and now have cases over 14 years in Haiti and two years in Nigeria without recurrence. We believe we have found a very simple and cost-effective way to cure malaria with little if any side effects.
Keywords
Artemisinin; Bioflavonoids; Curcumin; Cure; Immunization; Malaria; TriAntiMal
Introduction
Resistance to artemisinin derivatives (ARTs) in malaria disease is currently defined as a delayed parasite clearance following Artemisinin-derivative Combined Therapy (ACT). The resistance to the ar-temisinin -derivative is observed only in presence of resistance to the partner drug. The lack a mechanistic rationale to choose the partner drugs and the lack of markers with known specificity and sensitivity to monitor ART resistance, represent the most concern with ACT therapies [1].
However, the treatment of uncomplicated malaria has significantly reduced malaria-related mortality. ACTs have also played a significant role in the 18% decline in the incidence of malaria cases from 2010 to 2016. However, this progress is seriously threatened by the reduced clinical efficacy of artemisinin derivatives, where delayed parasitic clearance with a high rate of recrudescence was first reported in 2008 Western Cambodia. Resistance to ACTs has already spread to several countries in Southeast Asia. Furthermore, resistance to partner drugs has been shown in some instances to be facilitated by pre-existing decreased susceptibility to the artemisinin component of the ACT. A major concern is not only the spread of these multidrug-resistant parasites to the rest of Asia but also their possible appearance in Sub-Saharan Africa [2].
Materials and Methods
The studies were will be conducted at the general outpatient department of Ladoke Akintola University of Technology Teaching Hospital, Osogbo and State Specialist Hospital, Asubiaro, Osogbo.
The positive malaria study populations had fever above 38 C, HIV negative, not pregnant, and positive by the standard WHO thick blood film / 2.0% Giemsa staining for the number of asexual parasites per microliter of blood. These measurements were performed on days 0, 1, 2, 3, 7, 14, 30, and 60 for all patients for all patients.
Furthermore, venous blood was taken on days 0, 7, 14, 30, and 60 for the serum measure of immunologic components in future studies in three separate children studies (n=112; n=51; n=31), adults (n=21) and babies (n=16).
Buffy coats were taken from the children (n=31) and babies (n=16) at days 0, 5, 10, 16, 30, 60 as reported in the DNA amplification paper[3].
The study populations consisted of babies, children in three separate studies, and adults, which were reported separately in two recent publications [3,4], were used to determine the overall curative rate from P.falciparum infection and the immunologic functions in children and babies [4].
The TriAntiMal™ formulations were supplied by Dr. Jerry T. Thornthwaite, Director, Cancer Research Institute of West Tennessee, 114 East Main Street, Henderson, TN, USA. Each capsule contains a proprietary blend of 50 mg artemisinin (99%) and 50 mg antioxidants, bioflavonoids, synephrine, quercetin, curcuminoids, hesperetin, plus flavonoids (patent pending).
The oral dosage was two capsules for adults and children over 12 years old each day for 16 days. One capsule per day for 16 days was used for children between 12 – 2 years of age. Babies were given a proprietary blend of completely water soluble NutraNanoSphere™ (NNS) encapsulated artemisinin, curcumin and bilberry in 18 nm diameter NNSs as 10 drops in water or milk in a baby bottle every day for 16 days.
Though artemisinin and bioflavonoids are known to be very safe, adequate medical personnel were available to take care of any side effects. There were no noticeable adverse sides effects of the drug observed during or post treatment for any of the populations.
Confidentiality Data was handled by the researchers and the names for each patient coded as described in the approved protocol by the Human Subjects Committee. Alternative treatment Dihydroartemisinin/piperaquine fixed antimalarial combinations were administered to patients who withdrew from the study before parasitemia was cleared or patients that fail on the study drug. Ethical clearance this was obtained from the Ethical Committee, Osun State University in Osogbo, Nigeria. Data analysis All data were analyzed statistically using standard deviations and the analysis of True Population Proportion Curve Rate at 95% confidence limits and p values determined.
Results and Discussion
A summary of the treatments with TriAntiMal™ for babies, children and adults is shown in Table 1 (see references 3,4).Also, this table is a summary of the Nigerian studies with the Babies(Roberta) reflecting the baby data from the studies in Haiti, which along with the children and adults treated in Haiti (not shown) reflect the logterm immunity against P. falciparum infection in Haiti. Figure 1 shows Roberta Audate, the Sonlight Children’s Home Director, who used the TriAntiMal™ to cure babies and over 200 children and adults in Haiti working with Haitian doctors. The babies were treated daily by using a half of each gel cap expressed into a milk bottle and given to the babies by frequent mixing and administering. For Nigeria the TriAntiMal-NNS™ water-soluble-encapsulated formulation was used with great success.
The success of TriAntiMal™ treatment can be best explained by the following.
Rationale for using Natural Artemisinin
The natural Artemisinin was first isolated from the Sweet Wormwood plant in the 1970s with purity refinements now enable the routine purity to be analytical grade Artemisinin over 99% pure. Therefore, new research has allowed detailed protometric analysis to determine its mechanisms of action. Artemisinin kills the malaria parasite Plasmodium falciparum by indiscriminately binding to proteins in many of the organism’s key biochemical pathways. Recently, it was demonstrated that artemisinin attacks multiple parasitic targets, suggesting that mutations in drug targets are unlikely to cause high-level artemisinin resistance [5].
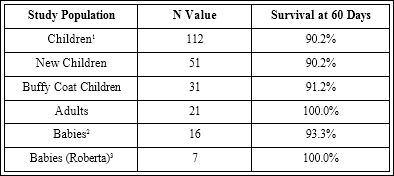
Table 1: Summary of the TriAntiMal Data shown in References 3 and 4 with results from Babies treated by Roberta Audate, Director of the Sunlight Children’s Home.
1Survival at 730 days 86.1% 219.9±8.7 mos. 3Seven Babies Figure 1

Figure 1: Roberta Audate with seven of her children from the Sunlight Children’s Home treated with TriAntiMal™ expressed from the gel caps and mixed constantly in milk for daily administration of TriAntiMal™ for 16 days. In Nigeria the NutraNanoSphere-TriAntiMal is used to give a completely water-soluble treatment using a baby bottle for delivery. The age when they were treated as babies and their age in 2015 are shown next to each child. The photograph is from May 2015. All children now four years later are still malaria free with no history of recurrence.
To identify the drug’s binding partners, synthesized biotin-alkyne-tagged artemisinin analog probes were incubated with live P.falciparum parasites. The proteins to which artemisinin binds were determined by using a mixture of streptavidin-labeled beads, which strongly attached to biotin. The purified proteins measured by mass spectrometry showed an extremely large number of 124 different proteins to which the native, pure artemisinin would bind [6].
Hanafy, et al. [7] used another chemical proteomic approach using a number of artemisinin-based protein profiling probes to identify proteins involved in glycolysis, hemoglobin metabolism, and redox defense within the malaria parasite that are alkylated by artemisinin, The data showed a variety of mechanisms of artemisinin in destroying the parasites, which included alkylated targets in the glycolytic, hemoglobin degradation, antioxidant defense, and protein synthesis pathways, which are essential for parasite survival.
The highly reactive and fast-acting nature of activated artemisinin suggests that it most likely has many targets, which explains the fact that the drug has developed only low-level resistance after decades of extensive use [5, 6].
Infection with P. falciparum can quickly progress to severe malaria and death. The main symptoms of severe malaria include severe breathing difficulties, low blood hemoglobin (severe anemia), low blood sugar, and coma. Children are particularly vulnerable since they have little or no immunity to the parasite. If untreated, severe malaria can lead cerebral malaria, which is only caused by P. falciparum.
Recrudescence as defined for infectious diseases, such as malaria, is the return of detectable symptoms in a patient whose infection has previously been at such a low level to be clinically detectable. An example is in P. falciparumparasites, where recrudescence easily occurs, because current, so-called Artemisinin Combination Therapies (ACT) do not work for long-term immunity against the parasite.
TriAntiMal™ works for long-term immunity, because it combines natural artemisinin (99% pure), not its derivatives, with citrus Bioflavonoids containing a variety of immune boosting components including curcuminoids. The Bioflavonoids work double duty by inhibiting three major enzymes that would lead to the degradation of artemisinin in the intestines. A reasonable explanation why the ACT treatment using the TriAntiMal™ results in long-term immunity (over 14 years for cases in Haiti and two years in Nigeria so far) is that very lows dosages are used daily over 16 days. The breakdown of the parasites occurs rapidly (24 hr or sooner) and supplies a continuous source of antigens for the full range of 16 days, which covers the normal transition of the IgM to IgG development. What can be called an “in vivo immunization” results in a very long-term immunity that lasts for years without any chance of the development of so-called artemisinin resistant parasites [3, 4].
On the contrary, the current ACT protocols do result in resistance treatment failures possibly based on erroneous conclusions using the so-called K13 gene mutation [8] that may lead to resistance for both the artemisinin DERIVATIVES and the chemotherapy drugs [9,10]. The problem with this measurement is the native artemisinin has many pathways to fight malaria. We certainly do not see resistance, because we are using a very low amount of native artemisinin in its native environment with the bioflavonoids and curcuminoids. So, the pharmaceutical industry marches on developing new malaria drugs with the fate begin resistance against their chemotherapy drugs and artificial artemisinin derivatives. Their logic for using derivatives of artemisinin was based on native artemisinin being water insoluble and bioavailability is decreased so more water-soluble derivatives would be better [11] These derivatives are only slightly more water soluble, but they do allow the pharmaceutical industry a way to patent new malarial drugs in the ACT format. Therefore, new ones are continually being developed to add to the arsenal of emerging ACT treatments with their sometimes-serious side effects. TriAntiMal™ components are all on the Generally Recognized as Safe (GRAS) list with the FDA with no side effects detected [12].
There is significant amount of data over the last 20 years, including our own papers, which show the immune system plays a major role in the treatment of malaria. Boosting the immunity of children with malaria is especially useful, since there are no major phenotypic and genotypic changes have yet been identified with P. falciparum. Therefore, a considerable time (over 20 years) and money (near $500 million) have been spent developing a vaccine against malaria with little success [13].
The Sweet Wormwood plant has been used in tea form as a source for natural artemisinin to treating malaria in China. If the World Health Organization (WHO) or anyone has proof of resistance developing among parasites in real live people against artemisinin, it should be made known. If not, there are not much logical reasons for this fear the WHO is inducing. If what we are doing is increasing the chance of developing an artemisinin resistant parasite, then why was it not done 1,000 years ago? It seems the pharmaceutical industry would not be interested in the TriAntiMal treatment, because there is no large profit in curing people with a cheap drug that may be in many cases, a onetime treatment protocol that effectively has gone back to the Sweet Wormwood plant and added back in chemical terms the key natural components of artemisinin, bioflavonoids, and curcuminoids.
Bioflavonoids for the treatment of Malaria
Certain common dietary flavonoids inhibit the in traerythrocytic growth of the 3D7 and 7G8 strains of P. falciparum. Their mode of action on mosquito larvae ranges from neurotoxic effects to inhibition of detoxification enzymes and larval development and/or midgut damages [13].
Some of these plants have their antimalarial efficacies demonstrated and the active compounds isolated with their probable mechanisms of action studied. Medicinal plant bioflavonoids are used to treat diseases where the biodiversity of plants occur in parallel with endemic transmission of malaria in Nigeria. Some show intense activity against malaria parasites in vitro and in experimentally infected mice [14].
Leaf flavonoids, also present in the tea, have shown a variety of biological activities and may synergize the effects of artemisinin against malaria and cancer. However, only a few studies have focused on the potential synergistic effects between flavonoids and artemisinin. Artemisinin and its semi-synthetic analogs might become more effective to treat parasitic diseases and cancer if simultaneously delivered with flavonoids. The flavonoids present in A. annua leaves have been linked to suppression of CYP450 enzymes responsible for altering the absorption and metabolism of artemisinin, but also, have been linked to a beneficial immunomodulatory activity in subjects affiicted with parasitic and chronic diseases. The high antioxidant activity of A. annua extract is most likely due to its high phenolic content [15].
A bioflavonoid fraction from Garcinia kola seeds has high antimalarial activities in P. berghei-infected mice, in addition to its known antioxidant properties [16].
Bioflavonoid extracts prepared from the Citrus limetta fruit peels showed promising anti-malarial activity by inhibiting the parasitaemia and inflammatory mediators (IFN-γ, TNF-α, IL-6) involved in malaria pathogenesis, able to improve the hemoglobin and glucose level and increase the survival time [17].
Commercial drugs, such as Accuvit®, contain flavonoids that are active in mice with malaria and in vitro against chloroquine-resistant p.falciparum [18].
New developments of flavonoids have made promising advances for the potential treatment of malaria, leishmaniasis, Chagas disease, and dengue, with less toxicity, high efficacy, and improved bioavailability [19].
Curcumin for the treatment of Malaria
Curcumin is not only effective in killing the parasite but is also able to prepare the immune system against recurrence of the disease caused by leftover parasites.
Turmeric possesses anti-microbial properties and it can kill the malaria parasite. Researchers have studied the role of turmeric with respect to various aspects of malaria.
Curcumin destroys P. falciparum
Curcumin has parasiticidal activity against many tropical parasites. Oral administration of curcumin to mice infected with malaria parasite (Plasmodium berghei) reduces blood parasitemia by 80-90% and enhances their survival significantly [20]. Cellular effects of curcumin are at least, in part, due to its perturbing effect on P. falciparum microtubules [21]. Consistent with findings in mammalian cell lines, curcumin’s prooxidant activity promoted the production in P. falciparum of reactive oxygen species (ROS), whose cytotoxic effect could be antagonized by coincubation with antioxidants and ROS scavengers. Curcumin treatment also resulted in damage of both mitochondrial and nuclear DNA, probably due to the elevation of intracellular ROS [22].
Curcumin was potent against both chloroquine-sensitive and resistant Plasmodium falciparum strains [23]. Curcumin inhibited histone acetyltransferase (HAT) activity of the P. falciparumn on derepressed 5 (PfGCN5) resulting in curcumin-induced killing by hypoacetylation viareducing nuclear HAT activity of a variety of histones. Another lethal activity against parasites was the result of curcumin’s prooxidant activity, resulting in damage to both mitochondrial and nuclear DNA in the P. falciparum parasites [24-28].
Curcumin increases the efficacy and overcomes the side effects of anti-malarial drugs
Curcumin and artemisinin act synergistically in inhibiting the growth of the malarial parasite. In combination with artemether, a derivative of artemisinin, which is used for drug resistant malaria and cerebral malaria, it not only cured the animals but also prevent recurrence. Along with bioflavonoids this three components result in a formulation designed to increase curcumin’s absorption and their combination offers better protection against malaria and prevents relapse [23-29].
Curcumin also increases the efficacy of conventional anti-malarial drugs. This duo turns out to be fast acting and even prevents relapse [27].
Curcumin is found to be therapeutic for cerebral malaria
Cerebral malaria is a fatal complication of Plasmodium falciparum infection. Parasite infected red blood cellsand platelets stick to the blood vessels and restrict blood flow to the brain. Additionally, inflammatory chemicals are secreted leading to swelling in the brain, convulsions, and coma. Cerebral malaria is the most severe and rapidly fatal neurological complication of P. falciparum infection and responsible for more than two million deaths annually. The current therapy is inadequate in terms of reducing mortality or post-treatment symptoms such as neurological and cognitive deficits. The pathophysiology of cerebral malaria is quite complex and offers a variety of targets which the TriAntiMal exploits for better therapeutic outcome. Jain, et al. [29] lists nine different targets that curcumin can modulate and serve as adjunctive therapy for cerebral malaria.
Conclusion
Based on the current state of so-called Artemisinin Combination Therapies (ACT), the following questions and conclusions can be made.
- Why use artemisinin derivatives? Artemisinin has been used for 1,000 years with no evidence of resistance in the form we present with bioflavonoids and curcuminoids, which can be described as a “back to the tea” approach;
- There are many pathways for artemisinin to work, not just the K13 defect;
- There is significance evidence that native artemisinin, bioflavo- noids, and curcumin in TriAntiMal cause long-term immunity;
- Why use immunosuppressive malaria drugs after their Artemis- inin DERIVATIVE treatments suppress the immune system? We rely on boosting the immune system for our cure;
- The current artemisinin derivatives and chemotherapeutic drugs all develop resistance in time, so new ACT drugs must be contin- ually developed.
- Current ACT treatments can be expensive and have severe side effects;
- We have an ACT treatment in which all components are directly anti-malaria and boost the immune system for long-term immu- nity against recurrence.
- The TriAntiMal™ treatments have shown that a low dose in the “na- tive” form is directly cytotoxic to the parasites, boosts the immune system, and results in a cure for over 90% of the patients;
- We have developed a completely water soluble TriAntiMal™ for- mulation using our NutraNanoSphere™ technology, in which ba- bies can be given drops in their milk daily for 16 days with a 3% cure rate [3, 30]
- Finally, we have a significant model for the treatment of cancer and viral diseases.
Epilogue: Remember Occam’s Razor
References
- Pantaleo A, Pau MC, Chien HD, Turrini F (2015) Artemisinin resistance, some facts and opinions. J Infect Dev Ctries 9:597-9.
- Ouji M, Augereau JM, Paloque L, Benoit-Vical F (2018) Plasmodium falciparum resistance to artemisinin-based combination therapies: A sword of Damocles in the path toward malaria Parasite 25: 24.
- Akanni E. Olufemi, Jerry T (2019) Thornthwaite, Ayankunle A. Ademola, Alli A.T. AntimalarialTreatment Study in South-Western Nigeria. Microbiol Infect Dis 3: 1-7.
- Jerry T, Thornthwaite JT, Akanni E Olufemi, Ayankunle A Ademola, Alli OAT(2019) DNA Gene expression to study the immunologic mechanisms for the long-term Cure of Malaria in Babies and Children in South-Western Nigeria. Adv Biol Chem 9: 1-20.
- Wang J, Xu C, Lun ZR, Meshnick SR (2017) Unpacking ‘Artemisinin Resistance’. Trends Pharmacol Sci 38: 506-511.
- Wang J, Lin Q (2016) Chemical proteomics approach reveals the direct targets and the heme-dependent activation mechanism of artemisinin in Plasmodium falciparum using an artemisinin-based activity probe. Microb Cell 3: 230-231.
- Hanafy M. Ismail, Victoria Barton, Matthew Phanchana, SitthivutCharoensutthivarakul, Michael H. L. Wong, et al. (2016) Artemisinin activity-based probes identify multiple molecular targets within the asexual stage of the malaria parasites Plasmodium falciparum PNAS 113: 2080-2085.
- Zaw MT, Emran NA, Lin Z (2018) Updates on k13 mutant alleles for artemisininresistance in Plasmodium falciparum. J Microbiol Immunol Infect 51:159-165.
- Martin RE, Shafik SH, Richards SN (2018) Mechanisms of resistance to the partnerdrugs of artemisinin in the malaria parasite. CurrOpinPharmacol 42:71-80.
- Duru V, Witkowski B, Ménard D (2016) Plasmodium falciparum Resistance to ArtemisininDerivatives and Piperaquine: A Major Challenge for Malaria Elimination inCambodia. Am J Trop Med Hyg 95:1228-1238.
- Li H, Zuo J, Tang W (2017) Water-soluble artemisinin derivatives as promisingtherapeutic immunosuppressants of autoimmune Cell Mol Immunol.
- Medina-Franco JL, Martínez-Mayorga K, Peppard TL, Del Rio A (2012) Chemoinformaticanalysis of GRAS (Generally Recognized as Safe) flavor chemicals and naturalproducts. PLoS One 7: 50798.
- Matuschewski K (2017) Vaccines against malaria-still a long way to go. FEBS J 284:2560-2568.
- Long Cui, Jun Miao, Liwang Cui (2007) Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: inhibition of histone acetylation and generation of reactive oxygen species. Antimicrob Agents Chemother 5: 488-494.
- Pavela R, Maggi F, Iannarelli R, Benelli G (2019) Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop 193:236-271.
- Adebayo JO, Krettli AU (2011) Potential antimalarials from Nigerian plants: a review. J Ethnopharmacol 133:289-302.
- Ferreira JF, Luthria DL, Sasaki T, Heyerick A (2010) Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 15: 3135-3170.
- Oluwatosin A, Tolulope A, Ayokulehin K, Patricia O, Aderemi K, et al. (2014) Antimalarial potential of kolaviron, a bioflavonoid from Garcinia kola seeds, against Plasmodium berghei infection in Swiss albino mice. Asian Pac J Trop Med 7:97-104.
- Mohanty S, Maurya AK, Jyotshna, Saxena A, Shanker K, et al. (2015) Flavonoids rich fraction of Citrus limetta fruit peels reduces proinflammatory cytokine production and attenuates malaria pathogenesis. Curr Pharm Biotechnol 16:544-552.
- Penna-Coutinho J, Aguiar AC, Krettli/ AU (2018) Commercial drugs containing flavonoids are active in mice with malaria and in vitro against chloroquine-resistant Plasmodium falciparum. Mem Inst Oswaldo Cruz 113:180279.
- Boniface PK, Ferreira EI (2019) Flavonoids as efficient scaffolds: Recent trends for malaria, leishmaniasis, Chagas disease, and Phytother Res.
- Reddy RC, Vatsala PG, Keshamouni VG, Padmanaban G, Rangarajan PN (2005) Curcumin for malaria therapy. Biochem Biophys Res Commun 326: 472-474.
- Chakrabarti R, Rawat PS, Cooke BM, Coppel RL, Patankar S (2013) Cellular effects of curcumin on Plasmodium falciparum include disruption of microtubules. PLoS One 8:57302.
- Cui L, Miao J, Cui L (2007) Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: inhibition of histone acetylation and generation of reactive oxygen species. Antimicrob Agents Chemother 51:488-494.
- Boyanapalli SS, Kong AT (2015) Curcumin, the King of Spices: Epigenetic Regulatory Mechanisms in the Prevention of Cancer, Neurological, and Inflammatory Diseases. CurrPharmacol Rep 1:129-139.
- Alam S, Panda JJ, Mukherjee TK, Chauhan VS (2016) Short peptide based nanotubes capable of effective curcumin delivery for treating drug resistant malaria. J Nanobiotechnology 14:26.
- Zhao J, Pan Y, Li X, Zhang X, Xue Y, et al. (2017) Dihydroartemisinin and Curcumin Synergistically Induce Apoptosis in SKOV3 Cells Via Upregulation of MiR-124 Targeting Cell Physiol Biochem 43:589-601.
- Wang M, Jiang S, Zhou L, Yu F, Ding H, et (2019) Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and miRNAs. Int J Biol Sci 15:1200-1214.
- Jain K, Sood S, Gowthamarajan K (2013) Modulation of cerebral malaria by curcumin as an adjunctive therapy. Braz J Infect Dis 17:579-591.
- Thornthwaite JT, Shah HR, England SR, Roland LH, Thibado SP, et al. (2017) Anticancer Effects of Curcumin,Artemisinin, Genistein, and Resveratrol, and Vitamin C: Free Versus Liposomal Forms. Advances inBiological Chemistry 7: 27-41.
Citation: Thornthwaite JT, Akanni EO (2019) Treating Malaria “Once and for All”. J Immuno Immunothe 2: 003.
Copyright: © 2019 Thornthwaite JT and Akanni EO. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.


