*Corresponding Author:
Bajaj A,
Consultant Histopathologist, A.B. Diagnostics, A-1, Ring Road, Rajouri Garden, New Delhi, India
Tel: +91 9811693956
E-mail: anubha.bajaj@gmail.com
Abstract
Atypical fibroxanthoma is an infrequent, low-grade, superficial sarcoma, contemplated as a dermal variant of undifferentiated pleomorphic sarcoma or malignant fibrous histiocytoma. Atypical fibroxanthoma is an exceptional dermal neoplasm of uncertain lineage. Of obscure aetiology, atypical fibroxanthoma probably arises from my fibroblasts or fibroblast-like cells.
vDisease Characteristics
Majority of lesions appear in Caucasians and are discerned upon sun damaged surfaces of head and neck on account of exposure to ultraviolet light. Genetic factors contribute towards the occurrence of atypical fibroxanthoma as genetic modifications are concurrent with undifferentiated pleomorphic sarcoma such as chromosomal deletions of 9p and 13q [1]. However, undifferentiated pleomorphic sarcoma delineates extensive genomic alterations and an aggressive clinical course. Possible factors contingent to appearance of atypical fibroxanthoma are cutaneous trauma, radiation therapy and conditions associated with immunosuppression such as diabetes, infection with human immunodeficiency virus or organ transplantation. The neoplasm demonstrates a variable age of occurrence, ranging between 41 years to 97 years with a median age at 74 years [1,2]. Tumefaction upon trunk and extremities commonly arise in younger subjects or children of around 13 years with a mean age of diagnosis at 39 years. In contrast, atypical fibroxanthoma situated upon head and neck appears in the elderly population with a mean age of disease discernment at 69 years. A male predominance is observed with nearly 76% of tumours arising in males [1,2]. Atypical fibroxanthoma is observed in association with predisposing disorders such as Li-Fraumeni syndrome or Xeroderma pigmentosum. Tumour incidence is enhanced in immune suppressed individuals or following therapeutic irradiation and an estimated incidence of 78 per 100,000 transplant recipients can be enunciated with the neoplasm [1,2].
Disease Pathogenesis
Atypical fibroxanthoma is a disorder of inadequately discerned pathogenesis. As the neoplasm depicts a predilection for emerging upon sun exposed body surfaces, ultraviolet light is possibly implicated. Atypical fibroxanthoma appearing in concurrence with Xeroderma pigmentosum is contingent to defective reparation of ultraviolet light induced cyclobutane pyrimidine dimers. Ultraviolet light induced pyrimidine dimers discerned in atypical fibroxanthoma appear in concordance with mutations within tumour suppressor gene p53. Several rare, genetic disorders associated with p53 induced atypical fibroxanthoma such as Xeroderma pigmentosum and Li-Fraumeni syndrome, delineate germline p53 mutations [2,3]. Genomic mutations of Harvey rat sarcoma (H-Ras) and Kirsten rat sarcoma( K-Ras) viral proto-oncogenes appear within undifferentiated pleomorphic sarcoma whereas aforesaid chromosomal mutations are absent in atypical fibroxanthoma. Consumption of arsenic can predispose to the emergence of atypical fibroxanthoma [2,3].
Clinical Elucidation
Atypical fibroxanthoma can arise as a gradually evolving or rapidly enhancing, miniature, well circumscribed, pinkishred or flesh coloured, dome shaped, polypoidal nodule or a plaque or an occasionally ulcerated tumefaction usually below<2 centimetre magnitude. Atypical fibroxanthoma demonstrates histological features simulating undifferentiated pleomorphic sarcoma although biologic behaviour is comparatively benign. Atypical fibroxanthoma is frequently discerned upon the head and neck of elderly Caucasian subjects. Atypical fibroxanthoma can also occur upon the trunk, shoulder, upper extremities and dorsum of hands [3,4].
Lesions of atypical fibroxanthoma can display superficial ulceration, crusting or scales and are typically miniature with a median diameter of 1 centimetre wherein below<5% of lesions exceed>2 centimetre magnitude. The nodule can develop rapidly although accompanying pain or pruritus is absent. Majority (>90%) of tumefaction occur upon head and neck whereas extremities and trunk are typically incriminated in almost <10% [3,4].
Histological Elucidation
On macroscopic examination, an occasionally ulcerated papule or nodule of beneath<2 centimetre magnitude is exemplified. Atypical fibroxanthoma manifests as a dermal neoplasm with specific features such as cellular and nuclear pleomorphism, atypical mitotic figures and a tumour architecture composed of spindle-shaped cells. The neoplasm can occasionally expand into the subcutaneous tissue. Multinucleated giant cells and solar elastosis are frequently discerned in atypical fibroxanthoma along with dissemination of a mixed inflammatory infiltrate within the tumour periphery. Atypical fibroxanthoma is predominantly a neoplasm confined to the dermis whereas spindle cell variant of squamous cell carcinoma is interconnected to superimposed epidermis and depicts foci of keratinization [4,5].
Morphologically, atypical fibroxanthoma represents a well-demarcated, nodular, exophytic tumefaction. The essentially dermal neoplasm lacks necrosis or an infiltrative pattern of growth wherein superimposed epidermis frequently demonstrates ulceration with the configuration of a collarette. On microscopy, a wellcircumscribed dermal nodule is delineated, usually situated upon cutaneous surfaces incriminated with solar-induced damage or solar elastosis. The tumefaction often abuts superimposed epidermis although occasionally a Granz zone of uninvolved dermis can be discerned. Spindle-shaped, spheroidal or epithelioid tumour cells demonstrating a haphazard or fascicular pattern are exhibited. Bizarre, multinucleated, pleomorphic cells are observed. Frequent mitosis and atypical mitotic figures are detected. The neoplasm, preponderantly centred upon the dermis, morphologically recapitulates undifferentiated pleomorphic sarcoma. However, extensive incrimination of dermis, subcutaneous tissue or deep-seated structures such as skeletal muscle or fascia is typically absent, features which aid in distinction of the neoplasm from pleomorphic dermal sarcoma [4,5]. Spindle cell variant of atypical fibroxanthoma is comprised of atypical monomorphic spindle-shaped cells articulating an intersecting, fascicular pattern. Atypical mitosis and frequent mitotic figures are enunciated. Morphological spectrum of atypical fibroxanthoma is comprised of several, diverse histological variants. Variants of atypical fibroxanthoma are contingent to diverse, specific histological features and are denominated as pigmented atypical fibroxanthoma depicting hemosiderin deposits instead of melanin pigment, granular cell atypical fibroxanthoma, sclerotic atypical fibroxanthoma, angiomatoid atypical fibroxanthoma, spindle cell non pleomorphic atypical fibroxanthoma, osteoid atypical fibroxanthoma, chondroid atypical fibroxanthoma, monoid atypical fibroxanthoma, colloidal atypical fibroxanthoma, osteoclast-like giant cell rich atypical fibroxanthoma and clear cell atypical fibroxanthoma (Figures 1-8) [4,5].
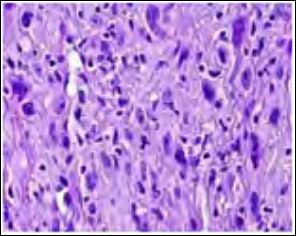
Figure 1: Atypical fibroxanthoma comprised of pleomorphic spindle- shaped cells with multinucleated cells, mitosis and atypical forms.
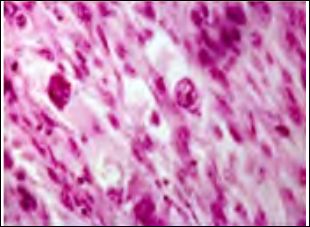
Figure 2: Atypical fibroxanthoma depicting spindle-shaped cell arranged in intersect- ing fascicles with pleomorphic nuclei and multinucleated giant cells.
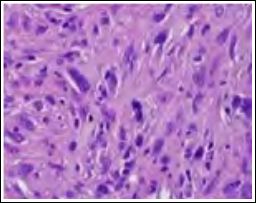
Figure 3: Atypical fibroxanthoma delineating interlacing bundles of spindle- shaped cells and nuclear pleomorphism with mitotic figures.
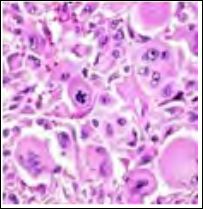
Figure 4: Atypical fibroxanthoma demonstrating spindle-shaped cellular configura- tions with multinucleated giant cells with mitotic and atypical figures.
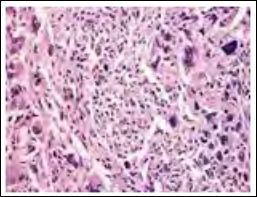
Figure 5: Atypical fibroxanthoma with spindle cells configuring whorls and fascicles, multinucleated giant cells and mitotic figures.
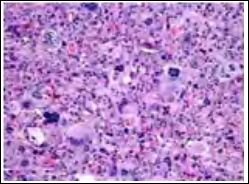
Figure 6: Atypical fibroxanthoma demonstrating spindle cell aggregates and bundles, cellular and nuclear pleomorphism with atypical mitosis and occasional giant cell.
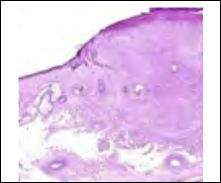
Figure 7: Atypical fibroxanthoma demonstrating nodules and fascicles of spindle – shaped cells depicting cellular and nuclear pleomorphism, prominent vascular ectasia and a superimposed squamous epithelium.
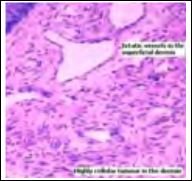
Figure 8: Atypical fibroxanthoma demonstrating patent vascular configurations with dermal aggregates of plump, spindle- shaped cells with moderate anisocytosis and pleomorphism with occasional mitosis.
Immune Histochemical Elucidation
Cogent immune histochemical reactions are imperative in segre- gating atypical fibroxanthoma from neoplasia recapitulating perti- nent, identical histological features such as spindle cell squamous cell carcinoma, malignant melanoma and undifferentiated pleomorphic sarcoma. Atypical fibroxanthoma lacks the delineation of a specif- ic immune reactive panel. Atypical fibroxanthoma demonstrates a non-specific immune reactivity to several stains such as CD10, p53, S100A6, vimentin and procollagen -1. Additionally, non-specific im- mune reactivity to smooth muscle actin (SMA), CD68 and CD99 is cogitated [5,6]. Thus, atypical fibroxanthoma is a diagnosis of ex- clusion. Atypical fibroxanthoma is immune non-reactive to human melanoma black (HMB-45) antigen, p40 (immune reactive in squa- mous cell carcinoma), desmin, pan- cytokeratin stains, CD31 besides a sparse immune reaction to S100 protein (immune reactive in malig- nant melanoma).
Immune reactivity to CD68, CD117, CD99, CD10, CD163, al- pha-1 antitrypsin, alpha1 antichymo-trypsin and focal immune reac- tion to Factor XIII a is exemplified. Besides, D2-40 is reactive in around 50% tumours, Calponin, desmin and smooth muscle actin (SMA) in nearly 30% neoplasia and CD31 in roughly 5% instances [5,6]. Typically, high molecular weight cytokeratin CK5/CK6 and p63 are immune non-reactive in atypical fibroxanthoma and immune reac- tive in spindle cell squamous cell carcinoma. S100 protein is non-re- active in atypical fibroxanthoma whereas a spindle cell malignant melanoma is immune reactive. Desmin is non-reactive in atypical fibroxanthoma whereas a pleomorphic leiomyosarcoma is immune reactive. CD10 is a non-specific marker which is immune reactive in atypical fibroxanthoma although several adjunctive neoplasia are also reactive. Vascular proliferation can be adequately discerned with cogent vascular markers such as ETS related gene (ERG) and CD31 which are immune non-reactive in atypical fibroxanthoma although reactive in spindle cell angiosarcoma [5,6].
Poorly differentiated sarcomatoid carcinomas can demonstrate a decimation of cytokeratin expression, thus employment of a variety of cytokeratin immune stains is advantageous. On ultrastructural ex- amination, myofibroblasts, fibroblasts and primitive mesenchymal cells are discerned. The neoplasm demonstrates a diploid chromo- somal pattern on cytogenetic analysis [5,6].
Differential Diagnosis
Atypical fibroxanthoma requires a separation from specific, atypical spindle cell neoplasia which recapitulates the histological features and abut superimposed epidermis. Aforesaid congregation is referred to as “SLAM” and indicate pertinent lesions such as spindle cell squamous cell carcinoma, leiomyosarcoma, atypical fibroxanthoma and spindle cell malignant melanoma [6]. Atypical fibroxanthoma requires a segregation from neoplasia such as basal cell carcinoma, Merkel cell carcinoma, amelanotic malignant melanoma, atypical or pseudo-sarcomatous dermatofibroma, pleomorphic dermal sarcoma and metastasis from intrinsic, visceral malignancies [6,7].
Spindle cell squamous cell carcinoma displays distinct foci of squamous differentiation and cytokeratin reactivity. Spindle cell squamous cell carcinoma is accompanied by tumour extension into deep-seated tissues besides immune reactivity to p63 and high molecular weight cytokeratin. Spindle cell or desmoplastic variant of malignant melanoma can be accompanied by an intra-epidermal proliferation of atypical melanocytic cells and is immune reactive to S100 protein whereas atypical fibroxanthoma is entirely immune non-reactive [6,7]. In contrast to atypical fibroxanthoma, pleomorphic dermal sarcoma is an expansive neoplasm with enhanced possibility of localized reoccurrence and metastasis. Pleomorphic dermal sarcoma is poorly-defined, infiltrative malignant neoplasm which invades deep-seated subcutaneous tissue and demonstrates tumour necrosis besides lympho-vascular invasion in addition to an estimated incidence of localized tumour relapse in 28% instances and distant metastasis in nearly 10% subjects. Atypical fibroxanthoma and pleomorphic dermal sarcoma are genetically interlinked and potentially represent a common category of tumour [6,7].
Extensive tumour implication of subcutaneous tissue or invasion of deep-seated anatomic structures such as skeletal muscle or fascia is indicative of pleomorphic dermal sarcoma [6,7]. Superficial cutaneous tissue specimen of a pleomorphic dermal sarcoma demonstrates the extraneous aspect of the neoplasm which can simulate and be misinterpreted as an atypical fibroxanthoma. However, cogent surgical excision of pleomorphic dermal sarcoma delineates an enlarged, deeply infiltrative lesion accompanied with an inferior prognosis [6,7].
Angiosarcoma, especially the spindle cell variant, displays prominent vascular spaces or red cell impaction and immune reactivity to specific vascular markers. Atypical fibrous histiocytoma also mandates a distinction from atypical fibroxanthoma. The neoplasm is composed of pleomorphic, plump, spindle-shaped or polyhedral cells with enlarged, hyperchromatic or bizarre nuclei, intermixed with multinucleated giant cells, around 5 to 15 mitotic figures per 10 high power fields and foci of necrosis. Cellular pleomorphism can be significant with the enunciation of foamy or hemosiderin-laden macrophages and multinucleated “monster” cells with bizarre nuclei. Aforesaid microscopic features are absent in atypical fibroxanthoma. Leiomyosarcoma, particularly the pleomorphic subtype, demonstrates an abundantly fascicular pattern of tumour evolution with immune reactivity to desmin [7].
Investigative Assay
Dermoscopic evaluation of atypical fibroxanthoma reveals the presence of polymorphic vessels as delineated with linear, dotted, hairpin, arborescent and/or extremely tortuous blood vessels radiating to centroidal zone along with intermingled white areas. A cogent cutaneous tissue specimen is a gold standard for diagnosis of atypical fibroxanthoma as the exceptional disorder clinically recapitulates basal cell carcinoma, squamous cell carcinoma, and Merkel cell carcinoma or amelanotic malignant melanoma. Pertinent immune reactivity is commonly performed for ascertainment of the disorder [8,9]. Metastatic or inaccessible, subungual atypical fibroxanthoma can be adequately delineated with a magnetic resonance imaging (MRI) which demonstrates intermediate T1 and T2 weighted signal intensity, in contrast to enhanced signal intensity demonstrable in squamous cell carcinoma and malignant melanoma [8].
Therapeutic Options
Atypical fibroxanthoma is infrequently metastatic and reappears in an estimated 6% to 10% instances. Tumour metastasis occurs in immune-compromised individuals, with enhanced tumour depth, presence of tumour necrosis besides vascular and perineural tumour invasion [8]. Tumour metastasis is frequently encountered in the parotid gland, lymph nodes and subcutaneous tissue. Metastases are typically discerned 12 months to 24 months following initial discernment. A competent surgical eradication with a broad perimeter of normal, uninvolved tissue is adopted. Preferable treatment option for managing an atypical fibroxanthoma is a comprehensive surgical extermination. Previously, tumour resection with a one centimetre tumour free perimeter was adopted. However, Moh’s micrographic surgery with a regular postoperative monitoring is currently contemplated as an efficacious and standardized treatment methodology accompanied by proportionate reoccurrence of up to 6.9% [7,8]. Following a comprehensive surgical excision, prognostic outcomes are superior. Candidates unfit for a surgical intervention can be managed with electronic brachytherapy, a technique considered to be effectual for treating atypical fibroxanthoma and beneficial with adoption of tumour debulking prior to therapy. Electrodessication and curettage is a modality which is appropriate for treating gradually evolving lesions beneath < 1 centimetre magnitude. Circumvention of atypical fibroxanthoma is pertinent to protection from ultraviolet radiation. An annual, comprehensive examination of cutaneous surfaces is required for preliminary discernment of the lesion. Thus, prevention of exposure to sunlight and ultraviolet light with regular physical examination is advantageous. Tumour reoccurrence can emerge although distant metastasis is exceptional [8-16].
References
- Kolb L, Schmieder GJ (2019) Atypical fibroxanthoma Stat Pearls Publishing.
- Merati M, Scott J, Honda, Bordeaux J (2018) Atypical fibroxantho- ma invading parietal bone. Dermatol Surg 44: 1644-1646.
- Klebanov N, Hoang MP (2018) Pleomorphic dermal sarcoma of the scalp cureus. 10: e2979.
- Winchester D, Lehman JJ, Tiffany T, Nicolette C, Thomas H, et al. (2018) Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol 79: 853-859.
- Lindsey NF, Trisha K, Jesse ML (2018) Atypical fibroxanthoma aris- ing in a burn scar treated with Moh’s micrographic Derma- tol Surg 44: 1229-1231.
- Moscarella E, Piana S, Specchio F, Kyrgidis A, Nazzaro G, et al. (2018) Dermoscopy features of atypical fibroxanthoma: A multi- centre study of the international dermoscopy society. Australas J Dermatol 59: 309-314.
- Nonaka D, Bishop PW (2014) Sarcoma-like tumour of the head and neck skin. Am J Surg Pathol 38: 956-965.
- Wang WL, Torres-Cabala C, Curry JL, Lvan D, McLemore M, et (2015) Metastatic atypical fibroxanthoma: A series of 11 cases including with minimal and no subcutaneous involvement. Am J Dermatopathol 37: 455-461.
- Figure 1 Courtesy: Pathology
- Figure 2 Courtesy: Medscape com.
- Figure 3 Courtesy: com.
- Figure 4 Courtesy: com.
- Figure 5 Courtesy: Libre
- Figure 6 Courtesy: Dermatology
- Figure 7 Courtesy: Research
- Figure 8 Courtesy: Pinterest.
Citation: Bajaj A (2020) The Smattery Metamorphoses- Atypical Fibroxanthoma. J Cell Mol Onco 2: 002.
Copyright: © 2020 Bajaj A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.