*Corresponding Author:
Guo-Hui Xu,
Department of Radiology, Si- chuan Cancer Hospital, No 55, Lane 4, Ren Min Road (South), Cheng- du 610041, Sichuan Province, China
Tel: +86 13708010123
E-mail: alexey.trenkin@gmail.com
Abstract
Purpose: The aim of this study was to find the optimal scan time of c-erbB2 antisense probe labeled with superparamagnetic iron oxide nanoparticles used for magnetic resonance imaging of BALB/c mice.
Procedures: The establishment of 30 SK-Br-3 tumor-bearing BAL- B/c mice was finished before the study. Each tumor-bearing BALB/c mouse underwent MR scan before injection, then the tumor tissues of 5 mice were immediately taken and fixed by 10% formalin and paraffin-embedded sections, underwent HE and Prussian blue staining, then were observed under microscope. The other tumor-bearing BALB/c mouse was injected with antisense probe (the concentration is 42.0 μg Fe/Kg), then underwent the MR scan in different time points (60 min,180 min,360 min,720 min and 1440 min). After every time point, the tumor tissues of 5 mice were immediately taken and fixed by 10% formalin and paraffin-embedded sections, underwent HE and Prussian blue staining, then were observed under microscope. The signal strength of tumor and adjacent muscles was measured, and the signal intensity changes were compared between different time points.
Results: The rate of establishment of SK-Br-3 tumor-bearing BAL- B/c mice was 100%. The signal intensity of 30 mice at different time points before and after injection were compared and the signal intensity changes of the tumor issue at 360 min after injection were most obvious. The pathological examination revealed that tumor issue structural disorder, a large number of special-shaped humor cell existed and arranged in the cancer nest shape. Before injection there were no blue iron particles scattered in the tumor issue, and a large number of punctuate blue iron particles scattered in the tumor issue in each time point after injection. The distribution of iron particles was most dense at 360 min after injection.
Conclusion: The optimal scan time of c-erbB2 antisense probe labeled with superparamagnetic iron oxide nanoparticles used for Magnetic resonance imaging of BALB/c mice is 360 min.
Keywords
Antisense probe; Live animal; Magnetic resonance imaging; Scan time; Superparamagnetic iron oxide
Introduction
Due to the lack of clinical method to early diagnosis, warning of metastasis, predict of the efficacy for malignancy, cancer has become a major cause of human death. Molecular imaging, which was developed in the end of the last century, has become a central issue in medical imaging research. By the preparation of the associated molecular probe, molecular imaging can make an early diagnosis of cancer in noninvasive and real-time way, and develop a dynamic, visual way for the warning of metastasis, predicting of the efficacy for treating malignancy [1].
Superparamagnetic Iron Oxide (SPIO), the frequently used para magnetism material, has magnetism aquare. SPIO can be arrangement freely along magnetic field which cause tremendous microcosmic phase difference in periphery proton, which resulting in the protonic T2 desphase relaxation quickening, the signal of tissue’s T2 weight imaging reducing distinctly and signal difference between tissues increasing distinctly. Its magnetism disappears rapidly when the magnetic field is taken out. Therefore, it is a super para magnetism. In addition, SPIO, which has lower toxin and less side effects, is an ideal and safety contrast medium and molecular carrier, thus extensively used in tracing vivo stem cells (such as nerve stem cell, embry stem cell and mesenchymal tissue stem cell) [2,3]. C-erbB2 oncogene is homologous gene of neu gene in human being, which amplify and expression only in malignant tumors [4-6]. Moreover, cerbB2 oncogene have close correlation with pathologic grade, lymph node metastasis and clinic stage of tumor, and frequently use as a major index of clinic chemical treatment plan and prognosis judgments [7-10]. Therefore, this gene provides a good situs for targeting diagnosis, once the amplification and over-expression of c-erbB2 oncogene can cause cellular mRNA increase and provide more conjunctive targeting situs. Because eugenic tumor cell membranes, have many transferrin receptors which can guide SPIO-labeled antisense probe into tumor cells, hence, the para magnetism materials increase [11-13]. After magnetic resonance imaging scanning, will get the imaging with clarity anatomic structure, high noise-signal ratio and well contrast is obtained, so oncogene specificity expression may be displayed from imaging.
In previous studies, reference to the antisense gene theory of Molecular Biology, we chose c-erbB2 oncogene as targeted gene and prepared the Antisense Oligodeoxynucleotide (ASODN) of complementary cerbB2 oncongene in gene synthesis technique and labeled Superparamagnetic Iron Oxide (SPIO) nanometer using chemical cross linking, and we prepared the c-erbB2 antisense probe labeled with SPIO nanoparticles successfully [14,15]. The basic group can complementarity and specifically combine with mRNA of oncogene when inducted in body, so it can distribution special in tumor tissue which have enlarge and over-expressive oncegene, and the location of tumor tissue can be demonstrated when magnetic resonance imaging. We also studied the pharmacokinetic and the distribution of c-erbB2 antisense probe labeled with superparamagnetic iron oxide nanoparticles in mice major organs [16]. Based on this, our study used the c-erbB2 antisense probe for the MR scan of SK-Br-3 tumor-bearing BALB/c mice, observed the signal intensity changes at different time points, expected to find the optimal scan time.
Materials and Methods
Materials
The c-erbB2 antisense probe labeled with SPIO nanoparticles which made by ourselves (Chinese patent no.: ZL 200710092512.5) [14,15]. Cancer cell Lines of SK-Br-3 which has high expression of c-erbB2 oncogene. Thirty BALB/c mice (weight 10 ~ 15 g, male or female) were obtained from the Chongqing Experimental Animal Center. The equipment used included a 3.0-T MR scanner with a wrist surface coil (GE Healthcare, Waukesha, WI, USA), which is widely used clinically.
Methods
The establishment of SK-Br-3 tumor-bearing BALB/c mice model
Referring to previous method, the SK-Br-3 Cancer cell Lines were cultivated with DMEM (low glucose) medium which containing 10% FBS in CO2-cubator, the cultivation temperature was 37 degrees and the volume fraction of carbon dioxide was 0.05, then took the SK-Br-3 Cancer cell in logarithmic growth phase, dissolve the cell with 0.25% trypsin, collected it to 5mL tube and had 1200r / min × 5 min centrifugation, made the cell suspension with culture solution which had no serum and antibiotic, then diluted it to 5×107/mL [17]. Thirty BALB/c mice, had a hypodermic in left forelimb with the cell suspension about 0.2ml (the number of cells about 1×107). 15-20 days after hypodermic, tumor diameter greater than 0.5cm, which prompt the success of model establishment, then we can use the model to MR scan.
The c-erbB2 antisense probe labeled with superparamagnetic iron oxide nanoparticles used for MR of BALB/c mice
Selection of SPIO dose: With reference to the literature and the preliminary results of preliminary experiments, we decided the dose of c-erbB2 antisense probe labeled with superparamagnetic iron oxide nanoparticles was 42.0μg Fe/kg0 [18,19].
Selection of magnetic resonance imaging scanning time points: MR scanning time has no uniform requirement in the other literatures, between each other, both have a huge difference. The earlier experimental results showed that the concentration of antisense probe in spleen and liver reached its peak in 180 min after injection, and peaked in 60 min after injection in kidney [16]. But the concentration of antisense probe in muscle tissue increased gradually, the distribution in 360 min after injection was different from the others. Based on the above, and reference the other literatures, we finally determined the MR scanning time points were before injection and 60 min, 180 min, 360 min, 720 min, 1440 min after injection (30 mice were randomly into six groups, 5 mice in each time point) [1,20-24].
The sequences and parameters of magnetic resonance imaging scan: The equipment used included a 3.0-T MR scanner with a wrist surface coil (GE Healthcare, Waukesha, WI, USA), according to the literatures, the sequence and parameters of MR scan were: (1) T1WI FSE: TE/TR MinFull/600, echo train length 3, bandwidth 31.25, freq 320, phase 192, NEX 2, (2) T2WI FSE: TE/TR 85/600, echo train length 18, bandwidth 41.67, freq 320, phase 256, NEX 2, (3) T2*GRE: TE/TR MinFull/300, flip Angle 20, bandwidth 20.83, freq 320, phase 224, NEX 2 [22,25,26]. Every sequence had the coronal and axial position scan, the slice thickness was 4mm, layer distance was 0.5mm, FOV was 14cm.
Magnetic resonance imaging scan: 30 mice were anesthetized by 2% Phenobarbital (30mg/kg), and inhale appropriate isoflurane to inhibit the breathing of mice which could reduce the image artifacts. Then we made the mice lay face upwards on the foam board, put it in the wrist surface coil, head in first. Each tumor-bearing BALB/c mouse underwent MR scan before injection, then the tumor tissues of 5 mice were immediately taken and fixed by 10% formalin and paraffin-embedded sections, underwent HE and Prussian blue staining, then were observed under microscope. After the MR scanning without injection, the other tumor-bearing BALB/c mouse was injected with antisense probe (the concentration is 42.0 μg Fe/Kg) in tail vein, then we had the MR scan with the same sequences, parameters and the parts of mice in the certain time points.
Analysis of the MR images: Measure the signal strength of tumor and adjacent muscles (blank control group), choice the area of interest in the biggest slice of the tumor, the range of pixels at least 50.
Pathological Examination
After the MR scanning in one-time point, immediately took the tumor tissue of 5 mice, make the tissue fixed by 10% formalin and paraffin-embedded sections, HE and Prussian blue staining, observed under microscope.
Statistical Analysis
The date is reported as means ± Standard Deviation (SD), the comparison of each time points use the analysis of variance for repeated measurements in single factor, P<0.05 was accepted as indicating a significant difference.
Results
The establishment of BALB/c mice model
30 mice were all alive, and all the left forelimbs of the mice had grown the tumor without ulceration, the rate of establishment of SK- Br-3 tumor-bearing BALB/c mice was 100%. The MR scan showed tumor tissue had equal signal in sequences T1WI, slightly high signal in sequences T2WI, there was no significant necrosis signal, so we can use the model in further experiments (Figure 1).

Figure 1: The model of tumor-burdened nude mice. A: 15d after inoculation. B: 20d after inoculation
The antisense probe labeled with SPIO used for MR of BALB/c mice
The changes of signal strength in sequence T1WI FSE: From the ta- ble 1 and figure 2, we can find, the signal strength of tumor tissue sig- nificantly increased between 0-60 min after injection, and slowly in- creasing between 60-360 min, then peaked in 360 min which time we can observed the changes in naked eye, after that the signal strength began to decline. The differences in signal strength between the group before injection and groups 60,180,360 min after injection were sta- tistically significant (P<0.01), moreover the statistical significance of group 360 min after injection was more notable. The signal strength of tumor tissue recovered to the normal level 720-1440 min after injec- tion (P>0.05). The signal strength of adjacent muscular tissue had an increase in a small range, which had the statistical difference (P<0.05), but it quickly reduced to the level same as before injection. The signal tendency of tumor tissue was different from the adjacent muscular tissue.
The changes of signal strength in sequence T2WI FSE: From the ta- ble 2 and figure 3, the signal strength of tumor tissue significantly de- creased between 0-60 min after injection, and slowly reduced between 60-360 min, then reached bottom value in 360 min, we can have ob- served it in naked eye, after that the signal strength began to rebound. The differences in signal strength between the group before injection and groups 60,180,360,720 min after injection were statistically sig- nificant (P<0.05), and the signal strength of group 360 min was the lowest. The signal strength of tumor tissue recovered to the normal level 1440 min after injection (P>0.05). The signal strength of adjacent muscular tissue had a small decrease in 60-360 min after injection, but the decrease wasn’t obvious as the tumor tissue, furthermore the signal strength of 720 min group didn’t have the statistical difference contrast with the group before injection (P>0.05). So the signal ten- dency of tumor tissue was different from the adjacent muscular tissue in sequence T2WI FSE too.
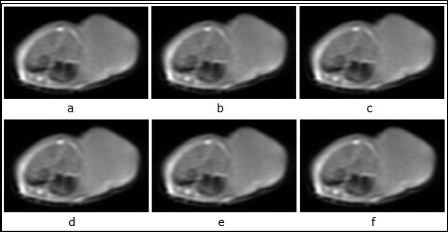
Figure 2: The signal change series of tumor tissue of each time point in T1WI FSE sequence after injection of antisense probe.
a: before injection. b: 60 min after injection. c: 180 min after injection. d: 360 min after injection. e: 720 min after injection. f: 1440 min after injection.
The changes of signal strength in sequence T2*GRE: The results are shown in table 3 and figure 4. The signal tendency of tumor tissue in sequence T2*GRE was basically same as the sequence T2WI FSE. The signal strength of adjacent muscular tissue had a small decrease in 60 min after injection, then turn to the normal level in 360 min after injection.
Pathological Examination
HE staining revealed that tumor issue structural disorder, there are a large number of special-shaped humor cell exist, arranged in the cancer nest shape, the tumor cell has scarce cytosolic, and had the mitotic figure 5. Each specimen basically had the same performance. With Prussian blue staining, there were no blue iron particles scattered in the tumor issue which had no injection, and a large number of punctuate blue iron particles scattered in the tumor issue in each time point after injection. And the distribution of iron particles is most densely in 360 min after injection (Figure 6).
Discussion
The early diagnosis is the most important purpose of Molecular Imaging Research, magnetic resonance imaging scanning has many advantages such as non-invasive, non-radiation, high-resolution multi-parameter and multi-faceted. With a variety of related probes have successful developed, MR Molecular Imaging will have a good application prospect in early diagnosis of the cancer [1,21,22,27-29].
Using SPIO in magnetic resonance imaging scanning alone, because of the phagocytosis of Kupffer cells, SPIO will gather in the liver, spleen, lymph nodes and other tissues, so there will be no specificity for tumor tissues [1]. Because of the biocompatibility of SPIO, our study used it be the part for imaging, and connected it with oligonucleotide which opposite to the c-erbB2 gene sequences in covalent bond, so we got the magnetic antisense probe, furthermore we chose the SK-Br-3 tumor cells which has the high expression of c-erbB2 oncogene to establish the tumor-bearing mice model [14,15]. According antisense gene theory, the oligonucleotide in antisense probe can make the targeted binding with cancer gene, and achieve specific enrichment at the tumor tissue, so the antisense probe can stay longer. With the analysis of signal intensity changes in every sequence, we can find that the signal intensity in tumor tissue has changed in 60 min after injection with antisense probe, and had a significant difference compared with the signal strength before injection (P<0.05). The following data revealed, the signal strength of tumor tissue peaked in 360 min after injection: With the signal of T2 sequence as an example, we can find that the signal intensity changes of the tumor tissue in 360 min after injection had a apparent decrease, and clearly displayed on the MR images, then it slowly recovered to normal level, the signal strength of adjacent muscle tissue also changed after injection, but the changes was not obvious, and we can’t find the vary in naked eye. With Prussian blue staining, a large number of punctuate blue iron particles scattered in the tumor issue, the distribution of iron particles is most densely in 360 min after injection. The signal intensity changes and pathological examination results could demonstrate of each other.

Table 1: The signal strength of each time points in T1WI FSE sequence (n=5).
*P<0.05, vs. before injection.

Table 2: The signal strength of each time points in T2WI FSE sequence (n=5).
*P<0.05, **P<0.001, vs. before injection.

Figure 3: The signal change series of tumor tissue of each time point in T2WI FSE sequence after injection of antisense probe. a: before injection. b: 60 min after injection. c: 180 min after injection. d: 360 min after injection. e:720 min after injection. f:1440 min after injection.

Table 3: The signal strength of each time points in T2*GRE sequence (n=5).
*P<0.05, **P<0.001, vs. before injection.

Figure 4: The signal change series of tumor tissue of each time point in T2*GRE sequence after injection of antisense probe.
a: before injection. b: 60 min after injection. c: 180 min after injection. d: 360 min after injection. e: 720 min after injection. f: 1440 min after injection.
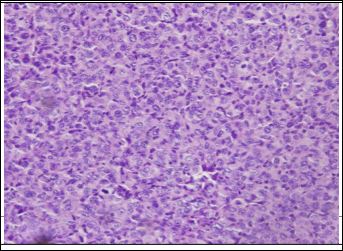
Figure 5: HE staining of tumor tissue 360 min after injection of antisense probe. (×200).
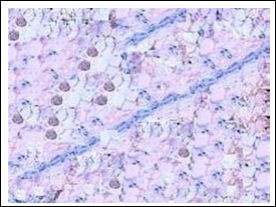
Figure 6: Staining with Prussian blue of tumor tissue 360 min after injection of antisense probe (×400).
From the above results, we speculated that the oligonucleotide of antisense probe makes the targeted binding with the c-erbB2 oncogene in tumor tissues, which made the antisense probe stay in tumor tissue longer and obvious. Of course, whether the antisense probe can make the antisense effect to c-erbB2 oncogene, and make the expression of oncogene was inhibited or blocked, we will take place in subsequent studies.
Conclusion
Our experimental results revealed, after the injection of antisense probe, the signal intensity of tumor tissue was significantly altered, and the signal intensity changes of the tumor issue in 360 min after injection were most obvious which we can observed it in naked eye. In summary, the optimal scan time of c-erbB2 antisense probe labeled with superparamagnetic iron oxide nanoparticles used for magnetic resonance imaging of BALB/c mice is 360 min.
Define the optimal scan time of c-erbB2 antisense probe labeled with superparamagnetic iron oxide nanoparticles used for magnetic resonance imaging of BALB/c mice, will be useful for the future study such as the optimal scan dose, the optimal scan sequence of c-erbB2 antisense probe labeled with superparamagnetic iron oxide nanoparticles used for Magnetic resonance imaging of BALB/c mice. And provide the foundation for the clinical diagnosis and treatment of c-erbB2 antisense probe labeled with superparamagnetic iron oxide nanoparticles.
Due to the limitations of laboratory equipment and conditions, the time interval between every point was relatively long, meanwhile might have a more accurate scan time, it also requires further experiments to be improved.
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Funding
This study was funded by National Natural Science Foundation of China (No. 30940021), the Natural Science Foundation of Chongqing City (CSTC 2008BB5209), and the Medicine Scientific Research Project of Chongqing Health Bureau (No. 062025).
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Qiao RR, Zeng JF, Jia QJ, Du J, Shen L, et al. (2012) Magnetic iron oxide nanoparticle--an important cornerstone of MR molecular imaging of tumors. Acta Physico - Chimica Sinica 28: 993-1011.
- Daldrup-Link HE, Rudelius M, Piontek G, Metz S, Bräuer R, et al. (2005) Migration of iron oxide-labeled human hematopoietic progenitor cells in a mouse model: in vivo monitoring with 1.5-T MR imaging equipment. Radiology 231: 197-205.
- Thrall JH (2004) Nanotechnology and Radiology 230: 315-318.
- Ming W, Zuo B, Shaolin L (2007) Tumor antisense gene imaging in Molecular Journal of Chongqing Medical University. 32: 554-557.
- Cherry SR (2004) In vivo molecular and genomic imaging: new challenges for imaging physics. Phys Med Biol 49: 13-48.
- Avitian TB (2000) In vivo antisense Q J Nucl Med 44: 236-255.
- Chen QQ, Chen XY, Jiang YY, Liu J (2005) Identification of novel nuclear localization signal within the ErbB-2 protein. Cell Res 15: 504-510.
- García I1, del Casar JM, Corte MD, Allende MT, García-Muñiz JL, et al. (2003) Epidermal growth factor receptor and c-erbB-2 contents in unresectable (UICC R1 or R2) gastric cancer. Int J biol Markers 18: 200-206.
- Soh LT, Heng D, Lee IW, Ho TH, Hui KM (2002) The relevance of oncogenes as prognostic markers in cervical Int J Gynecol Cancer 12: 465-474.
- Santin AD, Zhan F, Bellone S, Palmieri M, Cane S, et al. (2004) Gene expression profiles in primary ovarian serous papillary tumors and normal ovarian epithelium: identification of candidate molecular markers for ovarian cancer diagnosis and therapy. Int J Cancer 112: 14-25.
- Sahin AA (2000) Biologic and clinical significance of HER-2/neu (cerbB-2) in breast cancer. Adv Anat Pathol 7: 158-166.
- Burstein HJ, Kuter I, Campos SM, Gelman RS, Tribou L, et (2001) Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol 19: 2722-2730.
- Nagashima T, Shimodaira H, Ide K, Nakakuki T, Tani Y, et (2007) Quantitative transcriptional control of ErbB receptor signaling undergoes graded to biphasic response for cell differentiation. J Biol Chem 282: 4045-4056.
- Wen M, Li B, Bai W, Li S, Yang X (2008) Application of atomic force micros- copy in morphological observation of antisense probe labeled with magne- Mol Vis 14: 114-117.
- Wen M, Li B, Ouyang Y, Luo Y, Li S (2009) Preparation and quality test of su- perparamagnetic iron oxide labeled antisense oligodeoxynucleotide probe: a preliminary study. Ann Biomed Eng 37: 1240-1250.
- Wen Z, Liu H, He H, Tan S, Wen M, et (2013) Distribution of c-erbB2 anti- sense probe labeled with superparamagnetic iron oxide nanoparticles in the major organs of mice on MR imaging. Chin Pharm Abstr 46: 16-20.
- Xie J, Li S, Zhang Y, et al. (2005) The experimental study of c-erbB2 anti- sense oligodeoxynucleotide labeled with 99m technetium in SPECT imaging for breast cancer. National Medical Journal of China 85: 2940-2942.
- Thorek DL, Tsourkas A (2008) Size, charge and concentration dependent uptake of iron oxide particles by non-phagocytic Biomaterials 29: 3583- 3590.
- Boutry S, Brunin S, Mahieu I, Laurent S, Vander Elst L, et (2008) Magnetic labeling of non-phagocytic adherent cells with iron oxide nanoparticles: a comprehensive study. Contrast Media Mol Imaging 3: 223-232.
- Ming W, Lin S, Wei B, Shao-lin S, Bi-bo L (2007) Preparation of superpara- magnetic iron oxide nanoparticles and its acute toxicity to Acad J Sec- ond Mil Med Univ 28: 1104-1108.
- Shi X, Wang SH, Swanson SD, Ge S, Cao Z, et al. (2008) Dendrimer-func- tionalized shell-crosslinked iron oxide nanoparticles for in-vivo magnetic res- onance imaging of tumors. Adv Mater 20: 1671-1678.
- Lu J, Wang F, Jing ZY, Zhong DR (2009) Targeted Magnetic Nanoparticles Used as Probe for Magnetic Resonance Molecular Imaging of Zhong- guo Yi Xue Ke Xue Yuan Xue Bao 31: 124-128.
- Lin MM, Kim HH, Kim H, Dobson J, Kim DK (2010) Surface activation and targeting strategies of superparamagnetic iron oxide nanoparticles in can- cer-oriented diagnosis and therapy. Nanomedicine 5: 109-133.
- Schleich N, Po C, Jacobs D, Ucakar B, Gallez B, et (2014) Comparison of active, passive and magnetic targeting to tumors of multifunctional paclitaxel/ SPIO-loaded nanoparticles for tumor imaging and therapy. J Control Release 194: 82-91.
- Li X, Du X, Huo T, Liu X, Zhang S, et al. (2009) Specific targeting of breast tumor by octreotide-conjugated ultrasmall superparamagnetic iron oxide par- ticles using a clinical 0-Tesla magnetic resonance scanner. Acta Radiol 50: 583-594.
- Zhu W, Li X, Tang Z, Zhu S, Qi J, et (2007) Superparamagnetic Iron Oxide Labeling of Neural Stem Cells and 4.7T MRI Tracking in vivo and in vitro. J Huazhong Univ Sci Technolog Med Sci 27: 107-110.
- Chen K, Chen X (2010) Design and development of molecular imaging Curr Top Med Chem 10: 1227-1236.
- Padmanabhan P, Goggi J, Bejot R, Bhakoo KK (2011) Molecular targeting of breast cancer: imaging and Curr Pharm Biotechnol 12: 528-538.
- Seaman ME, Contino G, Bardeesy N, Kelly KA (2010) Molecular imaging agents: impact on diagnosis and therapeutics in oncology. Expert Rev Mol Med 12: 20.
Citation: Wen Z-P, He H, Wen M, Zhou P, Qing H-M, et al. (2018) The Investigation on the Optimal Scan Time of c-erbB2 Antisense Probe Labeled with Superparamagnetic Iron Oxide Nanoparticles Used for MR of BALB/c Mice. J Nanosci Nanomed Nanobio 2: 003.
Copyright: © 2018 Wen Z-P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.


