*Corresponding Author:
Fuchs O,
Department of Genomics, Institute of Hematology and Blood Transfusion, U Nemocnice 1, 12820 Prague 2, Czech Republic
Tel: +420221977512
E-mail: Ota.Fuchs@uhkt.cz
Abstract
Recent clinical and molecular studies of Myelodysplastic Syndrome (MDS) showed the contribution of abnormal activation of innate immune signals and associated inflammation to the pathogenesis of MDS. The presence of abnormal levels of cytokines, chemokines and growth factors (tumor necrosis factor alpha /TNF-α/, interferon gamma /IFN-γ/, transforming growth factor beta, myeloid growth factors /G-CSF and GM-CSF/, interleu- kin (IL)-6, IL-8) in the peripheral blood and in bone marrow of MDS patients has been described. These levels depend on analyzed MDS subtypes. Toll-Like Receptors (TLRs) and multiple downstream signaling mediators are overexpressed in MDS. NF-κB transcription factors are activated in response to inflammatory cytokines, pathogenic antigens, oxidative stress, DNA damage and the activation of pattern recognition receptors. Mesenchymal Stem Cells (MSCs) are primitive, non-hematopoietic stem cells that give rise to all of the various types of stromal cells that form bone marrow microenvironment (niche). The immunosuppressive capacity of MSCs is decreased in cells from low risk MDS. MDS-derived MSCs and bone marrow stromal cells are determinants of the fate of hematopoietic progenitors and have an important role in pathogenesis of MDS. Myeloid-Derived Suppressor Cells (MDSCs) are inflammatory and immunosuppressive effectors localized to the bone marrow that express the immune receptor CD33. MDS patients have increased numbers of MDSCs and they induce defects in myeloid and erythroid differentiation. Although hematopoietic cell transplantation can be curative, additional therapies are needed. Investigating CD33-targeted therapies in MDS and Chronic Myelomonocytic Leukemia (CMML) patients is justified by high frequency of CD33 expression. Blockade of immune checkpoints (programmed death-1 and its two ligands and cytotoxic T-lymphocyte associated antigen 4) can be a potential therapy in MDS and CMML patients. Bispecific Killer Cell Engager (BIKE) targeting CD16 expressed on effector natural killer cells and CD33 is able to facilitate elimination CD33+ MDS targets and immunosuppressive MDSC targets.
Keywords
Immunotherapies; Innate immune signaling; MDS; Microenvironment; NF-κB; Toll-like receptors
Introduction
Myelodysplastic Syndromes (MDS) are a heterogeneous group of clonal Hematopoietic Stem Cell (HSC) disorders characterized by ineffective hematopoiesis, peripheral cytopenias, frequent karyotypic abnormalities and risk of transformation to Acute Myeloid Leukemia (AML) [1-10]. The first MDS classification, the French-American-British (FAB) classification, published 33 years ago allowed scientific research of this disease [11]. FAB group system was modified and further defined to recognize and classify distinct sub-categories of MDS based on genetic features.
Chronic Myelomonocytic Leukemia (CMML) has long been recognized as a distinct clinico-pathological entity with features of both myelodysplastic and myeloproliferative syndromes resulting in different clinical presentations [12,13]. FAB group system classified CMML as part of MDS, given the morphologic evidence of dysplastic hematopoiesis. The FAB Group later proposed a reclassification of CMML patients into two subtypes based on White Blood Cell (WBC) count at diagnosis [14]. Patients with WBC counts of < 13x109/L were considered to have myelodysplastic CMML (MD-CMML) and those with > 13x109/L were considered to have myeloproliferative CMML (MPO-CMML). However, the two groups have overlapping features. Voglova et al., [15] analyzed 69 patients with CMML, 31 (45%) classified as MD-CMML and 38 (55%) classified as MP-CMML. Cytogenetic abnormalities were more frequent among MP-CMML patients. The median Overall Survival (OS) was significantly longer in the MD-CMML patients than in the MP-CMML group (30 vs. 16 months, respectively; p value < 0.01) and there was no significant difference in leukemic transformation. Over the course of disease, WBC count in 24 MD-CMML patients increased to more than 13x109/L. Therefore, MD-CMML and MP-CMML should be considered as different stages of the same disease. World Health Organization (WHO) in 2002 recognized CMML as a distinct entity and moved it to a new category called MDS/MPD [16]. WHO classification differentiates CMML-1 and CMML-2 according to blast procentages and more recently also CMML-0 with less than 5% medullary blasts [17].
Both, WHO classification and criteria for MDS are shown in Table 1 [18]. Using the International Prognostic Scoring System (IPSS), MDS is classified into low, intermediate-1, intermediate-2 and high-risk for progression towards AML [19]. Despite increasing insight into the tumor biology of MDS, the etiology of these syndromes remains undetermined. However, there is increasing evidence that cytopenia in MDS may, at least in part, be due to lymphocyte-mediated myelosupression suggesting that dysregulated immune mechanisms may be involved in the pathogenesis of MDS [20]. This hypothesis is supported by frequent association of MDS and CMML with clinical manifestations of Autoimmune Disorders (AD) and inflammatory response of the immune system [12,20,21-53]. Epidemiologic studies demonstrated that AD patients (suffering from rheumatoid arthritis, Sjӧgren syndrome, lupus erythematosus, seronegative arthritis, panarteritis nodosa, autoimmune hemolysis and pernicious anemia) have a higher risk to develop MDS or AML compared to general population [45].
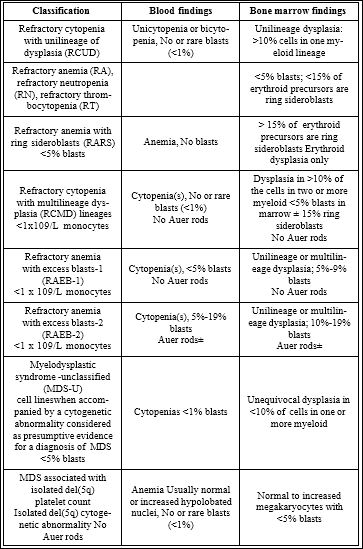
Table 1: World Health Organization MDS Classification and Criteria (2008).
Adapted according Nybakken and Bagg [18]
The Immune System Defects in Myelodysplastic Syndromes
Aberrant immunity, including abnormal immune cells and molecules, contributes to the development of MDS. Various immune molecules, including Interferon-γ (IFN-γ), Tumour Necrosis Factor-α (TNF-α) and Interleukins (ILs), produced by Antigen-Presenting Cells (APCs) and T lymphocytes generate a cytokine milieu that can lead to the destruction of HSCs. The excessive apoptosis was largely cytokine mediated with a number of proinflammatory and proapoptotic cytokines such as TNF-α, Transforming Growth Factor- β (TGF-β), and Interleukin 1β (IL1β) being overexpressed in the marrows of MDS patients. Stem/progenitor cells are impaired by these factors and exhibit marked deficiencies in proliferation and differentiation, high levels of apoptosis and dysfunctional responses to growth factor stimulation [42,54-56]. Overexpression of immune-related genes is widely reported in MDS [52]. Hyperactivation of innate immune/Toll- Like Receptor (TLR) signaling was described in MDS [50,53,57-60]. The innate immunity system is a conserved host defence mechamism that detects and eliminates pathogens [61,62]. Signals are mediated via downstream signaling mediators and eventually lead to activation of key intracellular molecular effectors such as transcription factor NF-кB and Mitogen-Activated Protein Kinases (MAPK). The resulting immune responses, including release of inflammatory cytokines, cause elimination of pathogens. Innate immunity responses are mediated by phagocytes such as macrophages and dendritic cells. However, TLR on hematopoietic progenitor cells stimulate innate immune system replenishment [63,64] and may be involved in the pathogenesis of MDS [50,53,65-69].
MDS are characterized not only by abnormal HSCs and immune system defects but also by changes in the hematopoietic microenvironment (niche) [70-74]. The pathogenesis of MDS likely depends on the interaction between aberrant hematopoietic cells and their microenvironment. Chronic immune stimulation in combination with senescence –dependent changes was observed in both, Hematopoietic Stem/Progenitor Cells (HSPC) and niche and seems to be critical to the pathogenesis of the disease. Inflammatory processes are regulatory stimulus promoting the proliferation and apoptotic death of hematopoietic progenitors in MDS. Immune system dysregulation, as a key driver of the pathological evolution of MDS, includes cytokine milieu abnormalities and inflammatory alterations in natural killer cells, T cells, and Myeloid-Derived Suppressor Cells (MDSC).
A detailed understanding of these mechanisms, which contribute to the pathogenesis of MDS, may help to find and to define novel targets for diagnosis and therapy in this disease.
The Association between Autoimmune Diseases and Myelodysplastic Syndromes
Immune and autoimmune biological anomalies have also been reported in MDS, such as the presence of antinuclear antibodies, antimitochondrial autoantibodies, Antineutrophil Cytoplasmic Autoantibodies (ANCA), autoantibodies rheumatoid factor, and cryoglobulins [32,75,76]. Approximately 10-30% of patients with MDS or CMML are associated with AD (Table 2), the most frequent being vasculitis, seronegative polyarthritis and specific skin lesions (Sweet´s syndrome, pyoderma gangrenosum) [21,23,26,43,76-84]. Distribution of AD among MDS subtypes is controversial [45]. It seems that is more involved in Refractory Anemia with Excess Blasts (RAEB), a MDS subtype characterized by increased myeloblasts or the presence of Auer rods, where in one study 86% MDS patients is associated with AD and 52% MDS patients is without AD. In a series of 235 MDS patients, 46 (19.6%) patients displayed features of AD [85]. In this study, distribution of MDS subtypes was similar between MDS cohorts with and without AD. MDS patients with AD are mostly male (up to 70%) and of older age (mean 78-83% years), which may be somewhat different from AD observed in general population, that predominate in females and in younger patients. Apart from trisomy 8 in patients with Behcet´s disease, frequently associated with MDS [81-83], there seems to be no significant association between karyotype and MDS associated with AD [45]. Distribution of MDS subtypes with del(5q) and del(7q)/ monosomy 7 are similar in association with AD or without AD. Association of MDS with vasculitis may independently predict adverse outcome [86]. Other AD do not influence outcome of MDS patients when associated with MDS [45].
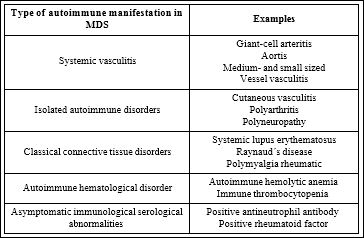
Table 2: Autoimmune diseases associated with MDS. Adapted according Oostvogels et al., [87]
A causal relationship is yet to be established between MDS and autoimmunity. The exact mechanism(s) underlying MDS and AD are not well established and are supposed to be related to immune dysfunction induced by MDS or by immunosuppressive therapy. Immune deregulation and synthesis of autoantibodies due to abnormalities in T and B cells with production of cytokines, defective macrophage clearance and neutrophil function, with subsequent prolonged circulation of immune complexes and activation of inflammatory mediators, reduced CD4 count, immature natural killer cells and impaired function of monocytes and dendritic cells with abnormal antigen presentation are observed. All these features may result from abnormal stimulation by dysplastic bone marrow stem cells [32,88-90]. Treatment options include treating both diseases concomitantly with 5-azacitidine [91,92].
T-cell and B-cell Abnormalities in MDS
The existence of functionally polarized human T cell responses based on their profile of cytokine secretion in both the CD4+ T helper (Th) and the CD8+ T cytotoxic cell subset has been established. Human Th1 and Th2 cells not only produce a different set of cytokines but also exhibit distinct functional properties. Deviation of type I and type II T cells and its negative effect on hematopoiesis in MDS in vitro was described [93]. In MDS, autologous T lymphocytes suppress both erythroid (CFU-E) and granulocytic (CFU-GM) progenitor cell growth in vitro [94,95]. CD8+ cells mediated this inhibition of hematopoiesis through the Major Histocompatibility Complex (MHC) class I molecules on target marrow cells. Removal of T cells from bone marrow often enhances colony formation [96,97]. Successful treatment with Anti-Thymocyte Globulin (ATG) eliminates or reduces the myelosuppressive effect of these autologous T cells [98]. The decline of CD8+ cells in high-risk MDS is related to the expression of the negative co-stimulatory T-cell receptor Programmed Death-1 (PD-1) and its ligand PDL-1. Higher levels of PD-1/PDL-1 in bone marrow cells are associated with resistance to therapy and with a poorer prognosis. It has recently been shown that T cell expression of the immunoinhibitory receptor PD-1 is regulated by DNA methylation. The hypomethylating agents (azacitidine and decitabine; HMAs) induced PD-1 expression on T cells in the MDS microenvironment, thereby likely hampering the immune response against the MDS blasts. Combination therapy using HMAs with a PD-1 pathway inhibitor can solve this problem.
Oligoclonal expansion of T cells in MDS was reported using flow cytometry and spectratyping [99-101]. Cukrova et al. [38] questioned the auto-reactivity of T-cells in MDS and found a defective in vitro cytotoxicity of these cells. The frequencies of activated T-cells were not related to characteristics of MDS patients [102]. However, the absolute lymphocyte count at diagnosis showed the adverse prognostic impact in a large cohort of MDS patients suggesting an influence of the host immunity on the disease in MDS patients [103].
More than 50% of early stage MDS patients have anti-erythroid autoantibodies in their bone marrow cultures. These autoantibodies are mainly directed against autologous erythroblasts and correlated with increased apoptosis [35].
The Involvement of Regulatory T-cells (Treg) in the MDS Pathogenesis
Treg are known to influence both the autoimmunity and tumour progression [104]. Kordasti et al. [105] evaluated the absolute number of both CD4+ and CD8+ Treg in the peripheral blood of 52 MDS patients. A significant correlation was shown between increased number of CD4+ Treg and several markers of disease aggressiveness (number of blasts in bone marrow, disease progression). None of these correlations was found for CD8+ Treg [105]. Kotsiniadis et al.[106] confirmed the effect of Treg on antitumour immunity in course of MDS. They found different Treg pattern in early and late stage of MDS. Treg were impaired in function and also in bone marrow homing in early stage MDS. However, Treg retained their function in late stage disease but were expanded [106]. In a retrospective study, Mailloux et al., [107] investigated the phenotypic features of Treg subsets including naive, cental memory and effector memory cells, in association with MDS progression. They found a significant shift from a central memory phenotype toward an effector phenotype connected with a higher percentage of abnormal bone marrow myeloblasts. The analysis of effector memory Tregs using flow cytometry may be a simple and useful method to predict an early immune escape in MDS patients [108]. Moreover, Treg were significantly reduced in MDS patients responding to treatment. The recruitment of CD8+ cytotoxic T-cells, the degree of dyserythropoiesis and the need for erythropoietin treatment were inversely related with the levels of Treg in the bone marrow of MDS patients [109].
Natural Killer Cells in MDS
Natural Killer (NK) cells interact with clonal cells. NK cytolytic function against different tumour targets was reduced in MDS patients in relation with increased risk of MDS, higher IPSS, abnormal karyotype and excess of blasts [110]. The percentage of NK cells was similar in MDS patients and in healthy controls. However, NK cells of MDS patients expressed increased levels of granzyme B and were mediators of cytotoxicity against dysplastic hematopoietic precursors [111]. Marcondes et al. [112] explored the relationship between NK function and IL-32 expression. NK cells from 48 MDS patients displayed impaired NK function utilizing distinct receptor-ligand interactions compared to healthy controls [111]. Reduced NK function showed global defects in NK receptor signaling. NK cell receptor proteins G2D (NKG2D), DNAX-Accessory Molecule-1 (DNAM-1) and natural cytotoxicity receptor NKp30 are involved in activation of NK cells. CD226 (Cluster of Differentiation 226), PTA1 (platelet and T cell activation antigen 1) or DNAM-1 is a protein that in humans is encoded by the CD226 gene which is located on chromosome 18q22.3. Reduced expression of all these activating receptors were associated with impaired NK function in MDS [111,113,114]. NK cells from MDS patients fail to exhibit appropriate effector response. Based on low levels of IL-32 in CMML and high levels in MDS but suppressed cytotoxicity function in both diseases, IL-32 levels did not correlate with cytotoxic function. Abnormal stroma in MDS may play an inhibitory role in NK cell differentiation and development. Loss of immunosurveilanced may then lead to the accumulation of cells with DNA damage in the intermediate stages of MDS progression. NK cells become more dysfunctional as MDS progresses. However, the direct cause and effect are unknown.
Abnormal Levels of Inflammatory Cytokines and Chemokines in the Pereipheral Blood and Bone Marrow of MDS Patients
A variety of immune molecules, including IFN-γ, TNF-α and ILs produced by Antigen-Presenting Cells (APCs) and T lymphocytes generate a cytokine milieu that can lead to destruction of HSCs. The secretion of TNF-α and and other related cytokines, such as IFN-γ or IL-6, is higher in low-risk MDS, whereas these and other cytokines are down-regulated in high-risk cases [50]. The overproduction of IFN-γ, TNF-α and ILs is hypothesised to conribute to the pathogenesis of MDS. Elevated amount of these secreted factors impair stem/ progenitor cells that exhibit marked deficiencies in proliferation and differentiation, high levels of apoptosis and dysfunctional responses to growth factor stimulation [42,115-123]. IL-17 enhances the production of IFN-γ and TNF-α by bone marrow T lymphocytes from patients with lower risk MDS and may be involved in the pathogenesis of lower risk MDS [124].
Increased rates of intramedullary apoptosis are the main cause of the cytopenias in MDS. Apoptosis is initiated by the death receptor Fas and its specific ligand (Fas-L), which is overexpressed and correlates with the rate of apoptosis in MDS [125-131]. Fas/Apo-1 (CD95) and Fas-L are measured by flow cytometry, quantitative PCR of cDNA generated from mRNA and immunohistochemistry. TNF-α - Related Apoptosis Inducing Ligand (TRAIL) is a member of the TNF family, which controls apoptosis by binding to agonistic receptors TRAIL-R1 and TRAIL-R2 and decoy receptors TRAIL-R3 and TRAIL-R4. TRAIL is present in normal marrow in negligible amounts, but is constitutively expressed in MDS marrow [119]. Fas-associated death-domain-Like interleukin-1β-converting enzyme Inhibitory Protein (FLIP) is important in controlling apoptosis in normal cells. Isoforms of FLIP are products of alternative mRNA splicing and have pro-apoptotic or anti-apoptotic properties. In early MDS, the anti-apoptotic isoform of FLIP downregulated and apoptosis is higher than in MDS with excess blasts, where resistance to apoptosis was described [132]. TNF-α receptor TRAIL-R2, which transmits cytoprotective signals via transcription factor NF-κB is also increased in late MDS and exert anti-apoptotic signal through regulation of bcl-2 and bcl-xL [133,134].
However, various cytokines, such as TGF-β, IFN-α and TNF-α itself, activate the p38 Mitogen-Activated Protein Kinase (MAPK) downstream signaling pathway in hematopoietic stem and progenitor cells, that increases apoptotic signaling in MDS bone marrow cells [135-140].
Toll-like Receptor Signaling and its Activation in MDS
The innate immune system is an evolutionarily conserved defense mechanism against pathogens which is implicated in the pathogenesis of MDS [65-68]. The Toll-Like Receptor (TLR) family (10 different TLRs in humans) plays a major role in the initial detection and subsequent elimination of foreign pathogens. This process is achieved through activation of intracellular signaling pathways, such as NF-κB and MAPK, which initiate a coordinated set of responses. Wei et al., [141] performed a genome-wide Chromatin Immunoprecipitation (CHIP) followed by Sequencing (Seq) analysis of H3K4me3 in MDS. This analysis identified multiple genes marked by increased H3K4me3 in bone marrow CD34+ cells. A large majority of the genes identified are known to be involved in TLR-mediated innate immunity signaling and NF-κB activation [141]. These authors showed in the same study that the histone H3K27me3 demethylase JMJD3/ KDM6B containing Jumonji domain 3 (Jmjd3) is significantly overexpressed in MDS bone marrow CD34+ cells and has an important role in the regulation of expression of genes involved in innate immunity. Thus JMJD3 demethylase is capable to remove the trimethyl group from histone H3 lysine 27 [142].
Gene expression and mutational analysis of eight human TLRs were performed in a large cohort of MDS [143]. TLR1, TLR2 and TLR6 are significantly overexpressed in MDS bone marrow CD34+ cells. TLR1 and TLR6 are known to form functional heterodimers with TLR2. Deep genetic sequencing identified a rare genetic variant of TLR2 (F217S) present in 11% of bone marrow mononuclear cells of patients with MDS where is associated with NF-κB activation. The level of this variant is in MDS is significantly higher than in normal population. Inhibition of TLR2 in cultured MDS bone marrow CD34+ cells from patients with lower risk of MDS results in increased formation of erythroid colonies. TLR2-mediated innate immune signaling has a role in pathophysiology of MDS and its targeting may have therapeutic potential.
Velegraki et al., [144] demonstrated increased expression of awide panel of genes involved in TLR4 signaling in bone marrow mononuclear cells. A gene expression microarray showed that TRAF6 is overexpressed in MDS CD34+ cells in comparison with healthy controls [145]. Furthermore, DNA arrays revealed the amplification of the TRAF6 locus (chromosome 11p12) and the TIRAP locus (chromosome 11q24.2) in MDS [146,147].
TLR4 is the receptor for Lipopolysaccharide (LPS), which induces the release of critical proinflammatory cytokines that are necessary to activate potent immune responses [148]. LPS/TLR4 signaling has been intensively studied in the past years. Two pathways diverge downstream of TLR4, the Myeloid Differentiation primary response gene 88 (MyD88)-dependent and -independent pathways, resulting in the expression of inflamatory cytokines of IFN-inducible genes. The MyD88-dependent pathway mediates a rapid and acute response, whereas MyD88-independent pathway is responsible for delayed response. MyD88 is a Toll-Interleukin 1 Receptor (TIR) containing adaptor protein that forms a complex on TIR domain of TLR4 (TIRAP). MyD88 recruits IL-1 Receptor Associated Kinase 4 (IRAK4), which then recruits IRAK1, resulting in subsequent autophosphorylation and disassociation from the TLR4-MyD88 complex. This complex then binds to TNF Receptor-Associated Factor (TRAF), key effector of the innate immune signaling complex. E3 ubiquitin ligase TRAF6 plays a key role in downstream activation of NF-κB. MyD88 is overexpressed in bone marrow progenitors of MDS and is associated with risk stratification and patient survival [60]. Rhyasen et al., [149,150] demonstrated IRAK1 upregulation in MDS bone marrow mononuclear cells and showed that targeting of IRAK1 is a therapeutic approach for MDS.
Bone Marrow Niche and its Involvement in MDS
Hematopoietic stem and progenitor cells reside within the “bone marrow niche”, which is cellular and molecular microenvironment, which maintains and regulates stem cell self-renewal, differentiation and proliferation. Mesenchymal Stem Cells (MSCs) are primitive, non-hematopoietic stem cells that give rise to all of the various types of stromal cells that form bone marrow microenvironment [50,70-74,151-160].MSCshave important roles in hematopoiesis and immune regulation.
Several studies have indicated that impaired MSCs propagate MDS [50,70-74,151-160]. Among the MSC impairments is altered expression of Aurora Kinase Genes (AURK) with an important role in the regulation of G2/M phase of cell cycle, centrosomes and cytokinesis. The expression of AURK is highly upregulated in MSCs in MDS patients. AURK is also targeted by microRNAs (miR-let-7a and miR-let-7b). Let-7 is a family of tumor suppressor microRNAs that are frequently down-regulated in malignant cells. Let-7 microRNA family members are downregulated in MDS with spliceosome mutations [161]. Dysregulated expression of AURK leads to increased number of centrosomes, gain or loss of chromosomes causing cell death of normal cells and survival of malignant cells.
Hematopoietic stem cells and mesenchymal stem cells undergo changes in response to induction factors like TNF-α, Fas and TGF-β in the bone marrow niche of MDS. However, these stem cells do not originate from the same neoplastic clone and often harbor different chromosomal aberrations, suggesting distinct genetic origin of MDS niche [162].
MDS MSCs release higher amount of IL-6 than normal MSCs [163]. Il6 is secreted by macrophages in MDS bone marrow and induces apoptosis in hematopoietic cells [163]. MDS MSCs inhibit T-cell proliferation in vitro and suppress the immune system in vivo. MSCs inhibit the proliferation of T-cells in normal healthy controls through secretion of TGF-β and Hepatocyte Growth Factor (HGF). However, in MDS this secretion is decreased and MSCs may increase the proliferation of T-cells, thereby reducing immunosuppression, which results in increased apoptosis of MDS cells.
CXCL12, a member of the CXC family of chemokines, also known as Stromal Cell Derived Factor 1 (SDF1), is thgough to have an important role in cell migration in and out of the bone marrow microenvironment. It is produced by bone marrow stromal cells, including endothelial cells and fibroblasts [152]. CXCL12 expression is lower in normal bone marrow than in MDS bone marrow [71,164]. Upregulated CXCL12 expression increases homing signaling for CXCR4 expressing hematopoietic cells, resuting in their hyperproliferation. This increased CXCL12 may be the reason for hypercellular bone marrow in MDS. CXCR4 high-expression group of MDS patients had a shorter overall survival time and shorter relapse-free survival time compared with those of the low-expression group [165,166]. There are positive correlations between CXCL12 and apoptosis in the low-grade MDS. For the high-grade MDS, there were positive correlations between CXCR4 and VEGF, and between CXCL12 concentration and bone marrow Microvessel Density (MVD). The apoptosis is one of the hallmarks for low-grade MDS and the angiogenesis for high-grade MDS. A refined understanding of the roles that CXCL12/CXCR4 axis and its correlation with angiogenesis and apoptosis play in MDS will fuel the development of therapies that can be targeted to the CXCL12/CXCR4 axis.
Perhaps the most striking evidence that bone marrow MSCs may play an important role in the induction of MDS is based on a study in mice, where selective deletion in osteoprogenitors of Dicer1, a RNaseIII endonuclease, essential for miRNA biogenesis and RNA processing, resulted in development of myelodysplasia and secondary leukemia [167]. Dicer1 was not deleted in hematopoietic stem cells.
As stromal cells in the endosteal niche, osteoblasts have important regulatory role in MDS bone marrow microenvironment. Osteoblasts regulate the maturation and proliferation of osteoclasts that are involved in Hematopoietic Stem Cells (HSCs) support. Strong adhesion between HSCs and osteoblasts maintains HSCs in bone marrow. In response to stress, infection and bleeding, HSCs migrate to vascular niche, resulting in their proliferation and differentiation. Within MDS bone marrow niche, malignant HSCs are found both in vascular niche and endosteal niche.
Induction of Myelodysplasia by Myeloid-Derived Suppressor Cells
Immature Myeloid-Derived Suppressor Cells (MDSCs), known to accumulate in tumor-bearing mice and cancer patients, are site-specific inflammatory and T cell immunosuppressive effector cells that contribute to cancer progression [168-172]. Their suppressive activity is in part driven by inflammation-associated signaling molecules, such as the Danger-Associated Molecular Pattern (DAMP) heterodimer S100A8/S100A9 (also known as myeloid-related protein 8 /MRP8/ and MRP14, respectively), which interact with several innate immune receptors that are involved in the biology of MDSCs activation [46,74,173,174].
Human MDSCs lack most markers of mature immune cells (LIN-, HLA-DR-) but possess CD33, the prototypical member of sialic acid-binding immunoglobulin-like super-family of lectins (Siglec) [169,175,176]. CD33 possesses an Immunoreceptor Tyrosine-based Inhibition Motif (ITIM) that is associated with immune suppression [175]. LIN-, HLA-DR-CD33+ MDSCs specifically accumulate in thebone marrow of MDS patients and impair hematopoiesis through a mechanism that involves S100A9 as an endogenous ligand for CD33-initiated signaling. S100A9 protein belongs to the family of S100 calcium-binding proteins that has an important role in inflammation. MDSCs did not expand in the S100A9 protein deficiency [169]. S100A9 protein activation is through the NF-κB signaling pathway. Therefore, inhibitors of the NF-κB signaling pathway may reduce MDSCs levels and be useful therapeutic agents in conjunction with active immunotherapy targeted against the cell surface CD33 antigen in MDS patients.
Transmembrane protein CD33 is frequently expressed in cases of MDS and CMML with elevated blast count [177]. In normal individuals, expression of CD33 is associated with myeloid maturation and is present on myeloid but also on some lymphoid cells [178-180].The CD33 antigen is not expressed in normal hematopoietic stem cells [181,182].
Immunotherapeutic Approaches in MDS
More than 40 years ago the observation that some patients given immunosuppressive conditioning with Antithymocyte Globulin (ATG) followed by bone marrow transplantation sometimes achieved full recovery of their autologous marrow prompted Gluckman et al., [183] to use ATG in patients with severe aplastic anemia. Horse ATG and more recently rabbit ATG have been also used to treat MDS [184-187]. Factors affecting responses included younger age, low IPSS score, and the presence of HLA-DR15 antigens. Complete responses were more common in patients with hypocellular MDS.
The first prospective studies using cyclosporine in patients with MDS was reported by Jonasova et al. [188] who treated 14 cytopenic patients with refractory and anemia and variable marrow cellularity. All responders achieved transfusion independence which sustained for up to 30 months. A study in China reported a 62.5% response rate in 32 patients with RA, RARS, and RAEB treated with cyclosporine [189]. Renal failure occured in a minority and this treatment needs careful followup of renal function.
The CD52 binding monoclonal antibody CAMPATH1 or alemtuzumabhas been shown to have efficacy in MDS treatment. Neukirchen et al., [190] reported experience with alemtuzumab in nine MDS RCMD patients. All patients had a hypocellular bone marrow with a blast count <5 % and were classified as intermediate-1 according to the IPSS. We found a response in five patients (60 %); three patients achieved a complete remission 3 and 6 months after the treatment with alemtuzumab, and two patients showed a haematological improvement. Alemtuzumab was administered in a 10-mg dosage for 10 days. Treatment was well tolerated, and no severe side effects were observed. We could confirm the finding that the alemtuzumab is effective and save selected MDS patients. Due to the promising results, further studies, especially with regard to long-term survival and risk of leukemic progression should be initiated.
Gemtuzumab ozogamicin (Mylotarg; CMA-676; Wyeth Laboratories, Philadelphia, PA) is a monoclonal antibody conjugated with the highly potent anthracycline calicheamicin and targeted against the cell surface CD33 antigen [191]. Gemtuzumab binds to the CD33 antigen and is internalized and hydrolyzed. The cytotoxic part of the molecule, calicheamicin, enters the nucleolus, binds DNA strands and causes breaks in DNA, resulting in cell death. US Food and Drug Administration (FDA) approved gemtuzumab ozogamicin for therapy of relapsed AML expressing the CD33 antigen in patients over 60 years of age [192,193]. Raza et al., [194] carried out an open-label, randomized, phase II study to evaluate the safety and efficacy of gemtuzumab ozogamicinmonotherapy in patients with the IPSS classification intermediate-2 or high risk MDS. No complete responses or partial responses were observed. Combination therapy with gemtuzumab ozogamicin and Interleukin-11 (IL-11) and gemtuzumab ozogamicin monotherapy were studied in a randomized study conducted at MD Anderson Cancer Center in patients 65 years of age or older with previously untreated AML or high-risk MDS [195]. None of 6 MDS patients randomized to gemtuzumab ozogamicin only arm had complete response. 25% of patients in the combination therapy arm attained complete response. However, this higher complete response did not translate into a survival advantage.
Bispecific Killer Cell Engager (BIKE) targeting CD16 expressed on effector natural killer cells and CD33 is able to facilitate elimination CD33+ MDS targets and immunosuppressive MDSC targets and may be therapeutically beneficial for MDS patients [196].
Cytotoxic chemotherapy has non-specific effects due to treatment related toxicities and patients also often relapsed due to residual cancer cells that are inherently resistant to cytotoxic therapy. Cancer immunotherapy has the capacity to overcome these both problems, directing a specific cytotoxic immune response against cancer cells, particularly residual cancer cells.The potential of the immune systém to eliminate malignant cell sis also demonstrated by allogeneic bone marrow transplantation. Moreover, relapsed disease following allogeneic bone marrow transplantation can be eradicted by the infusion of doner derived lymphocytes [197].Graft versus host disease remains a major cause of morbidity and mortality following allogeneic transplantation. Allogeneic hematopoietic stem cell transplantation remains the unique curative option for patients with MDS and AML at high risk of relapse.
The development of an effective cancer vaccine requires effective presentation of tumor antigen for effective T cell activation, and the concucurrent reversal of the immunosuppressive milleu in order to induce long-term immunity. Dendritic cells are bone marrow derived immune cells with potent antigen presenting abilities capable to induce primary immunity [198]. Dendritic cells are quantitatively and functionally deficient in MDS and AML patients [199]. Dendritic cells are good mediators of antileukemic aktivity and dendritic cells-vaccination strategies may be convenient for patients at relapse after allogeneic stem cell transplantation [200].
Over-expressed or aberrantly expressed cellular proteins including Wilms Tumor-1 (WT1) have been evaluated in phase I/II clinical trials of active immunotherapy with promising results [201]. The WT1 gene located on chromosome 11p13 encodes a zinc finger transcription factor that is important in cell growth and differentiation [202]. The WT1 gene expression is a good marker for diagnosis of disease progression of MDS [203]. WT1 is one of the antigens that triggers T cell-mediated myelosuppression in MDS [204]. Vaccination with WT1 peptide was found to be safe and well tolerated in MDS patients with only 8% of patients (7 out of 88 total patients with MDS) [204]. The isolated WT1 peptide vaccination may be insufficient to generate long-term protective immunity [204]. Coupling a vaccine approach with sequential blockadecheckpoint inhibition, such as cytotoxic T lymphocyte antigen 4 (CTLA-4) or PD1blockade, may be required to increase therapeutic benefit [205,206].
Recent findings regarding innate immune and inflammatory signals in MDS have been exploited for the development of novel therapeutic strategies in MDS. Preclinical studies with the specific inhibition of the activity or expression of TLR2 and its downstream effectors in primary MDS bone marrow cells by shRNA significantly improved differentiation, induced apoptosis and impaired their clonal generation potential, particularly in cells from lower risk MDS patients [50]. Opsona Therapeutics Ltd (Dublin, Ireland), the innate immune drug development company focused on novel therapeutic approaches to treat autoimmune, inflammatory diseases and oncology, announced the issuances of US Patent 8,734,794 and European Patent, EP2,451,842, which cover the Opsona developed antibody OPN-305, which is directed against TLR-2 (https://clinicaltrials.gov/ct2/show/NCT02363491). OPN-305 is the first humanized IgG4 monoclonal antibody against TLR2 and is studied in phase II clinical trial to improve erytroid differentiation of MDS bone marrow CD34+ cells.
Preclinical studies using MyD88 inhibition by inhibitory peptide (Invivogen, San Diego, CA), IL-8 inhibition by neutralizing antibody (ABCAM, Cambridge, MA) and IRAK1 inhibition by RNAint or specific inhibitor molecule were described [60,149]. Using a physics-based computational approach, Nimbus and their co-founding partner, Schrödinger Inc., uncovered the first truly selective small molecule IRAK4 (interleukin-1 receptor associated kinase 4) inhibitors. The three Nimbus novel compounds, ND-346, ND-2110 and ND-2158 demonstrated high selectivity against a panel of 334 kinases, and potent in vitro inhibition of cytokine production in cells and whole blood. Roche´s Genentech unit licenced IRAK4 inhibitors from Nimbus.
SCIO-469 is a small-molecule p38 mitogen-activated protein (MAP) kinase inhibitor developed by Scios Inc as a potential oral therapy for inflammatory diseases. Preclinical studies with SCIO-469 in MDS were described [137]. Phase II open-label study for patients with MDS has been completed (https://clinicaltrials.gov/ct2/show/ NCT00113893). ARRY-614, a potent, small-molecule dual p38/Tie2 inhibitor, developed by Biopharma, is being studied in patients with IPSS low and intermediate-1 risk MDS (Phase 1 study, https://clinicaltrials.gov/show/NCT01496495). In an initial dose-escalation study, using a powder-in-capsule formulation of ARRY-614, multi-lineage activity was observed. The most promising effects were seen in patients with thrombocytopenia and neutropenia, with transfusion independence frequently observed in platelet transfusion-dependent patients. ARRY-614 decreased the presence of phosphorylated p38 MAPK in bone marrow and reduced bone marrow apoptosis in most MDS patients while efficiently decreasing the levels of some inflammatory factors and erythropoietin in patients’ plasma [207].
Curis, Inc. in collaboration with Aurigene designed an orally bioavailable small molecule, which binds with high affinity to PD-L1 and disrupts the interaction between PD-L1 and PD1 receptors on T cells. Preliminary results generated by Aurigene demonstrate that in in vitro studies, such small molecule PD-L1 antagonists can induce effective T cell proliferation and IFN-γ production by T cells that are specifically suppressed by PD-L1 in culture. In addition, such small molecules also appear to have effects similar to anti-PD1 antibodies in in vivo tumor models, including IFN-γ production and inhibition of tumor growth. The anti-tumor effect of the oral PD-L1 antagonist is similar to that seen with a known anti-PD1 antibody in this mouse model. In early trials in patients with hematological malignancies, antibodies targeting CTLA-4 or PD1signaling pathway have displayed significant efficacy with minimal toxicity in patients [206]. A safety and pharmacology study of Atezolizumab (MPDL3280A, Anti-PD-L1 Antibody) administered alone or in combination with azacitidine in patients with myelodysplastic syndromes has been announced (https://www.clinicaltrials.gov/ct2/show/NCT02508870).
Sotatercept (formerly called ACE-011) is an investigational protein therapeutic that increases Red Blood Cell (RBC) levels by targeting molecules in the TGF-β superfamily. Acceleron is developing sotatercept in collaboration with Celgene Corporation for the treatment of anemia in rare blood diseases, including MDS. Sotatercept inhibits osteoclasts and promotes osteoblast survival in MDS bone marrow microenvironment. Phase 2 studies (https:// clinicaltrials.gov/ct2/show/NCT01736683) of Sotatercept for the treatment of anemia in low-or intermediate-1 risk myelodysplastic syndromes (MDS) or non-proliferative CMML is ongoing.
An oral small molecule inhibitor of TGF-β receptor I kinase, LY-2157299, galunisertib, is also being tested in a phase II trial (https:// clinicaltrials.gov/ct2/show/NCT02008318) in low and intermediate-1 risk MDS [208]. Inhibitor of IDO1 is an inhibitor of the enzyme Indoleamine 2,3-Dioxygenase (IDO). This inhibitor is proposed for the treatment of malignant diseases and has been used in phase II INCB024360 study for patients with MDS (https://clinicaltrials.gov/ct2/show/ NCT01822691) [209].
Conclusion and Perspectives
Great progress has been made in recent years in understanding the role of innate immune deregulation in the MDS pathogenesis. Constitutively activated innate immune and inflammatory pathways affect directly hematopoiesis; lead to altered cytokine secretion and impact T-cell immunity. All these biological effects contribute to the development and progression of MDS. Innate immune deregulation could be induced by cellular stresses asociated with senescent changes, genomic instability and other genetic and epigenetic abnormalities that occur in hematopoietic cells with aging, but could be also initiated by abnormal cellular interactions in the bone marrow environment (niche). However, it is necessary to identify the endogenous ligands responsible for Toll-like receptors activation and the conditions that contribute to their release. This information will help to develop new effective therapeutic approaches.
Acknowledgements
This work was supported by the project for conceptual development of research organisation No 00023736 from the Ministry of Health of the Czech Republic.
References
- Heaney ML, Golde DW (1999) N Engl J Med 340: 1649-1660.
- Corey SJ, Minden MD, Barber DL, Kantarjian H, Wang JC, et al. (2007) Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer 7: 118-129.
- Nimer SD (2008) Myelodysplastic Blood 111: 4841-4851.
- Tefferi A, Vardiman JW (2009) Myelodysplastic syndromes. N Engl J Med 361: 1872-1885.
- Tehranchi R, Woll PS, Anderson K, Buza-Vidas N, Mizukami T, et (2010) Persistent malignant stem cells in del(5q) myelodysplasia in remission. N Engl J Med 363: 1025-1037.
- Jädersten M, Karsan A (2011) Clonal evolution in myelodysplastic syndromes with isolated del(5q): the importance of genetic Haematologica 96: 177-180.
- Li J (2013) Myelodysplastic syndrome hematopoietic stem Int J Cancer 133: 525-533.
- Jaiswal S, Ebert BL (2014) MDS is a stem cell disorder after Cancer Cell 25: 713-714.
- Woll PS, Kjällquist U, Chowdhury O, Doolittle H, Wedge DC, et (2014) Myelodysplastic syndromes are propagated by rare and distinct human cancer stem cells in vivo. Cancer Cell 25: 794-808.
- Elias HK, Schinke C, Bhattacharyya S, Will B, Verma A, et (2014) Stem cell origin of myelodysplastic syndromes. Oncogene 33: 5139-5150.
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, et al. (1982) Proposals for the classification of the myelodysplastic Br J Haematol 51: 189-199.
- Saif MW, Hopkins JL, Gore SD (2002) Autoimmune phenomena in patients with myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk Lymphoma 43: 2083-2092.
- Benton CB, Nazha A, Pemmaraju N, Garcia-Manero G (2015) Chronic myelomonocytic leukemia: Forefront of the field in Crit Rev Oncol Hematol 95: 222-242.
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, et al. (1994) The chronic myeloid leukaemias: guidelines for distinguishing chronic granulocytic, atypical chronic myeloid, and chronic myelomonocytic leukaemia. Proposals by the French-American-British Cooperative Leukaemia Group. Br J Haematol 87: 746-754.
- Voglová J, Chrobák L, Neuwirtová R, Malasková V, Straka L (2001) Myelodysplastic and myeloproliferative type of chronic myelomonocytic leukemia--distinct subgroups or two stages of the same disease? Leuk Res 25: 493-499.
- Vardiman JW, Harris NL, Brunning RD (2002) The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 100: 2292-2302.
- Schuler E, Schroeder M, Neukirchen J, Strupp C, Xicoy B, et al. (2014) Refined medullary blast and white blood cell count based classification of chronic myelomonocytic leukemias. Leuk Res 38: 1413-1419.
- Nybakken GE, Bagg A (2014) The genetic basis and expanding role of molecular analysis in the diagnosis, prognosis, and therapeutic design for myelodysplastic syndromes. J Mol Diagn 16: 145-158.
- Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, et al. (1997) International scoring system for evaluating prognosis in myelodysplastic syn Blood 89: 2079-2088.
- Steurer M, Fritsche G, Tzankov A, Gotwald T, Sturm W, et al. (2004) Large-vessel arteritis and myelodysplastic syndrome: report of two cases. Eur J Haematol 73: 128-133.
- George SW, Newman ED (1992) Seronegative inflammatory arthritis in the myelodysplastic syndromes. Semin Arthritis Rheum 21: 345-354.
- Eng C, Farraye FA, Shulman LN, Peppercorn MA, Krauss CM, et al. (1992) The association between the myelodysplastic syndromes and Crohn dis Ann Intern Med 117: 661-662.
- Enright H, Jacob HS, Vercellotti G, Howe R, Belzer M, et (1995) Paraneoplastic autoimmune phenomena in patients with myelodysplastic syndromes: response to immunosuppressive therapy. Br J Haematol 91: 403-408.
- Raza A, Mundle S, Shetty V, Alvi S, Chopra H, et al. (1996) Novel insights into the biology of myelodysplastic syndromes: excessive apoptosis and the role of cytokines. Int J Hematol 63: 265-278.
- Gersuk GM, Lee JW, Beckham CA, Anderson J, Deeg HJ (1996) Fas (CD95) receptor and Fas-ligand expression in bone marrow cells from patients with myelodysplastic syndrome. Blood 88: 1122-1123.
- Enright H, Miller W (1997) Autoimmune phenomena in patients with myelodysplastic syndromes. Leuk Lymphoma 24: 483-489.
- Kook H, Zeng W, Guibin C, Kirby M, Young NS, et (2001) Increased cytotoxic T cells with effector phenotype in aplastic anemia and myelodysplasia. Exp Hematol 29: 1270-1277.
- Espinosa G, Font J, Muñoz-Rodríguez FJ, Cervera R, Ingelmo M (2002) Myelodysplastic and myeloproliferative syndromes associated with giant cell arteritis and polymyalgia rheumatica: a coincidental coexistence or a causal relationship? Clin Rheumatol 21: 309-313.
- Allampallam K, Shetty V, Mundle S, Dutt D, Kravitz H, et al. (2002) Biological significance of proliferation, apoptosis, cytokines, and monocyte/macrophage cells in bone marrow biopsies of 145 patients with myelodysplastic Int J Hematol 75: 289-297.
- Rizos E, Dimos G, Milionis HJ, Elisaf MS (2003) Temporal arteritis masquerading as chronic myelomonocytic leukemia. Clin Exp Rheumatol 21: 685-686.
- Giannouli S, Voulgarelis M, Zintzaras E, Tzioufas AG, Moutsopoulos HM (2004) Autoimmune phenomena in myelodysplastic syndromes: a 4-yr prospective study. Rheumatology (Oxford) 43: 626-632.
- Voulgarelis M, Giannouli S, Ritis K, Tzioufas AG (2004) Myelodysplasia-associated autoimmunity: clinical and pathophysiologic concepts. Eur J Clin Invest 34: 690-700.
- Pinheiro RF, Silva MR, Chauffaille Mde L (2006) The 5q syndrome and auto-immune phenomena: report of three cases. Leuk Res 30: 507-510.
- Kiladjian JJ, Fenaux P, Caignard A (2007) Defects of immune surveillance offer new insights into the pathophysiology and therapy of myelodysplastic Leukemia 21: 2237-2239.
- Barcellini W, Zaninoni A, Imperiali FG, Boschetti C, Colombi M, et (2007) Anti-erythroblast autoimmunity in early myelodysplastic syndromes. Haematologica 92: 19-26.
- Wu L, Li X, Chang C, Ying S, He Q, et al. (2008) Deviation of type I and type II T cells and its negative effect on hematopoiesis in myelodysplastic Int J Lab Hematol 30: 390-399.
- Barrett AJ, Sloand E (2009) Autoimmune mechanisms in the pathophysiology of myelodysplastic syndromes and their clinical Haematologica 94: 449-451.
- Cukrová V, Neuwirtová R, Dolezalová L, Belicková M, Bartůnková J, et al. (2009) Defective cytotoxicity of T lymphocytes in myelodysplastic Exp Hematol 37: 386-394.
- Sugimori C, List AF, Epling-Burnette PK (2010) Immune dysregulation in myelodysplastic syndrome. Hematol Rep 2: 1.
- Warlick ED, Miller JS (2011) Myelodysplastic syndromes: the role of the im- mune system in pathogenesis. Leuk Lymphoma 52: 2045-2049.
- Aggarwal S, van de Loosdrecht AA, Alhan C, Ossenkoppele GJ, Westers TM, et al. (2011) Role of immune responses in the pathogenesis of low- risk MDS and high-risk MDS: implications for Br J Haematol 153: 568-581.
- Calado RT (2011) Immunologic aspects of hypoplastic myelodysplastic syndrome. Semin Oncol 38: 667-672.
- Belizna C, Subra JF, Henrion D, Ghali A, Renier G, et (2013) Prognosis of vasculitis associated myelodysplasia. Autoimmun Rev 12: 943-946.
- Al Ustwani O, Ford LA, Sait SJ, Block AM, Barcos M, et al. (2013) Myelo- dysplastic syndromes and autoimmune diseases--case series and review of Leuk Res 37: 894-899.
- Braun T, Fenaux P2 (2013) Myelodysplastic Syndromes (MDS) and autoim- mune disorders (AD): cause or consequence? Best Pract Res Clin Haematol 26: 327-336.
- Chen X, Eksioglu EA, Zhou J, Zhang L, Djeu J, et (2013) Induction of my- elodysplasia by myeloid-derived suppressor cells. J Clin Invest 123: 4595- 4611.
- Yeung DF, Hsu R2 (2014) Expressive aphasia in a patient with chronic myel- omonocytic leukemia. Springerplus 3: 406.
- Serio B, Risitano A, Giudice V, Montuori N, Selleri C (2014) Immunological derangement in hypocellular myelodysplastic Transl Med UniSa 8: 31-42.
- Frietsch JJ, Dornaus S, Neumann T, Scholl S, Schmidt V, et al. (2014) Paraneoplastic inflammation in myelodysplastic syndrome or bone marrow failure: case series with focus on 5-azacytidine and literature review. Eur J Haematol 93: 247-259.
- Gañán-Gómez I, Wei Y, Starczynowski DT, Colla S, Yang H, et (2015) De- regulation of innate immune and inflammatory signaling in myelodysplastic syndromes. Leukemia 29: 1458-1469.
- Kulasekararaj AG, Kordasti S, Basu T, et (2015) Chronic relapsing remit- ting Sweet syndrome--a harbinger of myelodysplastic syndrome. Br J Hae- matol 170: 649-656.
- Peker D, Padron E, Bennett JM, Zhang X, Horna P, et al. (2015) A close association of autoimmune-mediated processes and autoimmune disorders with chronic myelomonocytic leukemia: observation from a single Acta Haematol 133: 249-256.
- Varney ME, Melgar K, Niederkorn M, Smith MA, Barreyro L, et al. (2015) Deconstructing innate immune signaling in myelodysplastic Exp Hematol 43: 587-598.
- Mundle SD, Ali A, Cartlidge JD, Reza S, Alvi S, et (1999) Evidence for in- volvement of tumor necrosis factor-alpha in apoptotic death of bone marrow cells in myelodysplastic syndromes. Am J Hematol 60: 36-47.
- Stifter G, Heiss S, Gastl G, Tzankov A, Stauder R (2005) Over-expression of tumor necrosis factor-alpha in bone marrow biopsies from patients with myelodysplastic syndromes: relationship to anemia and prognosis. Eur J Haematol 75: 485-491.
- Kondo A, Yamashita T, Tamura H, Zhao W, Tsuji T, et al. (2010) Interfer- on-gamma and tumor necrosis factor-alpha induce an immunoinhibitory mol- ecule, B7-H1, via nuclear factor-kappaB activation in blasts in myelodysplas- tic syndromes. Blood 116: 1124-1131.
- Maratheftis CI, Andreakos E, Moutsopoulos HM, Voulgarelis M (2007) Toll- like receptor-4 is up-regulated in hematopoietic progenitor cells and contrib- utes to increased apoptosis in myelodysplastic Clin Cancer Res 13: 1154-1160.
- Wei Y, Dimicoli S, Bueso-Ramos C, Chen R, Yang H, et (2013) Toll-like receptor alterations in myelodysplastic syndrome. Leukemia 27: 1832-1840.
- Velegraki M, Papakonstanti E, Mavroudi I, Psyllaki M, Tsatsanis C, et al. (2013) Impaired clearance of apoptotic cells leads to HMGB1 release in the bone marrow of patients with myelodysplastic syndromes and induces TLR4-mediated cytokine production. Haematologica 98: 1206-1215.
- Dimicoli S, Wei Y, Bueso-Ramos C, Yang H, Dinardo C, et (2013) Overex- pression of the toll-like receptor (TLR) signaling adaptor MYD88, but lack of genetic mutation, in myelodysplastic syndromes. PLoS One 8: 71120.
- Takeda K, Akira S (2004) TLR signaling Semin Immunol 16: 3-9.
- Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124: 783-801.
- Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, et al. (2006) Toll-like re- ceptors on hematopoietic progenitor cells stimulate innate immune system Immunity 24: 801-812.
- Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, et al. (2011) Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol 186: 5367-5375.
- Starczynowski DT, Karsan A (2010) Innate immune signaling in the myelodysplastic syndromes. Hematol Oncol Clin North Am 24: 343-359.
- Taganov KD, Boldin MP, Chang KJ, Baltimore D (2006) NF-kappaB-de- pendent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 103: 12481-12486.
- Starczynowski DT, Karsan A (2010) Deregulation of innate immune signaling in myelodysplastic syndromes is associated with deletion of chromosome arm 5q. Cell Cycle 9: 855-856.
- Starczynowski DT, Kuchenbauer F, Argiropoulos B, Sung S, Morin R, et al. (2010) Identification of miR-145 and miR-146a as mediators of the 5q- syn- drome phenotype. Nat Med 16: 49-58.
- Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, et (2011) miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med 208: 1189-1201.
- Kitagawa M, Kurata M, Yamamoto K, Abe S, Suzuki S, et al. (2011) Molec- ular pathology of myelodysplastic syndromes: biology of medullary stromal and hematopoietic cells (review). Mol Med Rep 4: 591-596.
- Abe-Suzuki S, Kurata M, Abe S, Onishi I, Kirimura S, et (2014) CXCL12+ stromal cells as bone marrow niche for CD34+ hematopoietic cells and their association with disease progression in myelodysplastic syndromes. Lab In- vest 94: 1212-1223.
- Bulycheva E, Rauner M, Medyouf H, Theurl I, Bornhäuser M, et al. (2015) Myelodysplasia is in the niche: novel concepts and emerging Leu- kemia 29: 259-268.
- Kim M, Hwang S, Park K, Kim SY, Lee YK, et (2015) Increased expression of interferon signaling genes in the bone marrow microenvironment of myelo- dysplastic syndromes. PLoS One 10: 0120602.
- Yang L, Qian Y, Eksioglu E, Epling-Burnette PK, Wei S (2015) The inflamma- tory microenvironment in MDS. Cell Mol Life Sci 72: 1959-1966.
- Mufti GJ, Figes A, Hamblin TJ, Oscier DG, Copplestone JA (1986) Immuno- logical abnormalities in myelodysplastic I. Serum immunoglobu- lins and autoantibodies. Br J Haematol 63: 143-147.
- Savige JA, Chang L, Smith CL, Duggan JC (1993) Myelodysplasia, vasculitis and anti-neutrophil cytoplasm antibodies. Leuk Lymphoma 9: 49-54.
- Green AR, Shuttleworth D, Bowen DT, Bentley DP (1990) Cutaneous vascu- litis in patients with myelodysplasia. Br J Haematol 74: 364-365.
- Fernández-Miranda C, García-Marcilla A, Martín M, Gil R, Vanaclocha F, et (1994) [Vasculitis associated with a myelodysplastic syndrome: a report of 5 cases]. Med Clin (Barc) 103: 539-542.
- Pirayesh A, Verbunt RJ, Kluin PM, Meinders AE, De Meijer PH (1997) Myel- odysplastic syndrome with vasculitic J Intern Med 242: 425- 431.
- Manganelli P, Delsante G, Bianchi G, Fietta P, Quaini F (2001) Remitting seronegative symmetrical synovitis with pitting oedema in a patient with my- elodysplastic syndrome and relapsing polychondritis. Clin Rheumatol 20: 132-135.
- Kimura S, Kuroda J, Akaogi T, Hayashi H, Kobayashi Y, et (2001) Trisomy 8 involved in myelodysplastic syndromes as a risk factor for intestinal ulcers and thrombosis--Behçet’s syndrome. Leuk Lymphoma 42: 115-121.
- Becker K, Fitzgerald O, Green AJ, Keogan M, Newbury-Ecob R, et (2009) Constitutional trisomy 8 and Behçet syndrome. Am J Med Genet A 149A: 982-986.
- Ahn JK, Cha HS, Koh EM, Kim SH, Kim YG, et al. (2008) Behcet’s disease associated with bone marrow failure in Korean patients: clinical characteris- tics and the association of intestinal ulceration and trisomy Rheumatology (Oxford) 47: 1228-1230.
- Omiya W, Ujiie H, Akiyama M, Izumi K, Shigematsu A, et al. (2012) Coex- istence of pustular and vegetative pyoderma gangrenosum in a patient with myelodysplastic syndrome. Eur J Dermatol 22: 711-712.
- de Hollanda A, Beucher A, Henrion D, Ghali A, Lavigne C, et (2011) Sys- temic and immune manifestations in myelodysplasia: a multicenter retro- spective study. Arthritis Care Res (Hoboken) 63: 1188-1194.
- Fain O, Hamidou M, Cacoub P, Godeau B, Wechsler B, et (2007) Vasculit- ides associated with malignancies: analysis of sixty patients. Arthritis Rheum 57: 1473-1480.
- Oostvogels R, Petersen EJ, Chauffaille ML, Abrahams AC (2012) Systemic vasculitis in myelodysplastic syndromes. Neth J Med 70: 63-68.
- Sinico RA, Meroni P (2013) The kaleidoscopic manifestations of systemic Autoimmun Rev 12: 459-462.
- Lopez FF, Vaidyan PB, Mega AE, Schiffman FJ (2001) Aortitis as a manifestation of myelodysplastic syndrome. Postgrad Med J 77: 116-118.
- Shetty V, Allampallam K, Raza A (1999) Increased macrophages, high serum M-CSF and low serum cholesterol in myelodysplasia and Kawasaki Br J Haematol 106: 1068.
- Pilorge S, Doleris LM, Dreyfus F, Park S (2011) The autoimmune manifesta- tions associated with myelodysplastic syndrome respond to 5-azacytidine: a report on three cases. Br J Haematol 153: 664-665.
- Al Ustwani O, Francis J, Wallace PK, Ambrus J Jr, Wetzler M (2011) Treating myelodysplastic syndrome improves an accompanying autoimmune disease along with a reduction in regulatory T-cells. Leuk Res 35: 35-36.
- Wu L, Li X, Chang C, Ying S, He Q, et al. (2008) Deviation of type I and type II T cells and its negative effect on hematopoiesis in myelodysplastic Int J Lab Hematol 30: 390-399.
- Baumann I, Scheid C, Koref MS, Swindell R, Stern P, et al. (2002) Autolo- gous lymphocytes inhibit hemopoiesis in long-term culture in patients with myelodysplastic syndrome. Exp Hematol 30: 1405-1411.
- Sloand EM, Rezvani K (2008) The role of the immune system in myelodysplasia: implications for therapy. Semin Hematol 45: 39-48.
- Molldrem JJ, Jiang YZ, Stetler-Stevenson M, Mavroudis D, Hensel N, et al. (1998) Haematological response of patients with myelodysplastic syndrome to antithymocyte globulin is associated with a loss of lymphocyte-mediated inhibition of CFU-GM and alterations in T-cell receptor Vbeta profiles. Br J Haematol 102: 1314-1322.
- Sloand EM, Mainwaring L, Fuhrer M, Ramkissoon S, Risitano AM, et al. (2005) Preferential suppression of trisomy 8 compared with normal hema- topoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome. Blood 106: 841-851.
- Kochenderfer JN, Kobayashi S, Wieder ED, Su C, Molldrem JJ (2002) Loss of T-lymphocyte clonal dominance in patients with myelodysplastic syn- drome responsive to immunosuppression. Blood 100: 3639-3645.
- Melenhorst JJ, Eniafe R, Follmann D, Nakamura R, Kirby M, et al. (2002) Molecular and flow cytometric characterization of the CD4 and CD8 T-cell repertoire in patients with myelodysplastic syndrome. Br J Haematol 119: 97-105.
- Epperson DE, Nakamura R, Saunthararajah Y, Melenhorst J, Barrett AJ (2001) Oligoclonal T cell expansion in myelodysplastic syndrome: evidence for an autoimmune process. Leuk Res 25: 1075-1083.
- Plasilova M, Risitano A, Maciejewski JP (2003) Application of the molecular analysis of the T-cell receptor repertoire in the study of immune-mediated hematologic diseases. Hematology 8: 173-181.
- Meers S, Vandenberghe P, Boogaerts M, Verhoef G, Delforge M (2008) The clinical significance of activated lymphocytes in patients with myelodysplastic syndromes: a single centre study of 131 patients. Leuk Res 32: 1026-1035.
- Jacobs NL, Holtan SG, Porrata LF, Markovic SN, Tefferi A, et (2010) Host immunity affects survival in myelodysplastic syndromes: Independent prog- nostic value of the absolute lymphocyte count. Am J Hematol 85: 160-163.
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA (2010) FOXP3+ regula- tory T cells in the human immune system. Nat Rev Immunol 10: 490-500.
- Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, et (2007) CD4+C- D25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS). Blood 110: 847-850.
- Kotsianidis I, Bouchliou I, Nakou E, Spanoudakis E, Margaritis D, et al. (2009) Kinetics, function and bone marrow trafficking of CD4+CD25+FOXP3+ reg- ulatory T cells in myelodysplastic syndromes (MDS). Leukemia 23: 510-518.
- Mailloux AW, Sugimori C, Komrokji RS, Yang L, Maciejewski JP, et (2012) Expansion of effector memory regulatory T cells represents a novel prognos- tic factor in lower risk myelodysplastic syndrome. J Immunol 189: 3198-3208.
- Mailloux AW, Epling-Burnette PK (2013) Effector memory regulatory T-cell expansion marks a pivotal point of immune escape in myelodysplastic syn- Oncoimmunology 2: 22654.
- Alfinito F, Sica M, Luciano L, Della Pepa R, Palladino C, et (2010) Immune dysregulation and dyserythropoiesis in the myelodysplastic syndromes. Br J Haematol 148: 90-98.
- Chamuleau ME, Westers TM, van Dreunen L, Groenland J, Zevenbergen A, et (2009) Immune mediated autologous cytotoxicity against hematopoietic precursor cells in patients with myelodysplastic syndrome. Haematologica 94: 496-506.
- Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, et al. (2007) Reduced natural killer (NK) function associated with high-risk myelodysplas- tic syndrome (MDS) and reduced expression of activating NK receptors. Blood 109: 4816-4824.
- Marcondes AM, Mhyre AJ, Stirewalt DL, Kim SH, Dinarello CA, et (2008) Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelo- monocytic leukemia modulates apoptosis and impairs NK function. Proc Natl Acad Sci USA 105: 2865-2870.
- Epling-Burnette PK, List AF (2009) Advancements in the molecular pathogenesis of myelodysplastic syndrome. Curr Opin Hematol 16: 70-76.
- Carlsten M, Baumann BC, Simonsson M, Jädersten M, Forsblom AM, et al. (2010) Reduced DNAM-1 expression on bone marrow NK cells associated with impaired killing of CD34+ blasts in myelodysplastic syndrome. Leuke- mia 24: 1607-1616.
- Serio B, Risitano A, Giudice V, Montuori N, Selleri C (2014) Immunological derangement in hypocellular myelodysplastic Transl Med UniSa 8: 31-42.
- Koike M, Ishiyama T, Tomoyasu S, Tsuruoka N (1995) Spontaneous cytokine overproduction by peripheral blood mononuclear cells from patients with my- elodysplastic syndromes and aplastic anemia. Leuk Res 19: 639-644.
- Raza A, Mundle S, Iftikhar A, Gregory S, Marcus B, et (1995) Simultane- ous assessment of cell kinetics and programmed cell death in bone marrow biopsies of myelodysplastics reveals extensive apoptosis as the probable basis for ineffective hematopoiesis. Am J Hematol 48: 143-154.
- Kitagawa M, Saito I, Kuwata T, Yoshida S, Yamaguchi S, et (1997) Over- expression of tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma by bone marrow cells from patients with myelodysplastic syndromes. Leuke- mia 11: 2049-2054.
- Zang DY, Goodwin RG, Loken MR, Bryant E, Deeg HJ (2001) Expression of tumor necrosis factor-related apoptosis-inducing ligand, Apo2L, and its re- ceptors in myelodysplastic syndrome: effects on in vitro Blood 98: 3058-3065.
- Deeg HJ, Gotlib J, Beckham C, Dugan K, Holmberg L, et al. (2002) Soluble TNF receptor fusion protein (etanercept) for the treatment of myelodysplastic syndrome: a pilot study. Leukemia 16: 162-164.
- Li X, Wu L, Ying S, Chang C, Pu Q (2008) Clonality investigation of morpho- logically dysplastic hematopoietic cells in myelodysplastic syndrome mar- Int J Hematol 87: 176-183.
- Kondo A, Yamashita T, Tamura H, Zhao W, Tsuji T, et al. (2010) Interfer- on-gamma and tumor necrosis factor-alpha induce an immunoinhibitory mol- ecule, B7-H1, via nuclear factor-kappaB activation in blasts in myelodysplas- tic syndromes. Blood 116: 1124-1131.
- de Bruin AM, Voermans C, Nolte MA (2014) Impact of interferon-γ on hematopoiesis. Blood 124: 2479-2486.
- Zhang Z, Li X, Guo J, Xu F, He Q, et (2013) Interleukin-17 enhances the production of interferon-γ and tumour necrosis factor-α by bone marrow T lymphocytes from patients with lower risk myelodysplastic syndromes. Eur J Haematol 90: 375-384.
- Bouscary D, De Vos J, Guesnu M, Jondeau K, Viguier F, et al. (1997) Fas/ Apo-1 (CD95) expression and apoptosis in patients with myelodysplastic Leukemia 11: 839-845.
- Kitagawa M, Yamaguchi S, Takahashi M, Tanizawa T, Hirokawa K, et al. (1998) Localization of Fas and Fas ligand in bone marrow cells demonstrat- ing myelodysplasia. Leukemia 12: 486-492.
- Gersuk GM, Beckham C, Loken MR, Kiener P, Anderson JE, et (1998) A role for tumour necrosis factor-alpha, Fas and Fas-Ligand in marrow failure associated with myelodysplastic syndrome. Br J Haematol 103: 176-188.
- Lepelley P, Grardel N, Erny O, Iaru T, Obein V, et al. (1998) Fas/APO-1 (CD95) expression in myelodysplastic Leuk Lymphoma 30: 307- 312.
- Gupta P, Niehans GA, LeRoy SC, Gupta K, Morrison VA, et al. (1999) Fas ligand expression in the bone marrow in myelodysplastic syndromes cor- relates with FAB subtype and anemia, and predicts survival. Leukemia 13: 44-53.
- Deeg HJ, Beckham C, Loken MR, Bryant E, Lesnikova M, et (2000) Neg- ative regulators of hemopoiesis and stroma function in patients with myelo- dysplastic syndrome. Leuk Lymphoma 37: 405-414.
- Claessens YE, Bouscary D, Dupont JM, Picard F, Melle J, et (2002) In vi- tro proliferation and differentiation of erythroid progenitors from patients with myelodysplastic syndromes: evidence for Fas-dependent apoptosis. Blood 99: 1594-1601.
- Sloand EM, Pfannes L, Chen G, Shah S, Solomou EE, et al. (2007) CD34 cells from patients with trisomy 8 myelodysplastic syndrome (MDS) express early apoptotic markers but avoid programmed cell death by up-regulation of antiapoptotic proteins. Blood 109: 2399-2405.
- Sawanobori M, Yamaguchi S, Hasegawa M, Inoue M, Suzuki K, et (2003) Expression of TNF receptors and related signaling molecules in the bone marrow from patients with myelodysplastic syndromes. Leuk Res 27: 583- 591.
- Barkett M, Gilmore TD (1999) Control of apoptosis by Rel/NF-kappaB tran- scription factors. Oncogene 18: 6910-6924.
- Katsoulidis E, Li Y, Yoon P, Sassano A, Altman J, et (2005) Role of the p38 mitogen-activated protein kinase pathway in cytokine-mediated hematopoi- etic suppression in myelodysplastic syndromes. Cancer Res 65: 9029-9037.
- Zhou L, Opalinska J, Verma A (2007) p38 MAP kinase regulates stem cell apoptosis in human hematopoietic failure. Cell Cycle 6: 534-537.
- Navas T, Zhou L, Estes M, Haghnazari E, Nguyen AN, et (2008) Inhibition of p38alpha MAPK disrupts the pathological loop of proinflammatory factor production in the myelodysplastic syndrome bone marrow microenviron- ment. Leuk Lymphoma 49: 1963-1975.
- Peng H, Wen J, Zhang L, Li H, Chang CC, et (2012) A systematic model- ing study on the pathogenic role of p38 MAPK activation in myelodysplastic syndromes. Mol Biosyst 8: 1366-1374.
- Bachegowda L, Gligich O, Mantzaris I, Schinke C, Wyville D, et al. (2013) Signal transduction inhibitors in treatment of myelodysplastic syndromes. J Hematol Oncol 6: 50.
- Gañán-Gómez I, Bohannan ZS, Garcia-Manero G (2015) p38 MAPK in MDS. Aging (Albany NY) 7: 346-347.
- Wei Y, Chen R, Dimicoli S, Bueso-Ramos C, Neuberg D, et (2013) Global H3K4me3 genome mapping reveals alterations of innate immunity signaling and overexpression of JMJD3 in human myelodysplastic syndrome CD34+ cells. Leukemia 27: 2177-2186.
- Xiang Y, Zhu Z, Han G, Lin H, Xu L, et (2007) JMJD3 is a histone H3K27 demethylase. Cell Res 17: 850-857.
- Wei Y, Dimicoli S, Bueso-Ramos C, Chen R, Yang H, et al. (2013) Toll-like receptor alterations in myelodysplastic Leukemia 27: 1832-1840.
- Velegraki M, Papakonstanti E, Mavroudi I, Psyllaki M, Tsatsanis C, et al. (2013) Impaired clearance of apoptotic cells leads to HMGB1 release in the bone marrow of patients with myelodysplastic syndromes and induces TLR4-mediated cytokine production. Haematologica 98: 1206-1215.
- Hofmann WK, de Vos S, Komor M, Hoelzer D, Wachsman W, et al. (2002) Characterization of gene expression of CD34+ cells from normal and myelo- dysplastic bone marrow. Blood 100: 3553-3560.
- Starczynowski DT, Vercauteren S, Telenius A, Sung S, Tohyama K, et al. (2008) High-resolution whole genome tiling path array CGH analysis of CD34+ cells from patients with low-risk myelodysplastic syndromes reveals cryptic copy number alterations and predicts overall and leukemia-free sur- Blood 112: 3412-3424.
- Gondek LP, Tiu R, O’Keefe CL, Sekeres MA, Theil KS, et (2008) Chromo- somal lesions and uniparental disomy detected by SNP arrays in MDS, MDS/ MPD, and MDS-derived AML. Blood 111: 1534-1542.
- Lu YC, Yeh WC, Ohashi PS (2008) LPS/TLR4 signal transduction Cytokine 42: 145-151.
- Rhyasen GW, Bolanos L, Fang J, Jerez A, Wunderlich M, et al. (2013) Tar- geting IRAK1 as a therapeutic approach for myelodysplastic Can- cer Cell 24: 90-104.
- Rhyasen GW, Bolanos L, Starczynowski DT (2013) Differential IRAK signaling in hematologic malignancies. Exp Hematol 41: 1005-1007.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem Science 284: 143-147.
- Shiozawa Y, Havens AM, Pienta KJ, Taichman RS (2008) The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia 22: 941-950.
- Bianco P (2011) Bone and the hematopoietic niche: a tale of two stem Blood 117: 5281-5288.
- Kastrinaki MC, Pontikoglou C, Klaus M, Stavroulaki E, Pavlaki K, et (2011) Biologic characteristics of bone marrow mesenchymal stem cells in myelo- dysplastic syndromes. Curr Stem Cell Res Ther 6: 122-130.
- Geyh S, Oz S, Cadeddu RP, Fröbel J, Brückner B, et al. (2013) Insufficient stromal support in MDS results from molecular and functional deficits of mes- enchymal stromal cells. Leukemia 27: 1841-1851.
- Ferrer RA, Wobus M, List C, Wehner R, Schönefeldt C, et al. (2013) Mes- enchymal stromal cells from patients with myelodyplastic syndrome display distinct functional alterations that are modulated by Haemato- logica 98: 1677-1685.
- Boulais PE, Frenette PS (2015) Making sense of hematopoietic stem cell niches. Blood 125: 2621-2629.
- Teofili L, Martini M, Nuzzolo ER, Capodimonti S, Iachininoto MG, et (2015) Endothelial progenitor cell dysfunction in myelodysplastic syndromes: possi- ble contribution of a defective vascular niche to myelodysplasia. Neoplasia 17: 401-409.
- Cogle CR, Saki N, Khodadi E, Li J, Shahjahani M, et (2015) Bone marrow niche in the myelodysplastic syndromes. Leuk Res 39: 1020-1027.
- Rankin EB, Narla A, Park JK, Lin S, Sakamoto KM (2015) Biology of the bone marrow microenvironment and myelodysplastic Mol Genet Metab 116: 24-28.
- Aslan D, Garde C, Nygaard MK, Helbo AS, Dimopoulos K, et al. (2016) Tu- mor suppressor microRNAs are downregulated in myelodysplastic syndrome with spliceosome mutations. Oncotarget .
- Blau O, Hofmann WK, Baldus CD, Thiel G, Serbent V, et (2007) Chromo- somal aberrations in bone marrow mesenchymal stroma cells from patients with myelodysplastic syndrome and acute myeloblastic leukemia. Exp He- matol 35: 221-229.
- Zhao ZG, Xu W, Yu HP, Fang BL, Wu SH, et (2012) Functional character- istics of mesenchymal stem cells derived from bone marrow of patients with myelodysplastic syndromes. Cancer Lett 317: 136-143.
- Kastrinaki MC, Pavlaki K, Batsali AK, Kouvidi E, Mavroudi I, et al. (2013) Mesenchymal stem cells in immune-mediated bone marrow failure syn- Clin Dev Immunol 2013: 265608.
- Zhang Y, Zhao H, Zhao D, Sun L, Zhi Y, et al. (2012) SDF-1/CXCR4 axis in myelodysplastic syndromes: correlation with angiogenesis and Leuk Res 36: 281-286.
- Zhang Y, Guo Q, Zhao H, Zhao D, Wu X, et (2013) Expression of CXCR4 is an independent prognostic factor for overall survival and progression-free survival in patients with myelodysplastic syndrome. Med Oncol 30: 341.
- Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, et (2010) Bone progenitor dysfunction induces myelodysplasia and secondary leukae- mia. Nature 464: 852-857.
- Kusmartsev S, Gabrilovich DI (2006) Role of immature myeloid cells in mechanisms of immune evasion in Cancer Immunol Immunother 55: 237-245.
- Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regu- lators of the immune system. Nat Rev Immunol 9: 162-174.
- Ostrand-Rosenberg S, Sinha P (2009) Myeloid-derived suppressor cells:linking inflammation and cancer. J Immunol 182: 4499-4506.
- Condamine T, Gabrilovich DI (2011) Molecular mechanisms regulating my- eloid-derived suppressor cell differentiation and function. Trends Immunol 32: 19-25.
- Diaz-Montero CM, Finke J, Montero AJ (2014) Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol 41: 174-184.
- Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, et al. (2008) Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol 181: 4666-4675.
- Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J (2009) The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol 86: 557-566.
- Crocker PR, Paulson JC, Varki A (2007) Siglecs and their roles in the immune system. Nat Rev Immunol 7: 255-266.
- Cao H, Crocker PR (2011) Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation? Immunology 132: 18-26.
- Sanford D, Garcia-Manero G, Jorgensen J, Konoplev S, Pierce S, et al. (2016) CD33 is frequently expressed in cases of myelodysplastic syndrome and chronic myelomonocytic leukemia with elevated blast Leuk Lymphoma.
- Nakamura Y, Noma M, Kidokoro M, Kobayashi N, Takei M, et (1994) Ex- pression of CD33 antigen on normal human activated T lymphocytes. Blood 83: 1442-1443.
- Hernández-Caselles T, Martínez-Esparza M, Pérez-Oliva AB, Quintanil- la-Cecconi AM, García-Alonso A, et al. (2006) A study of CD33 (SIGLEC-3) antigen expression and function on activated human T and NK cells: two iso- forms of CD33 are generated by alternative J Leukoc Biol 79: 46-58.
- Tanimoto M, Scheinberg DA, Cordon-Cardo C, Huie D, Clarkson BD, et al. (1989) Restricted expression of an early myeloid and monocytic cell surface antigen defined by monoclonal antibody M195. Leukemia 3: 339-348.
- Naito K, Takeshita A, Shigeno K, Nakamura S, Fujisawa S, et al. (2000) Calicheamicin-conjugated humanized anti-CD33 monoclonal antibody (gemtuzumab zogamicin, CMA-676) shows cytocidal effect on CD33-posi- tive leukemia cell lines, but is inactive on P-glycoprotein-expressing Leukemia 14: 1436-1443.
- Imrichova D, Messingerova L, Seres M, Kavcova H, Pavlikova L, et (2015) Selection of resistant acute myeloid leukemia SKM-1 and MOLM-13 cells by vincristine-, mitoxantrone- and lenalidomide-induced upregulation of P-gly- coprotein activity and downregulation of CD33 cell surface exposure. Eur J Pharm Sci 77: 29-39.
- Gluckman E, Devergie A, Faille A, Bussel A, Benbunan M, et al. (1979) An- tilymphocyte globulin treatment in severe aplastic anemia--comparison with bone marrow Report of 60 cases. Haematol Blood Transfus 24: 171-179.
- Molldrem JJ, Caples M, Mavroudis D, Plante M, Young NS, et al. (1997) Antithymocyte globulin for patients with myelodysplastic syndrome. Br J Haematol 99: 699-705.
- Killick SB, Mufti G, Cavenagh JD, Mijovic A, Peacock JL, et (2003) A pilot study of antithymocyte globulin (ATG) in the treatment of patients with ‘low- risk’ myelodysplasia. Br J Haematol 120: 679-684.
- Steensma DP, Dispenzieri A, Moore SB, Schroeder G, Tefferi A (2003) Anti- thymocyte globulin has limited efficacy and substantial toxicity in unselected anemic patients with myelodysplastic syndrome. Blood 101: 2156-2158.
- Lim ZY, Killick S, Germing U, Cavenagh J, Culligan D, et (2007) Low IPSS score and bone marrow hypocellularity in MDS patients predict hematologi- cal responses to antithymocyte globulin. Leukemia 21: 1436-1441.
- Jonásova A, Neuwirtová R, Cermák J, Vozobulová V, Mociková K, et al. (1998) Cyclosporin A therapy in hypoplastic MDS patients and certain re- fractory anaemias without hypoplastic bone marrow. Br J Haematol 100: 304-309.
- Chen S, Jiang B, Da W, Gong M, Guan M (2007) Treatment of myelodysplas- tic syndrome with cyclosporin Int J Hematol 85: 11-17.
- Neukirchen J, Platzbecker U, Sockel K, Tsamaloukas A, Haas R, et al. (2014) Real life experience with alemtuzumab treatment of patients with lower-risk MDS and a hypocellular bone marrow. Ann Hematol 93: 65-69.
- McGavin JK, Spencer CM (2001) Gemtuzumab Drugs 61: 1317- 1322.
- Sievers EL, Larson RA, Stadtmauer EA, Estey E, Löwenberg B, et (2001) Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-pos- itive acute myeloid leukemia in first relapse. J Clin Oncol 19: 3244-3254.
- Sievers EL, Linenberger M (2001) Mylotarg: antibody-targeted chemothera- py comes of age. Curr Opin Oncol 13: 522-527.
- Raza A, Fenaux P, Erba H, Mandelli F, Schiller G et al. (2002) Preliminary analysis of a randomized phase 2 study of of the safety and efficacy of 1 vs 2 doses of Gemtuzumab Ozogamicin(Mylotarg ®) in patients with high risk myelodysplastic syndrome. Blood 100: 3140.
- Estey EH, Thall PF, Giles FJ, Wang XM, Cortes JE, et (2002) Gemtuzum- ab ozogamicin with or without interleukin 11 in patients 65 years of age or older with untreated acute myeloid leukemia and high-risk myelodysplastic syndrome: comparison with idarubicin plus continuous-infusion, high-dose cytosine arabinoside. Blood 99: 4343-4349.
- Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, et al. (2014) CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood 123: 3016-3026.
- Chang YJ, Huang XJ (2013) Donor lymphocyte infusions for relapse after allogeneic transplantation: when, if and for whom? Blood Rev 27: 55-62.
- Avigan D (1999) Dendritic cells: development, function and potential use for cancer immunotherapy. Blood Rev 13: 51-64.
- Grabrucker C, Liepert A, Dreyig J, Kremser A, Kroell T, et al. (2010) The quality and quantity of leukemia-derived dendritic cells from patients with acute myeloid leukemia and myelodysplastic syndrome are a predictive fac- tor for the lytic potential of dendritic cells-primed leukemia-specific T J Immunother 33: 523-537.
- Pyzer AR, Avigan DE, Rosenblatt J (2014) Clinical trials of dendritic cell- based cancer vaccines in hematologic malignancies. Hum Vaccin Immuno- ther 10: 3125-3131.
- Kuball J, de Boer K, Wagner E, Wattad M, Antunes E, et (2011) Pitfalls of vaccinations with WT1-, Proteinase3- and MUC1-derived peptides in com- bination with MontanideISA51 and CpG7909. Cancer Immunol Immunother 60: 161-171.
- Di Stasi A, Jimenez AM, Minagawa K, Al-Obaidi M, Rezvani K (2015) Review of the Results of WT1 Peptide Vaccination Strategies for Myelodysplastic Syndromes and Acute Myeloid Leukemia from Nine Different Studies. Front Immunol 6: 36.
- Tamaki H, Ogawa H, Ohyashiki K, Ohyashiki JH, Iwama H, et (1999) The Wilms’ tumor gene WT1 is a good marker for diagnosis of disease progres- sion of myelodysplastic syndromes. Leukemia 13: 393-399.
- Sloand EM, Melenhorst JJ, Tucker ZC, Pfannes L, Brenchley JM, et al. (2011) T-cell immune responses to Wilms tumor 1 protein in myelodysplasia responsive to immunosuppressive therapy. Blood 117: 2691-2699.
- Brayer J, Lancet JE, Powers J, List A, et al. (2015) WT1 vaccination in AML and MDS: A pilot trial with synthetic analog Am J Hematol 90: 602- 607.
- Xia Y, Medeiros LJ, Young KH (2015) Immune checkpoint blockade: Releasing the brake towards hematological malignancies. Blood Rev .
- Garcia-Manero G, Khoury HJ, Jabbour E, Lancet J, Winski SL, et (2015) A phase I study of oral ARRY-614, a p38 MAPK/Tie2 dual inhibitor, in patients with low or intermediate-1 risk myelodysplastic syndromes. Clin Cancer Res 21: 985-994.
- Zhou L, McMahon C, Bhagat T, Alencar C, Yu Y, et al. (2011) Reduced SMAD7 leads to overactivation of TGF-beta signaling in MDS that can be reversed by a specific inhibitor of TGF-beta receptor I kinase. Cancer Res 71: 955-963.
- Liu X, Shin N, Koblish HK, Yang G, Wang Q, et al. (2010) Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 115: 3520-3530.
Citation: Fuchs O (2016) The Immune Mechanisms Involved in the Pathogenesis and Pathophysiology of Myelodysplastic Syndromes and Immunotherapeutic Strategies. J Hematol Hemother 1: 001.
Copyright: © 2016 Fuchs O. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.