*Corresponding Author:
Michael Halim,
University of Salford, MSc Biomedical Sci- ence, Greater Manchester, United Kingdom
Tell: 081387656882
Email: michael-halim1000@gmail.com
Abstract
P53 tumor suppressor gene is widely known for its critical behavior that is related to damaging nuclear metabolism in the human body, which further contributes in increasing chronic activations. It is also explored that as there is an increase in the changing lifestyle and eating patterns, a number of chronic diseases are induced due to which human body cells are damaged and thus, incurable diseases are diagnosed. China is reported to lie in the category of most diabetic vulnerable countries in the world due to a number of patients being reported having type 1 or type 2 diabetes. Both types occur due to different reasons but are mainly treated using similar medical tools and techniques. In this literature review, P53 tumor suppressor gene, which plays a major role in diabetes mellitus (type2 diabetes), is also investigated.
Keywords
Diabetes mellitus; Metabolic pathways; P53
Introduction
Across the globe, diabetes has become one of the most common diseases, which is prevalent due to a number of reasons including increasing life expectancy, changed lifestyle, as well as growing obesity problems. It is investigated in WHO projects that diabetes is predicted to be 7th most leading cause of cancer by 2030, which is now becoming a challenging situation for entire nations [1]. Also, there are a number of cases that have been reported in international hospitals which clearly indicates that most of the patients are diagnosed with diabetes due to which there are growing chances of spread of the disease in coming years. International Diabetic Federation (IDF) reports that at global level [2], there are approximately 382 million patients suffering from diabetes whereas there is an expected growth in the rate by 2035 [3].
Through investigating Asian countries, India is reported to diagnose approximately 65.1 million people with diabetes whereas China is the second largest country in the world which has almost 98.4 million diabetic patients that are diagnosed at its medical institutes [4]. There is an extensive research on diabetes and thus, statistics and information in these researches are still in an upgrade phase due to changes in its nature and acting bodies that result in further complications for metabolism [5]. Furthermore, it is also revealed that most of the researchers have identified that diabetes is basically an initial step towards chronic diseases, in which some of them are treated while rest of them transform into cancerous nature. Palmer illustrates that diabetes is misinterpreted due to its relation with obesity however, apart from its integral relation with latter disease; there are a number of reasons that cause diabetes in a human body. Table 1 shows distribution of affected populations of different regions in the world.

Table 1: High Blood Glucose Age-Standardized in Different Regions (Source: Glob- al Report, WHO).nutrients; Hb=Hemoglobin; Vit= Vitamin.
Major diabetic cases in different regions have indicated that there are some molecular conditions which contribute in metabolic disorder. It is found that are there some proteins which contains anti-diabetic effects with which transcriptional activation of genes is regulated. Among all these proteins, P53 tumor protein is identified as one of the diabetes pathogenesis which regulates at posttranslational level [6]. The following paper is developed to understand pivotal role of P53 tumor protein in type 2 diabetes. Moreover, it aims to investigate background of diabetes by illustrating its types and major complications while discussing complete structure of the protein that exhibits anti-diabetes effects. Furthermore, it is also important to investigate some of the medicinal practices that are involved in determining activation with respect to metabolic disorder [4].
Types of Diabetes Mellitus
According to the study proposed by [7], the author explains that diabetes mellitus is one of the primary diseases, which is often associated with prevention of the body from useful energy that is gained through eating food and experiencing basic lifestyle activities.
It is also illustrated in the paper that diabetes often occur in different situations and thus, an individual has to suffer from various health issues that affect its metabolic system completely. Pancreas in the body is related to producing little to no insulin through beta cells and thus, requires the body to use excessive sugar or energy [8]. However, when the pancreas does not function appropriately, it increases chances of diabetes in the body [3].
The mechanism of diabetes is also described by [5], which stresses on the cells that contribute in making energy in the body. The paper explains that the food eaten by individual is broken down into sugary substances, often known as glucose, which is responsible in developing energy in the body that further enable it to perform daily activities in an active manner [4]. In the body, a number of blood vessels are present that are responsible in transporting sugar to different organs of the body where there are different types of nutrients are stored, such as stomach, liver, muscles etc [2]. However, it is medically proved that sugar does not travel itself to the cells due to which pancreas is explored to have certain functions in triggering transportation of glucose [1]. In the whole process, pancreas has the role of producing insulin in the blood, which further acts as a key to help sugar penetrating in the body for developing internal energy.
The process of internal sugar transmission and energy development is further added by [9], which states that sugar level is identified to be lowered when sugar leaves the blood stream and penetrates in the cell during the function. In this way, pancreas is unable to produce insulin and thus, enough sugar is not supplied to body cells that are responsible in developing energy in the body [6]. The consequences of such malfunctioning process results in increased sugar level, which causes individual to experience major health disabilities. The paper also illustrates that diabetes is developed in two different stages, which have different malfunctioning processes and affects human body in different ways [10] (Table 2).
Table 2: Characteristics of Diabetes Mellitus [11].
Types of Diabetes
Based on the study developed by [5] the author emphasizes on inadequate function of pancreas that increases diabetic symptoms and thus, an individual suffers from such disability. It is explored in the research that there are two main types of diabetes which are the consequence of inadequate insulin production in the body or even impaired response to insulin. From medical perspectives and information collected from different healthcare institutes, the author indicates that two types of diabetes are present, which have different functions, symptoms, and treatment mechanisms [12]. Both of the types are associated with chronic disabilities of body and hence, people have to undergo numerous phases for the identification of the type of diabetes [13].
Type 1 diabetes is primary internal disability of the human body which is related to damage of beta cells that are responsible for producing insulin in the cells of pancreas. Ryan claims that there are almost 10% of the cases in every region which often leads to chronic conditions when pancreatic beta cells are dead or even insulin production is greatly affected. It is further explored that type 1 diabetes patients have to use insulin injections in most of the times so that their blood glucose could be controlled easily [14]. The paper also mentions that type 1 diabetes is common among the people who are under 30 years of age however, due to increasing internal adverse functions, the disease can occur at any age [15].
Another type of disease is type 2 which is related to adult diabetic conditions and thus, mostly adults have to suffer from pancreatic malfunctioning processes. According to [2] type 2 cases are reported to include pancreas producing insulin but in limited or insufficient quantity. However, sometimes, medical reports indicate that if pancreas is producing enough insulin then it is not performing in a way that it should be. Apart from this, it is also explored that out of 10 patients, 9 of them are diagnosed with type 2 diabetes and mainly occurs above 40 years of age [4]. Despite the facts about internal disturbances among functions of pancreas, type 2 is likely to occur in childhood as well only if there are increasing risk factors related to the disease. In terms of lessening the effects of type 2, doctors recommend controlling diet, excessive workout, and weight management [3]. However, treatment of such disease is related to oral intake of glucoselowering medications and eve insulin injections [1].
Structure of p53 Tumor Suppressor Protein
As discussed in latter section, P53 tumor protein has the antidiabetes effects, which contributes in regulating transactional activities [16]. Defines the structure of tumor protein that is built upon N-terminal transcriptional activation domain, DNA binding domain, and C terminus with oligomeric and similar activities. The author explains that main function of P53 is to perform according to transcription while it has the ability to regulate more than 500 genes. As illustrated in Figure 1, 393 residue polypeptide form N-terminal to C-terminal are present in its functional domains which are responsible in regulating stress-activated and sequence-specific DNA binding which is also linked with transcription factor [17].
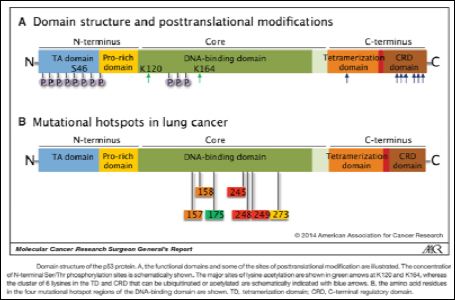
Figure 1: P53 Tumor Structure.
From the above figure, it is noticed that N-terminal comprises of 1-43 residues, which mainly contains transcription functions [15]. However, 364-393 residues in C-terminal are termed as negative regulatory domain, which have the capability to bind DNA based sequencing process. Apart from this, 100-300 residues are present in the central domain that are identified as DNA binding activators ad thus, their location also contributes in containing oncogenic P53 mutations [12]. There is another important domain in P53 i.e. proline rich which has the ability to bind Mdm2 with N-terminal and C-terminal domains. It is studied in the paper proposed by Barnes that P53 contains binding functions which binds DNA with Zinc as cofactor while the connection is established as homotetramer [18].
During inavtive state, P53 is transformed as cytoplasmic that contains exposure to stress and thus, accumulation in the nucleus is resultant while biochemical effects in the nucleus are evident as well [4]. Basically, responses with respect to P53 are often subjected to controlled and finely-tuned functions that are present at different levels. When activating signals does not function in the process, P53 is then repressed by the oncoprotein with respect to Mdm2 and Mdm4. It is explored in the research developed by [19] Mdm2 repressor is mainly linked with masking transactivation domain and comprises of E3 ligase which is not present in Mdm4 but has the ability to repress transactivation of P53.
It is also found in the paper of [20]. That P53 plays a major role in gene expression and their stability and thus, performs significant functions that are closely linked with these types of genes. It is investigated in the research that P53 tumor suppressor has capability to become activated and stabilize cellular stress and its various types [2]. Based on the illustrated mechanism of P53 in Figure 2, it is identified that there are different signaling pathways that have covered major area of P53/Mdm2/Mdm4 that is able to release p53 from its repressors and thus, its functions are able to regulate downstream transcription in which genes are involved in attaining cellular responses in various ways [3]. Soon after translocation is performed in the nuclear side, P53 starts to act as transcriptor that is related to binding tetramer to cis-regulatory areas of genes. In addition to this, P53 also involves general transcription factors of RNAPII initiation machinery with the aim to relocate target genes [15].
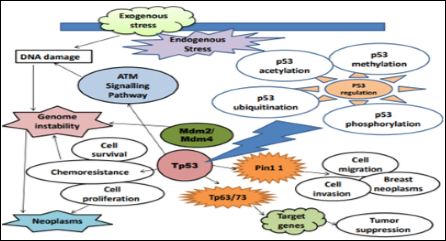
Figure 2: Cellular Mechanism of P53.
The structure of P53 tumor suppressor has other properties as well which are associated with many other proteins involved in the mechanism and thus, they are responsible in interacting with each other in different phases of the RNAPII transcription cycle [1]. In addition, there is also a physical interaction identified in P53 that occurs within cyclin-dependent kinase CDK9 and thus, Churm reports its inactivation of its catalytic activity through tiny molecules including Flavopiridol (FP), which is found to emphasize on blockage held among mRNA synthesis in living cells while it in turn triggers P53 activation.
Influence of P53 on Type 2 Diabetes
P53 in type 2 diabetes was first investigated in 2009 when there was an experimental practice conducted by Minamino and his colleagues with respect to insulin and diet resistance in Ay transgenic mice that are mainly vulnerable to type 2 diabetes while it is mediated by P53 [21]. The investigation revealed that inhibition of P53 activity is due to disturbances in siRNA cells or by TP53 gene. These in turn were witnessed to alleviate senescence while also lessened amount of inflammatory cytokine expression in the adipose tissue of the mice on which the experiment was actually conducted. The findings of the results declare that due to the activation of these cells, development of insulin was hindered [12].
The research developed by [22]. indicates that chronic hyperglycameia is often associated with mitochondrial dysfunction which is basically induced while increases production of glycation end products (AGEs), as well as protein kinase C activation, and other pathways, which altogether has immediate effect on the Reactive Oxygen Species (ROS) overproduction and oxidative stress [15]. It is also ensured by the author that ROS has also capability of damaging cells due to which oxidative lipids, proteins, and DNA are also affected [3]. The findings of the research reveal that due to these changes oxidative damages at the nuclear level, P53 tumor is activated which in turn regulates genes, such as apoptotic, pro-inflammatory, and metabolic [4]. Thus, hyperglycaemia is responsible in increasing P53 tumor level in the genes which are considered to play a vital role in the development of metabolism disabilities while other vascular complications related to type2 diabetes are recorded.
In another study conducted by [23] the author addresses that type 2 diabetes patients are main targets of P53 tumor, which are found to be in higher level as compared to other primary controls. The study further describes that in type 2 diabetes patients, level of P53 tumor increases with age and time and thus, chronic hyperglycaemia is found to be most chronic in terms of increasing P53 tumor. It is further explored in the study of [24] that increasing levels of glucose also affects apoptosis which are primarily associated with large amount of P53 tumor expression and proapoptotic factor (BAX). In the experiment conducted in 2009, [25] also relates that pancreatic beta cells are also exposed to higher level of glucose which is found to be chronic while also relates with stabilizing P53 tumor that is caused by low level of Mdm2 expressions ad degradation of P53 tumor [15].
There are other researches as well which sheds light on major influence of P53 tumor in affecting type 2 diabetes patients and thus, their relevant evidence supports the process in a positive way As mentioned [26,27]. Illustrates in the research that human endothelial cells are treated with increasing amount of glucose, which induces P53 tumor in endothelial senescence and thus, it gradually decreases SIRT1 expression. In another area of the research, the author describes that SIRT1 contributes in deregulating P53 tumor activity by using deacetylation. In relation to this, in human endothelial Eahy cells, AGE is induced in the cells which as a result reduces level of SIRT1 while increases P53 tumor level Furthermore [2,28]. Studied about the impact of P53 tumor on humans for which different levels of effects are reported.
In the previous researches, the influence of P53 tumor in type 2 diabetes is evident which were compared with the patients of impaired glucose tolerance, as well as normoglycaemics [29,30]. Explains that results of these relationships were identified as contrast. The results show that relevant indications were not adequately determining extent of influence that is caused by P53 tumor due to which, researches in the following area might differ with those that have outlined significant details [12]. Thus, it is noticed in the research that level of type 2 diabetes patients and its controls are not significant enough to prove the claim [1].
The influence of P53 tumor is also explored in the research developed by different regulators associated with metabolic pathways, such as tumor development caused by metabolic pathways while they are known to enhance metabolic pathways that are also considered to be anti-tumorigenic including fatty acid oxidation. By understanding various concepts and internal mechanism with respect to P53 tumor [31,32] also highlights that in type 2 diabetes patients, P53 tumor acts as a promoting agent which increases expression of gluconeogenesisrelated genes while further develops hepatic glucose production thorough incorporating liver cells in a human body that comprises of varying statuses of P53 tumor. Hence, the study concludes that the role of P53 tumor is critical in the pathogenesis of obesity and thus, type 2 diabetes patients are primarily investigated in this regard [3].
The paper conducted by [33]. Also shows that due to excessive intake of calories, there is an increase in the level of obesity which further results in the stress oxidation in the adipose tissue of an individual’s metabolism and thus, type 2 diabetes like diseases become popular in the health risks [15]. This is also responsible in promoting senescence-like changes for instance, increased level of P53 tumor, as well as large production of pro-inflammatory cytokines [34]. Also supports the findings that 12-wk high fat diet in people are also known as basic causes of increased P53 tumor level which occur in tissues, such as adipose, muscle, and even small intestine. In relation with latter findings [35] also explores that when an individual takes high amount of calories in their diet then there are the chances of increased endothelial expression of P53 tumor among them and thus, major effects are resulted in organs and tissues. In this way, it is clear that such diabetic condition also results into different expressions, including hyperglycaemia and hyperinsulinaemia which contributes in increased regulation of P523 tumor. This also shows that a number of different tissues are involved through an increased amount of oxidative stress [4].
As it can be seen that in order to understand the relation between P53 and type 2 diabetes patients, an experiment was associated, this ensures that human internal mechanisms are completely similar to that of a mouse [2]. A number of theorists and researchers have been investigated to gather information regarding basic links between the states and thus, it is explored that P53 tumor trigger expressions of different activators and cells in the body that results in increasing diabetes level and thus, type 2 cells are mainly determined in the organs and tissues [3]. These researches have widely provided evidence of the influence which is significant as it not only affects nuclear performances but also damages complete functions of cells that are important in retaining metabolism [31].
From the present results of research, it is also explored that each of the concept and claims are aligned with the findings associated with animal models induced with diabetes and obesity, and thus, it is clear that type 2 diabetes patients are found to have higher level of P53 tumor in their cells that affects their tissues and organs greatly [12]. Moreover, increased duration and value of WHR is also related to the consequence of P53 tumor that affects diabetes to the greatest extent. Hence, the study provides clear evidence of the nature of tumor that plays a critical role in developing diabetic and obesityrelated mutations in the body.
It is also reviewed in the article produced by Halim and Alice Halim that role of P53 in type2 diabetes in terms of mitochondrial, regulation, and glycolysis is controversial and contradictory. The article includes some of the complexities related to P53 which are mainly due to the induction of target genes that are related to different types of cells while in the presence or absence from different extents of stresses. It is also studied in the proposed research that P53 tumor involves various processes which includes different mechanisms in choosing target genes while also whether cell fate is complex or elusive. Furthermore, there are other factors as well which have a major role in the selection of target genes including P53 expression, posttranslational modifications, presence of cofactors, and sequencebased affinities in P53 binding [36].
Conclusion
In human physiology, glucose is considered as one of the significant substance that is required by the body to increase internal energy with respect to contributing in the daily routine activities in a more appropriate way. It is explored in the research that glucose plays a vital role in organisms and thus, it is transported from one energy source to another through various cellular organisms, such as bacteria, yeasts, and even worms. It is studied in the research that diabetes is one of the most common diseases in the healthcare, which has impacted economies to the greatest extent. By investigating statistical analysis of diabetes, China is under threat of increasing disease, which is reported to further increase by 2035. The main reason behind conducting the study was to explore different types of diabetes that are common among patients and how P53 tumor suppressor influences molecular cells in the body that further activates negative functions and thus, human body is induced with chronic tumor cells. A number of researches have been explored in the research which emphasizes on the structure of P53 tumor and its internal domains that contribute in increasing internal metabolism of negative cells. In this regard, it is studied that P53 tumor comprises of three different domains while each domain has a total of 393 residues that have varying functions. Later in the research, influence of P53 on type2 diabetes is studies which ensure that it has a direct relation in increasing the internal metabolism and thus, P53 tumor works to increase diabetic level in the body, causing tissues and organs to damage.
References
- Papatheodorou K, Papanas N, Banach M, Papazoglou D, Edmonds M (2016) Complications of diabetes Journal of diabetes research 2016.
- Itahana Y, Itahana K (2018) Emerging roles of p53 family members in glu- cose metabolism. International Journal of Molecular Sciences 19: 776.
- Kung CP, Murphy ME (016) The role of the p53 tumor suppressor in me- tabolism and diabetes. The Journal of endocrinology 231: R61.
- Murtaza I, Laila O (2016) P53 TRANSCRIPTION FACTOR AND DIABE- TES: IS THERE ANY LINK?.
- Sliwinska A, Kasznicki J, Kosmalski M, Mikołajczyk M, Rogalska A, et (2017) Tumour protein 53 is linked with type 2 diabetes mellitus. The Indi- an journal of medical research 146: 237-243.
- Ryan JG (2015) Emerging Diabetes Research: Prevention, Risk and Man- Clinical therapeutics 37: 1169-1171.
- Samarghandian S, Farkhondeh T, Samini F (2017) Honey and health: A review of recent clinical research. Pharmacognosy research 9: 121.
- Carrasco-Garcia E, Moreno M, Moreno-Cugnon L, Matheu A (2017) In- creased Arf/p53 activity in stem cells, aging and Aging cell 16: 219- 225.
- Zhao N, Li J, Li L, Niu XY, Jiang M, et (2015) Molecular network-based analysis of Guizhi-Shaoyao-Zhimu decoction, a TCM herbal formula, for treatment of diabetic peripheral neuropathy. Acta Pharmacologica Sini- ca 36: 716.
- Belgardt BF, Ahmed K, Spranger M, Latreille M, Denzler R, et (2015) The microRNA-200 family regulates pancreatic beta cell survival in type 2 diabetes. Nature medicine 21: 619.
- Jin G, Xiao W, Ding X, Xu X, An L, et (2018) Prevalence of and Risk Fac- tors for Diabetic Retinopathy in a Rural Chinese Population: The Yangxi Eye Study. Investigative Ophthalmology & Visual Science 59: 5067-5073.
- Krug EG (2016) Trends in diabetes: Sounding the The Lancet 387: 1485-1486.
- Hameed I, Masoodi SR, Mir, Nabi M, Ghazanfar K, et al. (2015) Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condi- World journal of diabetes 6: 598-612.
- Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, et al. (2015) Cellular senescence in type 2 diabetes: A therapeutic opportunity. Diabe- tes 64: 2289-2298.
- Halim M, Halim A (2019) The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes & Metabolic Syndrome 13: 1165-1172.
- Gong H, Yang X, Zhao Y, Petersen RB, Liu X, et (2015) Amyloidogenic- ity of p53: A hidden link between protein misfolding and cancer. Current Protein and Peptide Science 16: 135-146.
- Gorin Y, Cavaglieri RC, Khazim K, Lee DY, Bruno F, et al. (2015) Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. American Journal of Physiology-Renal Physiology 308: F1276-F1287.
- Barnes PJ (2015) Mechanisms of development of multimorbidity in the European Respiratory Journal 45: 790-806.
- Gordon MW, Yan F, Zhong X, Mazumder PB, Xu-Monette ZY, et (2015) Regulation of p53‐targeting microRNAs by polycyclic aromatic hydrocarbons: Implications in the etiology of multiple myeloma. Molecular carcinogenesis 54: 1060-1069.
- Capone F, Guerriero E, Colonna G, Maio P, Mangia A, et al. (2015) The cytokinome profile in patients with hepatocellular carcinoma and type 2 » PLoS One 10: e0134594.
- Kuricová K, Pácal L, Šoupal J, Prázný M, Kaňková K (2016) Effect of glucose variability on pathways associated with glucotoxicity in diabetes: Evaluation of a novel in vitro experimental approach. Diabetes research and clinical practice 114: 1-8.
- Busquets O, Ettcheto M, Pallàs M, Beas-Zarate C, Verdaguer E, et (2017) Long-term exposition to a high fat diet favors the appearance of β-amyloid depositions in the brain of C57BL/6J mice. A potential model of sporadic Alzheimer’s disease. Mechanisms of ageing and development 162: 38-45.
- Basu S, Murphy ME (2016) Genetic modifiers of the p53 pathway. Cold Spring Harbor perspectives in medicine 6: a026302.
- Tugay K, Guay C, Marques AC, Allagnat F, Locke JM, et (2016) Role of microRNAs in the age-associated decline of pancreatic beta cell function in rat islets. Diabetologia 59: 161-169.
- Mantovani A, Targher G (2017) Type 2 diabetes mellitus and risk of hepatocellular carcinoma: Spotlight on nonalcoholic fatty liver disease. Annals of translational medicine 5: 270.
- Lu Z, Liu N, Wang F (2017) Epigenetic regulations in diabetic Journal of diabetes research 2017.
- Sturmlechner I, Durik M, Sieben CJ, Baker DJ, Deursen JMV, et al. (2017) Cellular senescence in renal ageing and disease. Nature Reviews Nephrology 13: 77-89.
- Stefani GP, Baldissera G, Nunes RB, Heck TG, Rhoden CR (2015) Metabolic Syndrome and DNA Damage: The Interplay of Environmental and Lifestyle Factors in the Development of Metabolic Open Journal of Endocrine and Metabolic Diseases 5: 65.
- Churm R, Davies JS, Stephens JW, Prior SL (2017) Ghrelin function in human obesity and type 2 diabetes: A concise Obesity reviews 18: 140-148.
- Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, et al. (2017) Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox biology 11: 637-645.
- Deng D, Yang Y, Tang X, Skrip L, Qiu J, et (2015) Association between metformin therapy and incidence, recurrence and mortality of prostate cancer: Evidence from a meta‐analysis. Diabetes metabolism research and reviews 31: 595-602.
- Suarez AA, Felix AS, Cohn DE (2017) Bokhman redux: endometrial cancer “types” in the 21st century. Gynecologic oncology 144: 243-249.
- Gonzalez-Menendez P, Hevia D, Mayo JC, Sainz RM (2018) The dark side of glucose transporters in prostate cancer: Are they a new feature to characterize carcinomas?. International journal of cancer 142: 2414-2424.
- Kung CP, Basu S, Murphy ME (2016) A link between TP53 polymorphisms and metabolism. Molecular & cellular oncology 3: e1173769.
- Hardeland R (2017) Melatonin and the pathologies of weakened or dysregulated circadian oscillators. Journal of pineal research 62: e12377.
- ter Braak B, Siezen C, Speksnijder EN, Koedoot E, Steeg Hv, et al. (2015) Mammary gland tumor promotion by chronic administration of IGF1 and the insulin analogue AspB10 in the p53 R270H/+ WAPCre mouse model. Breast cancer research 17: 14.
Citation: Halim M, Halim A, Trivosa V (2020) The Crucial Role of P53 Tumor Sup- pressor Gene in Regulating Metabolic Pathways That Reduce the Risks of Developing Diabetes Mellitus. J Diab Meta Syndro 3: 012.
Copyright: © 2020 Halim M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


