*Corresponding Author:
Desmond Alexander Jackson,
Division of Rheumatology, University Hospitals Cleveland Medical Center, Cleve- land, OH, USA
Tel: +216 8442289
Fax: +216 8442288
Email: Desmond.Jackson@uhhospitals.org
Abstract
Granulomatosis with Polyangiitis (GPA) is an anti-neutrophil cytoplasmic auto anti body (ANCA)-associated vasculitis (AAV) that can have different systemic presentations, with a limited form that can isolate to upper respiratory tract, nasal tract, sinuses, ears, or trachea. Hearing loss is often caused by otitis media or less commonly sensorineural hearing loss. Rituximab is one agent studied for use as an induction therapy in GPA and is also one option for maintenance therapy. We demonstrate a case of a 69 year old female with sudden, mixed bilateral sensorineural and conductive hearing loss on audiometry studies, and arthralgia that was diagnosed as having early, limited GPA based on the European Medicines Agency criteria and expert rheumatology opinion. Due to difficulty titrating down on high dose prednisone and the fact she was refractory to azathioprine, we utilized rituximab for her disease, which worked well as demonstrated by the key indicators of prevention of relapse and substantially decreased need for glucocorticoids over her last two years of treatment. Review of the literature shows a few cases of the use of cyclophosphamide and one case of rituximab used in cases of GPA with hearing loss as its predominant feature. Unlike in those cases, our patient presented with early, limited GPA with predominantly sudden hearing loss as her concern, but no pulmonary-renal findings, making it a unique case in which rituximab served as an effective alternative to treat the otologic manifestation of GPA. It is up to the clinician’s discretion to evaluate the risk versus benefits of rituximab. Most notable risk or side effects of rituximab include infusion reactions, infection, and immunodeficiency. More research and trials with rituximab as a potential maintenance therapy for limited vasculitis are required.
Background
Granulomatosis with Polyangiitis (GPA), formerly known as Wegener’s Granulomatosis, is a multisystem autoimmune disease of unknown etiology that features necrotizing granulomatous inflammation and pauci-immune vasculitis in small blood vessels that are characterized by the presence of circulating Anti-neutrophil Cytoplasmic Antibodies (ANCAs) in 80-94% of affected patients [1]. In GPA patients with positive ANCA, 80% to 90% are c-ANCA positive with PR-3 specificity, while 10% to 20% are p-ANCA positive with MPO specificity [2].
Although often recognizable due to its systemic and characteristic pulmonary-renal syndrome, more than 70% of presentations involve the nasal tract, sinuses, ears, or trachea, with upper respiratory tract findings often preceding pulmonary or renal disease. Conductive hearing loss is the most common audiologic finding, usually caused by otitis media [3,4]. Sensorineural Hearing Loss (SNHL) is less common. If caught early enough, treatment of this limited GPA manifestation can lead to improvement or at least preservation of a patent’s hearing [5].
Rituximab, an anti-CD20 monoclonal antibody that targets B-cells, has become an attractive therapeutic choice given the role of ANCAs in the pathogenesis of GPA (short-lived plasma cells of B-cell origin being the producers of this antibody). Furthermore, two randomized clinical trials have shown rituximab is not inferior to cyclophosphamide for induction of remission in GPA, with the favorable side effect profile of rituximab helping to increase its use by clinicians. Lally and colleagues show in a retrospective study that rituximab significantly reduces the otolaryngologic manifesations of GPA [6,7]. In the 99 patients studied, the likelihood of active otolaryngologic disease in patients treated with rituximab was 11-fold less than for those who received other treatments, such as methotrexate, azathioprine, cyclophosphamide, or trimethoprim-sulfamethoxazole. A search of Pubmed, Google scholar, and MEDLINE showed a few case reports and no trials that specifically address the role of rituximab in stopping hearing loss [8]. We are presenting a case demonstrating that not only was rituximab useful in inducing remission of GPA-related hearing loss, but for the last 2 years of treatment, has continued to maintain our patient’s hearing level.
Report of the Case
We report a case of a 69 year old female referred from Ear, Nose, and Throat (ENT) clinic for bilateral progressive hearing loss for the past one year. She was initially found to have fluid in her right ear with concern for otitis media. It was initially treated by her Primary Care Physician (PCP) with an anti-histamine, antibiotics, and short courses of prednisone, but unfortunately symptoms did not improve. She was sent to ENT when she noticed hearing loss developing in the right ear.
Audiometric evaluation revealed a severe, mixed hearing loss through 500 Hz sloping to a profound mixed hearing loss 1000-8000 Hz with word recognition ability estimated to be poor (6%) based on an NU-6 recorded 50-word list of the right ear, and moderately-severe sensorineural hearing loss through 2000 Hz sloping to a severe to profound sensorineural hearing loss 3000-8000 Hz with word recognition ability estimated to be good (84%) based on an NU-6 recorded 50word list of the left ear. Given the lack of improvement, audiometry in the right ear showing mixed conductive and sensorineural hearing loss and left ear showing sensorineural hearing loss, there was concern for autoimmune ear disease. Rhinoscopic exam was normal, including the septum and the turbinates, and there was no evidence of nasal polyps or nasopharyngeal obstruction. The oropharyngeal exam was normal. Both ears showed middle ear inflammation with no evidence of cholesteatoma. CT scan of the temporal bones without contrast and with reconstruction showed middle ear inflammation. She had mild temporomandibular joint (TMJ) pain on the left radiating to the left lower jaw. She had no other respiratory complaints, chest pain, joint pain, joint swelling, or rashes.
Her renal function was normal and her anti-nuclear antigen (ANA) panel, rheumatoid factor, and anti-cyclic citrullinated protein (anti-CCP) testing were all negative. Of note, she was found to have a positive c-ANCA of 1:20 and Myeloperoxidase (MPO) antibody of 33 AU/mL, which were both elevated. The rest of the patient’s laboratory data is presented in the following table 1.
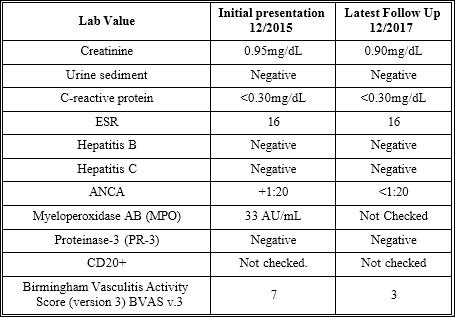
Table 1: Patient lab findings at initial presentation and upon most recent follow up.
She was started her on prednisone 60 mg oral daily with which she reported a significant improvement of approximately 85% in her hearing on the left, although not much in the right ear. After being seen by us in the rheumatology clinic, we determined the most likely etiology behind her autoimmune hearing loss was GPA. The other differential diagnoses of middle ear inflammation include Cogan’s syndrome, Sjogren’s Syndrome (SS), Rheumatoid Arthritis (RA), Systemic Lupus Erythematosus (SLE), infection, and malignancy. RA, SLE, and SS were excluded due to negative serology and lack of systemic inflammatory findings common in these entities. Cogan’s syndrome has mean age of onset of 30 and typically includes interstitial keratitis with vestibule auditory dysfunction, therefore it is unlikely. Patient was treated with antibiotics with persistent mixed hearing loss, excluding infection [9]. Finally, the patient’s CT scan did not show evidence of any cranial masses to suggest malignancy. As such, the most likely etiology is GPA given the patient had a positive ANCA serology, met the European Medicines Agency Algorithm (EMA) criteria, and expert clinical experience of the attending rheumatologist.
She was initiated on azathioprine 50 mg daily, eventually requiring 50 mg three times daily. The patient’s Liver Function Tests (LFTs) became elevated with an Alanine Aminotransferase (ALT) 342 U/L and an Aspartate Aminotransferase (AST) 185 U/L. Therefore, the patient was contacted and informed to reduce her azathioprine to 50 mg daily with repeat LFTs showing improvement with an ALT of 146 U/L and AST of 41 U/L. However, the patient reported no improvement in her hearing, which was confirmed by subsequent angiography during her next outpatient ENT visit. Azathioprine was discontinued given the refractory nature of her GPA and some concern of medication side effects. She was not a candidate for methotrexate due to interaction with alcohol and recent transaminitis. After further testing and screening exams for hepatitis and tuberculosis came back negative, she was started on a steroid-sparing regimen of rituximab 1000 mg IV day 0 and day 15. Her prednisone was slowly tapered from 60 mg daily to 20 mg daily with increments of 10 mg every 4 weeks. She was noted to have a mild flare of her symptoms upon reaching a dose of less than 20 mg of prednisone though (felt increased fatigue with left ear fullness). She was continued on a slower taper of prednisone from 20 mg daily (by 2.5 mg every 4 weeks), and we decided to continue her on rituximab 1000 mg IV every 6 months to prevent relapse.
On her most recent visit, patient reported stability of symptoms despite lowering prednisone to 4 mg daily and is set to continue rituximab RA protocol for now (received 4th dose without adverse effects reported). ENT also repeated audiometry exam prior to our most recent visit. Audiometric evaluation revealed a moderately-severe hearing loss at 250 Hz sloping to a profound mixed hearing loss 750-8000 Hz with word recognition ability estimated to be poor (18%) based on an NU-6 recorded 50-word list of the right ear and a moderately-severe mixed hearing loss through 2000 Hz sloping to a profound mixed hearing loss 4000-8000 Hz with word recognition ability estimated to be excellent (94%) based on an NU-6 recorded 50-word list of the left ear. These results confirmed stability of her hearing.
Discussion
Otologic involvement occurs in 25-40% of patients during the course of GPA. Otitis media is the most common form of ear involvement, occurring in 40-70% of cases and may antedate upper and lower airway disease by months. Otitis media may be the presenting feature and sole manifestation of GPA. Sensorineural hearing loss is less common. In 70% to 90% of cases of sudden SNHL, the etiology is not identified and thus classified as idiopathic. In 30% of cases, immune-mediated SNHL may be associated with a systemic autoimmune disease such as GPA, SLE, antiphospholipid syndrome, RA, SS, Behcet’s syndrome, sarcoidosis, Cogan’s syndrome, among others. However, the incidence of involvement of the inner ear in the systemic autoimmune disease varies greatly. The cause is unclear, but suggested mechanisms include cochlear nerve compression by adjacent granuloma, cochlear immune-complex deposition, and local vasculitis that involve cochlear vessels. SNHL loss is usually bilateral and progression is generally rapid [10]. The condition, however, is occasionally reversible with glucocorticoids or cytotoxic agents [11].
As our case demonstrates, patients with bilateral middle ear symptoms that cannot be explained by infection should have GPA high on list of differential diagnoses. The yield of a biopsy, though an important part in diagnosing GPA, depends on the sampled organ and the ability to target it for a procedure, thus can vary considerably. This highlights the fact that there is no absolute test or clinical criteria by itself that will confirm the diagnosis of vasculitis.
Therefore, our patient was given her diagnosis based off of clinical and laboratory presentation [12]. This is not unheard of, as in cases of limited GPA in early stages without systemic vasculitis features, such as those with ANCA negativity or lack of Small-vessel Vasculitis (SVV) and granulomatous inflammation on pathology. In these instances, it is often left to the expertise of a rheumatologist to make a diagnosis of GPA by looking at the complete picture. Our patient did meet the EMA criteria, which even in the absence of biopsy, qualifies as GPA by having a positive ANCA and upper airways (ENT) findings [13].
In regards to our choice of treatment modality, we looked at the role of B-cell elimination using rituximab given its favorable effect on AVV by removing the cells responsible for ANCA production. Limited GPA such as in our patient can occur in up to 25 percent of cases, although many of these patients may subsequently develop renal disease [14]. Patients with limited GPA are usually younger when disease starts, more likely to be female, have a longer disease onset on average compared to those with systemic disease, and have a higher likelihood of recurrence. Given the patient’s arthralgia, conductive and sensorineural hearing loss, the patient had a Birmingham Vasculitis Activity Score version 3 (BVAS v.3) score of 7 [15]. This score is usually used in research studies and not commonly used in clinical practice. Because of the early presentation of this patient, she did not demonstrate many manifestations that would occur later in the disease process, which may explain the low score. There was a study in 2009, in which Mukhtyar C and colleagues found that the subject with the highest BVAS (BVAS=37) had active disease in five organ systems. In the same token, we did not use the Vasculitis Damage Index (VDI) score because she presented early in her disease course, thus it would not provide clinical utility [12].
The neutrophil/lymphocyte ratio was not assessed because in a paper by Abaza NM and colleagues, the neutrophil/lymphocyte ratio applied more to patients with uveitis, proptosis, cutaneous manifestations and ischemic heart disease, with no mention of it use in early, limited GPA or specifically sensorineural hearing loss. In 2016, Kim and colleagues presented a patient that had systemic GPA manifestations [16]. This patient had renal and lung involvement that was refractory to cyclophosphamide and glucocorticoids, thus, rituximab was used, leading to dramatic improvement in symptoms. Our case involved isolated mixed hearing loss with no systemic manifestations that was also refractory to prior immunosuppressive therapy [17]. In order to prevent further hearing loss, we elected to use rituximab, which proved to be efficacious. According to Stone JH and colleagues, rituximab therapy was not inferior to daily cyclophosphamide treatment for induction of remission in severe AVV and may be superior in relapsing disease. As such, with increasing use of rituximab for induction and further studies looking at its use as maintenance therapy, we wanted to demonstrate a case showing steroid-sparing effect, control from relapses, and tolerable safety profile in a patient who has received two years of treatment with rituximab for her limited GPA that had led to hearing loss [6].
We reviewed another case report involving a patient with progressive hearing loss and fever of unknown origin that was discovered to be due to GPA, and thus was treated with intravenous methylprednisolone, oral prednisolone and cyclophosphamide resulting in resolution of symptoms. Reviewing a case report in Japan involving a patient with GPA who demonstrated a one year progression of hearing loss confirmed by hearing testing, we note the patient had objective improvement via repeat hearing tests after being treated with steroids and cyclophosphamide [18]. With this in mind, we wanted to demonstrate that like in systemic GPA, rituximab is another alternative agent that can be considered for treating hearing loss due to GPA [19]. It is to the clinician’s discretion to evaluate the risk versus benefits of rituximab as the potential side effects can be serious. Approximately 10% to 35% of patients can have an infusion reaction that is usually not severe with the use of premedication. About 1% can have a serious allergic reaction. Infection risk, which includes reactivation of resolved hepatitis B, JC virus, tuberculosis, and herpes zoster are increased with rituximab infusions. Hypogammaglobulinemia usually only occurs in patients after multiple courses of rituximab therapy with IgG becoming low in 3.5% to 12% of patients and IgM becoming low in 22% to 26% of patients. Patients who develop low IgG are more likely to get serious infection [20].
Conclusion
GPA is an AVV that can be isolated to the upper respiratory tract, nasal tract, sinuses, ears, or trachea with otologic involvement resulting in sensorineural hearing loss. Clinical trials have shown rituximab is not inferior to cyclophosphamide for induction of remission in GPA. There is not much literature indicating specific immunosuppressant therapies for treatment of GPA-induced hearing loss, especially in cases without the other more significant pulmonary-renal manifestations seen. Similar case reports involving progressive hearing loss were treated with intravenous steroid therapy along with cyclophosphamide resulting in resolution of symptoms with only one case showing refractory GPA that failed to respond to cyclophosphamide, but that did respond to rituximab per Kim et al. With our case, we show that rituximab was able to prevent sensorineural hearing loss relapse as well as substantially decrease the need for glucocorticoids. Like in systemic GPA, rituximab can therefore be considered as another alternative agent to be used for hearing loss related to GPA. More research and trials with rituximab as a potential maintenance therapy for limited vasculitis are required.
References
- Finkielman JD, Lee AS, Hummel AM, Viss MA, Jacob GL, et (2007) ANCA are detectable in nearly all patients with active severe Wegener’s granuloma- tosis. Am J Med 120: 9-14.
- Ferraro AJ, Hassan B, Savage CO (2007) Pathogenic mechanisms of an- ti-neutrophil cytoplasm antibody-associated Expert Rev Clin Immu- nol 3:543-555.
- Klippel JH (2001) Primer on the Rheumatic Diseases (12th). Ed: Atlanta GA Arthritis Foundation. 392-394.
- Finley JC, Bloom DC, Thiringer JK (2004) Wegener granulomatosis present- ing as an infiltrative retropharyngeal mass with syncope and hypoglossal pa- Arch Otolaryngol Head Neck Surg 130: 361-365.
- Cadoni G, Prelajade D, Campobasso E, Calŏ L, Agostino S, et al. (2005) Wegener’s granulomatosis: a challenging disease for Acta Otolaryngol. 125: 1105-1110.
- Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, et (2010) Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221-232.
- Jones RB, Tervaert JWC, Hauser T, Luqmani R, Morgan MD, et al. (2010) Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 363: 211-220.
- Lally L, Lebovics RS, Huang WT, Spiera RF (2014) Effectiveness of rituximab for the otolaryngologic manifestations of granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res 66: 1403-1409.
- Mazlumzadeh M, Matteson EL (2007) Cogan’s syndrome: an audiovestibu- lar, ocular, and systemic autoimmune Rheum Dis Clin North Am 33: 855-874.
- Rossini BAA, Penido N de O, Munhoz MSL, Bogaz EA, Curi RS (2017) Sud- den Sensorioneural Hearing Loss and Autoimmune Systemic Diseases. Int Arch Otorhinolaryngol 21: 213-223.
- Gubbels SP, Barkhuizen A, Hwang PH (2003) Head and neck manifestations of Wegener’s granulomatosis. Otolaryngol Clin North Am 36: 685-705.
- Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, et (2009) Modifi- cation and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 68: 1827-1832.
- Abari IS (2016) 2017 ACR/EMA revised criteria for too early diagnosis of gran- ulomatosis with polyangiitis (GPA). Autoimmune Dis Ther Approaches Open Access 3: 2.
- Keogh KA, Wylam ME, Stone JH, Specks U (2005) Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cyto- plasmic antibody-associated vasculitis. Arthritis Rheum 52: 262-268.
- Stone JH, Wegener’s Granulomatosis Etanercept Trial Research Group (2003) Limited versus severe Wegener’s granulomatosis: baseline data on patients in the Wegener’s granulomatosis etanercept trial. Arthritis 48: 2299-2309
- Abaza NM, El-Latif EMA, Gheita TA (2017) Clinical significance of neutrophil/ lymphocyte ratio in patients with granulomatosis with polyangiitis. Reumatol Clin 17: 30277-30272.
- Kim SH, Jung AR, Kim SI, Yeo SG (2016) Refractory Granulomatosis with Polyangiitis Presenting as Facial Paralysis and Bilateral Sudden J Audiol Otol 20: 55-58.
- Debski MG, Zycińska K, Czarkowski M, Zukowska M, Wardyn KA, et al. (2007) Progressive hearing loss as the leading sign of Wegener’s Granulo- Pol Arch Med Wewn 117: 266-269.
- Okamura KO, Ohtani I, Anzai T (1992) The Hearing Loss in Wegener’s Granu- lomatosis: Relationship Between Hearing Loss and Serum AurisNasus Larynx 19: 1-6.
- Engel P, Gómez-Puerta JA, Ramos-Casals M, Lozano F, Bosch X (2011) Therapeutic targeting of B cells for rheumatic autoimmune diseases. Phar- macol Rev 6: 127-156.
Citation:Jackson DA, Ahmad HI, Askari AD (2018) Sudden Deafness due to Granulomatosis with Polyangiitis Responding to Rituximab. J Case Repo Imag 2: 007.
Copyright: © 2018 Jackson DA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


