*Corresponding Author:
Golam Sarower,
Fisheries and Marine Resource Technology Discipline, Khulna University, Khulna -9208, Bangladesh
Email: sarower@yahoo.com
Abstract
Total coliform indicates the presence of Citrobacter spp., Enterobacter spp., Escheriechia spp., Klebsiella spp., Salmonella spp., Shigella spp. and Vibrio spp. In Bangladesh, traditional shrimp culture farms are highly susceptible to contamination with pathogenic bacteria because of poor sanitation near to these farms. The aim of the present study was to quantify total coliform and detect enterovirulent groups (EHEC, enterohaemorrhagic, EPEC, enteropathogenic and ETEC, enterotoxigenic) of E. coli in shrimp farms in winter and summer seasons at Khulna district of Bangladesh. All the farms were contaminated with coliform but E.coli was present only in 55% farms. In winter season, average number of coliform and E. coli in hygienic farms were 4.94×102 (cfu/ mL) and nil whereas that in unhygienic farms were 8.17×102 (cfu/ mL) and 4.15×102 (cfu/mL), respectively. However, in summer, the number of coliform and E. coli in hygienic farms were 8.26×103 (cfu/ mL) and 8.51×102 (cfu/mL) whereas that in unhygienic farms were 1.11×104 (cfu/mL) and 4.52×103 (cfu/mL), respectively. Using cultural, biochemical and PCR techniques, 76 E. coli were identified. Among them, EPEC and ETEC groups were 22% and 29%, respectively whereas EHEC group was absent. The results of this study revealed that water contaminated in shrimp farms of Khulna district resulted from insufficient sanitation that represent unhygienic products.
Keywords
Coliform; Escherichia coli; PCR; Sanitation; Shrimp farm; Target gene
Introduction
Bangladesh is endowed with natural shrimp resources and shrimp farming has been recognized as a part of Blue Revolution for the geographic features of southwest coastal area [1,2]. In the early seventies, Bangladesh entered the world’s export market for shrimp and since then this crustacean has suddenly become a very high-priced commodity. The shrimp sector has undergone dramatic changes in terms of area, production, and marketing [3]. Currently, shrimp industry is one of the most important contributors for economic sustenance and is the second largest export commodity of the country. Sometimes, the importing countries reject shrimp consignments claiming them unfit for consumption due to the presence of antibiotics, filth, unexpected foreign materials and pathogenic microbes such as E. coli, Salmonella sp., Vibrio sp. etc. The abundance of E. coli, however, has been reported to be more dangerous than that of coliforms [4,5].
Coliform and E. coli, which are widely distributed pathogens in aquatic environment have been universally accepted as an indicator of fecal pollution, because of their presence in high numbers in mammalian gut. Most of the E. coli strains are harmless, only a small percentage is pathogenic to humans [6,7].
To date, several types of enterovirulent E. coli have been recognized as the etiologic agents of various gastrointestinal infections in humans. The most commonly encountered are those belonging to the enterohaemorrhagic E. coli (EHEC) producing shigatoxin (causes bloody diarrhea), enterotoxigenic E. coli (ETEC) producing heat-stable or heat-labile enterotoxins, enteroinvasive E. coli (EIEC) producing cytotoxin and enterotoxin (causes bacillary dysentery), and enteropathogenic E. coli (EPEC) having the mechanism of virulence unrelated to the excretion of typical E. coli enterotoxins [6].
Given the importance of the detection of E. coli in water and product quality monitoring, a number of culture-based and immunological methods have been developed for its rapid detection. A fluorogenic method based on the enzymic cleavage of 4-methylumbelliferyl-β- D-glucuronide (MUG) [8], used widely has several disadvantages especially in relation to its lack of specificity. Firstly, it does not distinguish pathogenic from nonpathogenic strains of E. coli. Secondly, some other strains of Salmonella, Shigella and Yersinia can be detected by splitting MUG [9] and thirdly, phenotypically MUG-negative E. coli [10], for instance, EHEC strains is not detected by this method [11]. For the specific detection of pathogenic ETEC and EHEC strains, therefore, several ELISA-based methods have been developed including detection of heat-labile LT [12], heat- stable ST1 [13] and ST2 [14] enterotoxins of ETEC; and verotoxins VT1 and VT2 of EHEC strains [15]. However, some of the tests are known to have variable sensitivities, so depends on the relative levels of gene expression of the target gene products under selective culture conditions, makes disadvantage of rendering unculturable cells non- detectable [16].
With the new era of molecular tools, PCR and gene probe technology have provided rapid and highly sensitive methods for the specific detection of pathogenic ETEC [17] and EHEC strains [18]. Various multiplex PCR protocols to simultaneously detect segments of different toxin genes of enterovirulent strains of E. coli have also been developed viz., multiplex detection of lt1/vt1/vt2 genes of ETEC and EHEC strains [19], lt1/lt2 (ETEC) [20], lt1/st1 (ETEC) [21], elt/ est (ETEC), eae (EPEC), ipaH (EIEC) [22] and lt/sth/stp (ETEC), stx1/ stx2 (EHEC), and eae/bfp (EPEC) [23].
The enterovirulent strains of this pathogen may create enormous loss for the fishery industry due to the unacceptability by international consumers. It is, therefore, highly prerequisite to detect whether any of enterovirulent strains of E. coli is present in the traditional and improved traditional shrimp farms in a reliable and rapid way. The objective of this study was to address this safety issue through enumeration of coliform quantification and enterovirulent E. coli detection in shrimp farms by PCR technique.
Materials and Methods
Study area and sampling
In this study, water samples were collected from 15 farms consisting of 6 hygienic and 9 unhygienic farms in winter (November to February) and from 16 farms consisting of 8 hygienic and 8 unhygienic farms in summer (May to August) at different sites of Khulna district. Using visual observation farms were categorized into unhygienic farm and hygienic farm. Criteria for unhygienic and hygienic farms are listed in Table 1. After collection, the water samples were brought to the laboratory carefully and preserved in the refrigerator for immediate use; however, for long term usage, samples were stored at 4°C.
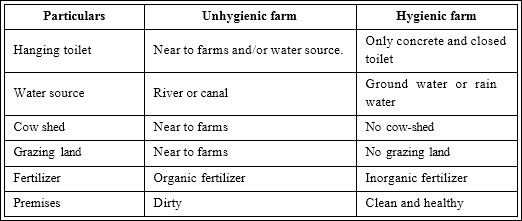
Table 1: Criteria for unhygienic and hygienic shrimp farm.
Counting of coliform and detection of E. coli
MacConkey agar was used to enumerate total coliform. A known quantity of collected aqueous samples was spread on MacConkey agar media and the plates were incubated at 37 oC for 24 hours. Lactose positive colonies showed red color on MacConkey agar plate and the number of coliforms was equal to the number of lactose positive colonies.
Then 5-10 red colonies on MacConkey agar plate were finally selected and isolated for detection of E. coli. The selected red colonies were subcultured onto nutrient agar until the pure cultures with homogenous colonies were obtained. Finally, for identification of E. coli, characteristics of isolated red colonies were studied using the following test-Eosine Methylene Blue (EMB) Agar Test [24], Lactose Fermentation Test, Methyl Red (MR) Test, Voges–Proskauer Reaction (VP) Test and Citrate Agar Test as described by [25]. When the selected red colonies were streaked on EMB plate, E. coli was identified with a characteristics green metallic sheen along the streak after a 24-48-hour incubation period. E. coli was also characterized by their ability to ferment lactose, positive for MR Test and negative for VP and Citrate utilization Test.
Culture of organisms and extraction of total DNA
Bacterial strains in water collected from 31 selected farms were cultured in 5 ml of Luria-Bertani broth (per litre: 10 g Bacto-tryptone, 5 g NaCl, 5 g yeast extract (Difco Laboratories, MI, USA) for overnight at 37°C. Cells were harvested by centrifugation at 14 000 rpm for 1 min and washed five times with sterile distilled water.
Total DNA extraction was carried out from amplified bacterial cells using DNAZOL® Reagent (Invitrogen Life technologies, USA), ethanol and sodium hydroxide (Difco Laboratories, MI, USA). The concentration and purity of DNA samples were determined from the ratio of absorbance at A260 and A280 (absorbance at 260 nm and 280 nm) using a spectrophotometer against NaOH blank cuvette. DNA sample containing cuvette was washed properly before loading next sample.
Target Genes and primer designation
The target genes chosen of this experiment were: alkaline phosphatase phoa, housekeeping gene (present in all E. coli); the heatlabile lt1, lt2 genes and heat-stable st1 genes of ETEC group; verotoxin vt gene of EHEC group and attachment and effacement eae gene of EPEC group.
Six pairs of specific primers were designed from gene sequence to amplify the target genes. The primer designation was done completely based on the verified sequences in the gene bank NCBI (Table 2). As a marker, 100 bp DNA sized was used to compare and ensure the size of PCR amplified product
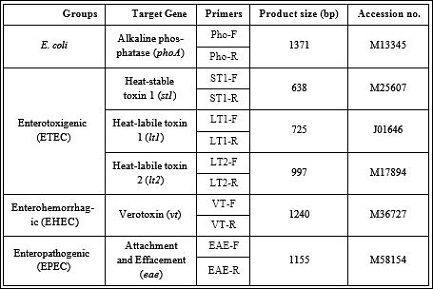
Table 2: Primers and expected size of PCR-amplified gene targets of pathogenic E. coli.
PCR Amplification and analysis of PCR products
A thermal cycler (C1000TM, BIO-RAD, USA) was used for PCR. The amplification program included an initial denaturation step at 94°C for 2 min and 35 cycles of denaturation at 94°C for 1 min, annealing at 58 °C for 1 min and final extension at 72°C for 1 min. The reactions were performed in a 0.2 ml PCR tube (Eppendorf, Humburg, Germany) in which 25μl reaction mixture containing 2μl extracted DNA sample (0.5µg/50µl), 2μl oligonucleotide primers (1µM), 5μl 5X green reaction buffer (1.5mM MgCl2), 2μl dNTPs (0.2mM), 1μl Top DNA polymerase (1.25U) and 13μl de-ionized distilled water (Bioneer Corporation, Daejeon, Korea). PCR products were analyzed by gel electrophoresis in 2% agarose (Bioneer Corporation) containing 0.5μg ml-1 ethidium bromide in TAE buffer. DNA bands were visualized by High Performance UV Transilluminator (UVP, CA, USA) and photographed using the DigiDoc-It 120 gel documentation system (UVP). To compare the size of double stranded DNA from 100 to 2,000 base pairs, 100 bp designed DNA markers were used. The DNA marker consists of 13 double stranded DNA fragments ranging in sizes from 100 to 1,000 (Bioneer, Korea).
Results and Discussion
In this study, 31 shrimp farms were selected to detect the occurrence of coliform and enterovirulent E. coli. The average number of coliforms in unhygienic farms was twofold higher than that in hygienic farms in winter season and these loads in both types of farms became highly increased in summer season (Table 3)

Table 3: Average number of total coliform and E. coli of 31 shrimp farms in two seasons.
All coliform contaminated water samples were subjected to investigation to detect the presence of E. coli. Among 31 shrimp farms, 17 farms (55% farms) showed contamination of E. coli. No contamination of E. coli was found in hygienic farms in winter, but found in unhygienic farms. Whereas, both hygienic and unhygienic farms were highly contaminated with E. coli (Table 3).
Following bacteria culture and DNA extraction, initially PCR analysis of 76 isolates of E. coli screened from 17 contaminated farms was carried out with a pair of primers targeted at phoa gene specific for E. coli. As shown in Figure 1.
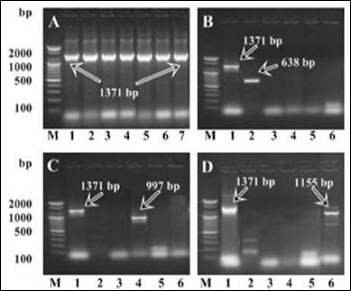
Figure 1: Electrophoretic analysis of PCR-amplified target genes from E. coli and its different strains obtained under optimal conditions of PCR. A, PCR-amplified phoa gene from E. coli isolates of contaminated farms. Lane M, 100 bp DNA marker; lane 1, positive control; lane 2 4, isolates from hygienic farms; lane 5 7, isolates from unhygienic farms. B, C and D, mobility of the different target genes is indicated on the right. Lane M, 100 bp DNA marker; lane 1, Pho-A amplimers; lane 2, ST1 amplimers; lane 3, LT1 amplimers; lane 4, LT2; lane 5, VT amplimers; lane 6, EAE amplimers. Gels are representative of independent experiments of 76 isolates.
A PCR bands corresponding to phoa genes were detected in seven representative isolates. The results indicated E. coli that is positive for this gene was present in all 76 isolates. Additional five pairs of oligonucleotide primers were designed in this study to simultaneously amplify a house-keeping as well as various virulence-associated genes of ETEC, EHEC and EPEC groups in a single tube. Seventy six isolates of E. coli screened from farms water were characterized by PCR and the results showed that st1 and lt2 toxin gene of group ETEC and eae virulence gene of EPEC group were present whereas toxin lt1 of group ETEC and vt verotoxin of group EHEC were totally absent among the isolates of E. coli. B-D shows the PCR bands of 1371 bp, 638 bp, 997 bp and 1155 bp which clearly indicated the presence of phoA gene, st1 gene, lt2 gene and eae gene, respectively (Figure.1 B-D). In both seasons, contamination of ETEC, EHEC and EPEC groups were not found in hygienic farms where as ETEC and EPEC were found to be contaminated in unhygienic farms.
Among the 76 isolated E. coli, EPEC and ETEC groups were 22% and 29%, respectively (Table 4).

Table 4: PCR results summery of sampling farms in the winter and summer seasons. nd: Not detected
EHEC strain was not detected in any farms that were contaminated with E. coli. This observation is consistent with previous reports claiming that enterotoxigenic E. coli strains that are lt2+ are rarely found [26], and so far, ETEC strains that are lt2+ have been isolated only in Brazil, Thailand and Bangladesh [27-29].
[29] were able to identify the virulent strains lt2 genes of ETEC group and EAE gene of EPEC group from shrimp farms. In the current experiment, the PCR was performed without screening and removing detritus to avoid target loss [26] and to reduce extra time needed assuming that PCR is able to amplify target DNA if present in the sample. Overall, the PCR analyses confirmed that st1+, lt2+ (of ETEC origin) and eae+ E. coli strains were present in water of unhygienic farms. [30] claimed same objects such as hanging toilet, nearest cattle field as mentioned in the traditional sanitation to be the sources of E. coli. The result in this study revealed that the improved sanitation of shrimp farms attracted less E. coli than less sanitation ones.
In shrimp culture ecosystem, most of the bacteria play a negative role as they compete with shrimps for food and oxygen, causing stress and disease [31]. Generally, gram-negative bacteria were found to be the dominant forms in the shrimp culture ponds [32]. The presence of coliform indicates that water supply may be vulnerable to contamination by harmful microorganisms. E. coli is one of the members of total coliform that is found only in the intestines of mammals, including humans. Although only a small percentage of E. coli is pathogenic to humans, their presence in water indicates recent fecal contamination and may indicate the possible presence of diseasecausing pathogens, such as bacteria, parasites and viruses. To better determine the health risks associated with exposure to pathogenic E. coli in the environment, the frequency at which pathogenic E. coli occurs in the shrimp farms must be assessed. Our results above indicate the potential of the PCR assay as a versatile and efficient means to identify and differentiate pathogenic from non-pathogenic E. coli strains in shrimp farms. Conceivably, this method can also be used as a cost-effective means to determine the prevalence or frequency of occurrence of these organisms in diverse ecological niches by enabling rapid identification and typing of clinical and environmental isolates of ETEC, EHEC and EPEC strains.
Conclusion
The findings reported in this study describe a versatile, reliable and highly sensitive PCR for the rapid detection of ETEC, EHEC and EPEC strains of E. coli in shrimp farms. The preincubation of cells from water samples in LB broth for 8 h prior to PCR also greatly enhanced the detection sensitivity of the system for ETEC and EPEC strains. Overall, the data indicated that the PCR scheme is a potentially very useful and powerful technique for the microbiological assessment of shrimp farm hygienicity. This method would be particularly useful for the assessment of health risks that may be associated with exposure to ETEC, EHEC and EPEC pathogens, which this study has shown are commonly found in various samples of traditional shrimp farms which are highly susceptible for pathogenic bacterial contamination.
Declarations
Funding statement
This work was supported by Ministry of Education, Bangladesh with funds from USDA.
Competing interest statement
The authors declare no conflict of interest.
Ethical statement
This material is the authors’ own original work, which has not been previously published elsewhere.
References
- Islam MS (2008) From pond to plate: Towards a twin-driven commodity chain in Bangladesh shrimp aquaculture. Food Policy 33: 209-223.
- Pokrant B (2014) Brackish water shrimp farming and the growth of aquatic monocultures in coastal Bangladesh. In: Christensen J, Tull M (eds) Historical perspectives of fisheries exploitation in the Indo-Pacific. Springer, Netherlands 107-132.
- FRSS (2014) Fisheries Statistical Yearbook of Bangladesh 2012-2013, Fisheries Resources Survey System (FRSS), Department of Fisheries, Bangladesh 30: 59.
- Fewtrell L, Bartram J (2001) Water Quality: Guidelines, Standards and Health. World Health Organization Water Series IWA Publishing, Lon- don, UK.
- Rodrigues C, Cunha MA (2017) Assessment of the microbiological qual- ity of recreational waters: indicators and methods. Euro-Mediterr J En- viron Integr 2: 25.
- Kong RYC, So CL, Law WF, Wu RSSA (1999) Sensitive and versatile multiplex PCR system for the rapid detection of enterotoxigenic (ETEC), enterohaemorrhagic (EHEC) and enteropathogenic (EPEC) strains of Escherichia coli. Mar Pollut Bull 38: 1207-1215.
- Li B, Liu H, Wang W (2017) Multiplex real-time PCR assay for detection of Escherichia coli O157:H7 and screening for non-O157 Shiga tox- in-producing coli. BMC Microbiol 17: 215.
- Edberg SC, Allen MJ, Smith DB, Kriz NJ (1990) Enumeration of total co- liforms and Escherichia coli from source water by the defined substrate Appl Environ Microbiol 56: 366-369.
- Bettelheim KA (1992) The genus Escherichia coli. In The Prokaryotes. Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (eds.). Spring- er Verlag, New York, USA.
- Chang GW, Brill J, Lun R (1989) Proportion of β-glucuronidase-nega- tive Escherichia coli in human fecal Appl Environ Microbiol 55: 335-339.
- Doyle MP, Schoeni JL (1984) Survival and growth characteristics of Escherichia coli associated with hemorrhagic colitis. Appl Environ Mi- crobiol 48: 855-856.
- Ristaino PA, Levine MM, Young CR (1983) Improved GM1-enzyme- linked immunosorbent assay for detection of Escherichia coli heat-labile J Clin Microbiol 18: 808-815.
- Carroll PJ, Woodward MJ, Wray C (1990) Detection of LT and STIa tox- ins by latex and EIA tests. Vet Rec 127: 335-336.
- Urban RG, Pipper EM, Dreyfus LA, Whipp SC (1990) High-level produc- tion of Escherichia coli STb heat-stable enterotoxoin and quantification by a direct enzyme-linked immunesorbent assay. J Clin Microbiol 28: 2383-2388.
- Downes FP, Green JH, Greene K, Strockbine N, Wells JG, et (1989) Development and evaluation of enzyme-linked immunosorbent assays for detection of shiga-like toxin I and shiga-like toxin II. J Clin Microbiol 27: 1292-1297.
- Roszak DB, Colwell RR (1987) Survival strategies of bacteria in the nat- ural environment. Microbiol Rev 51: 365-379.
- Victor T, Du Toit R, Van Zyl J, Bester A, Van Helden P (1991) Improved method for the routine identification of toxigenic Escherichia coli by DNA amplification of a conserved region of the heat-labile toxin A J Clin Microbiol 29: 158-161.
- Woodward MJ, Carroll PJ, Wray C (1992) Detection of entero- and vero- cyto-toxin genes in Escherichia coli from diarrhoeal disease in animals using the polymerase chain reaction. J Vet Microbiol 31: 251-261.
- Lang AL, Tsai YL, Mayer CL, Patton KC, Palmer CJ (1994) Multiplex PCR for detection of the heat-labile toxin gene and shiga-like toxin I and II genes in Escherichia coli isolated from natural waters. Appl Environ Microbiol 60: 3145-3149.
- Kong RYC, Dung WF, Vrijmoed LLP, Wu RSS (1995) Co-detection of three species of waterborne bacteria by multiplex PCR. Mar Pollut Bull 31: 317-324.
- Stacy-Phipps S, Mecca JJ, Weiss JB (1995) Multiplex PCR assay and simple preparation method for stool specimens detect enterotoxigen- ic Escherichia coli DNA during course of infection. J Clin Microbiol 33: 1054-1059.
- Nessa K, Ahmed D, Islam J, Kabir F, Hossain M (2007) Usefulness of a Multiplex PCR for Detection of Diarrheagenic Escherichia coli in a Di- agnostic Microbiology Laboratory Setting. Bangladesh J Med Microbiol 1: 38-42.
- Tobias J, Vutukuru SR (2012) Simple and rapid multiplex PCR for iden- tification of the main human diarrheagenic Escherichia coli. Microbiol Res 167: 564-570.
- Quinn PJ, Carter ME, Markey BM, Carter GR (2004) Clinical Veterinary Microbiology. Mosby, USA.
- Cheesbrough M (2000) District laboratory practice in tropical countries (part 2), Cambridge University Press, UK.
- Tengs T, Dahlberg OJ, Shalchian-Tabrizi K, Klaveness D, Rudi K, et al. (2000) Phylogenetic analyses indicate that the 19’Hexanoyloxy-fucox- anthin-containing dinoflagellates have tertiary plastids of haptophyte origin. Mol Biol Evol 17: 718-729.
- Guth BE, Pickett CL, Twiddy EM, Holmes RK, Gomes TA, et al. (1986) Production of type II heat-labile enterotoxin by Escherichia coli isolated from food and human feces. Infect Immun 54: 587-589.
- Ahmed MS, Biswas B, Roy D, Raseduzzaman M, Rahi L, et al. (2013) Polymerase chain reaction technique for rapid check of virulency of Escherichia coli from shrimp farms. Int J Eng Appl Sci 3: 1-7.
- Roy D, Biswas B, Islam HR, Ahmed MS, Rasheduzzaman M, et al. (2013) Rapid Identification of Enterovirulent Escherichia coli Strains using Polymerase Chain Reaction from Shrimp Farms. Pak J Biol Sci 16: 1260-1269.
- Karunasagar I, Reily A (1999) Aquaculture and Biotechnology. Oxford and IBH publishing Co. Pvt. Ltd. New Delhi, India.
- Moriarty DJW (1997) The role of microorganisms in aquaculture Aquaculture 151: 333-349.
- Sung HH, Lin SC, Chen WL, Ting YY, Chao WL (2003) Influence of Timsen™ on Vibrio populations of culture pond water and hepatopancreas and on the hemocytic activity of tiger shrimp (Penaeus monodon). Aquaculture 219: 123-133.
Citation: Sultana S, Sayeduzzaman, Shams FI, Hossain SJ, Sarower G (2021) Quan- tification of the Coliform Bacteria and Detection of Enterovirulent Escherichia coli Strains Using Strain Specific genes in Shrimp Farms. J Aqua Tech Deve 4: 006.
Copyright: © 2021 Sultana S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


