*Corresponding Author:
Chukwu MN,
Department of Food Technology, Abia State Polytechnic, Aba, Abia State, Nigeria
E-mail: mchukwu61@gmail.com
Abstract
The proximate composition and organoleptic attributes of legume-yoghurt fermented by lactic acid bacteria were studied. About 500g of whole legume seeds were sorted, washed, boiled for 30 minutes and then dehulled manually. The dehulled seeds were soaked separately overnight and thoroughly washed. The seeds were blended separately in a blender with hot water (seed: water ratio=1:5) till a very smooth consistency was achieved. The resultant slurry from each seed was filtered through cheese cloth to yield the respective legume-milk. About 100ml of the resultant legume milk were pasteurised at 90oC for 15 minutes in triplicates. The first set had no glucose, the second set had 2% glucose while the third had 5% glucose added. Each legume-milk was inoculated with 0.5g of the starter culture and incubated at 42oC for 48 hours in a water bath. After fermentation, the samples were stored for 0-10 day(s) between 4oC and 28oC. Proximate composition and sensory properties of three legumes-yoghurt samples with 2% glucose were determined. Triplicate data obtained were subjected to statistical analysis using SPSS software of version 21. Mean values were determined using One- Way ANOVA and Fisher’s Least Significant Difference was used to separate the means at (p ≤ 0.05). The proximate compositions of the milk samples showed that soymilk had protein (3.40%), fat (2.26%), ash (0.53%), fibre (0.45%), moisture (92.05%) and carbohydrate (1.31%) which were significantly (p ≤ 0.05) different from Lima milk which had protein (3.00%), fat (0.97)%, ash (0.45%), fibre (1.53%), moisture (91.25%) and carbohydrate (2.80%); and Bambara nut milk had protein (3.06%), fat (0.64%), ash (0.35%), fibre (1.45%), moisture (90.40%) and carbohydrate (4.10%); and cow milk had protein (3.29%), fat (3.61%), ash (0.76%), moisture (88.00%) and carbohydrate (4.34%). The result showed no significant difference in colour and mouthfeel but significant difference existed for taste, aroma and overall acceptability, with bambara nut yoghurt rated highest.
Keywords
Bambara groundnut; Carbohydrate; Fat; Lima bean; Milks; Moisture; Protein; Soybean; Starter culture
Introduction
Legumes play an important role in human nutrition as they are rich source of protein, calories, certain minerals and vitamins [1]. They are crops of the family Leguminosae that is also called Fabacae [2-4]. It is well documented that cereal proteins are deficient in certain essential amino acids, particularly in lysine [5] whereas legumes contained adequate amount of lysine [6]. It is advisable to enhance the protein content of the diet through easily available and accessible plant protein sources especially legumes to improve the nutritional status of the low-income groups of population [7].
Soybean (Glycine max) is a rich source of protein and also rich in all essential amino acids. Yet soybean-based foods are not widely acceptable due mainly to the beany flavour and also the belief that they cause flatulence. Preparation of fermented foods using lactic acid bacteria has been suggested to improve their acceptability [8-10].
Lima bean (Phaseolus lunatus) is a legume with limited use. In recent times, plant geneticists have improved the lima beans enormously and a number of early maturing, disease and pest resistant, non-toxic cultivars are widely available commercially. Lima beans are rich in niacin, thiamine and riboflavin. It also contains high levels of potassium, phosphorous, calcium and iron [11].
Bambara groundnut (Voadezia subterranean) is also a legume with limited use. It is neglected due to storage induced defects and anti-nutritional factors such as trypsin inhibitors, hemagluttinins, cyanogenic glucosides, anti-coagulants, toxic histones [12]. However, most of these toxins can be reduced to tolerable levels by simple preparative procedures such as fermentation, germination, roasting, soaking in warm water and thorough cooking. Bambara groundnut is a nutritionally balanced seed containing essential amino acids such as isoleucine, leucine, lysine, methionine, phenyl-alanine, threonine and valine. They are also rich in iron [13]. Bambara groundnut are not oil seeds. Hence, they are grown for their edible seeds used as nutritional pulse [14].
Milk is an excellent source of all nutrients except iron and ascorbate. Milk has long been recognized as an important food for infants and growing children. The scarcity of milk supply in developing countries perhaps led to the development of alternative milk from vegetable sources. However, prior to the development of such vegetable milks like soymilk which serve as a less expensive substitute for dairy milk, direct milk consumption as a beverage was not common in Nigeria. The development of milk substitutes extracted from legumes serves as an alternative way of producing an acceptable nutritious food based on vegetable. Vegetable milks can be used for babies in communities where babies are not given dairy milk for ethical reasons such as with vegans or for medical reasons as in milk allergies and galactosemia. Among the sources of vegetable milk, soybean has received very high research attention and more research is still being designed to improve the quality of soymilk [15,16].
Vegetables do not produce literal milk, like a cow, however there are products made from oil seeds, legumes and cereals that resemble cow milk in appearance and nutrition. Tradition and economic reasons that limit the use of dairy products promote the idea of reducing dairy products as vehicles of the probiotic agents or even replacing with milk from other media, such as cereals, fruits and vegetables [17]. Most prominent of these is soybean milk, its nutritional content is very close to that of cow milk [18]. It can be coagulated to make cheese and fermented to make yoghurt. The production of vegetable milk using legumes and oil seeds is an old technology. However, the technology has been improved to include development of vegetable alternatives to dairy milk, especially in the formulation of infant foods because they are high in protein, mineral and vitamins. Legumes that have been used in vegetable milk production include soybean, cowpeas, winged bean, groundnut and melon seeds [15]; chickpea, pigeon peas, black graw, mung beans, coconut, lupin, peanut, and sunflower seeds [19] and bambara groundnut.
Vegetable milk and vegetable milk products have nutritional benefits for young and old people because of their extreme richness in protein, minerals, essential fatty acids, which are considered to be highly valuable for human nutrition [20]. It is also suitable for both religious (vegetarians) and health (children who are allergic to cow milk protein, people on cholesterol free, lactose free, and dairy free diets) reasons [17]. The current interest in vegetable milk has been motivated by dairy and dairy products being priced too high for the low-income earners [21]. Peanut milk is a low-cost edible product with a high nutritional value. In this regard researchers have focused on products, resulting from fermentation such as yoghurt, buttermilk and ripened cheese analogues [21].
Fermented foods can be included in the category of functional foods, owing to their calcium content and other health promoting components [18]. There is a greater interest in the potential beneficial effects of the fermented milk on health resulting in the increase of available varieties and amount consumed around the world. Dairy products have been used traditionally as vehicles of probiotics in humans [17]. Soybean has received attention from the researchers due to its protein quality. Soymilk is suitable for the growth of the lactic acid bacteria [22]. Several studies have mentioned the production and use of the fermented soymilk drinks as probiotic, mainly soybean yoghurt, which further can be supplemented with oligofructose and inulin [20]. Lima beans and bambara groundnut have potential for use in probiotic beverage owing to their similarity to soybean milk.
Non-dairy probiotic products represent a huge growth potential for the food industry, and may be widely explored through the development of new ingredients, processes, and products. There are a wide variety of traditional non-dairy fermented beverages produced around the world. Much of them are non-alcoholic beverages manufactured with cereals as the main raw material [18]. Nevertheless, fruit juices, desserts and legume-based products can also be used featuring probiotics [23]. The utilization of lactic acid bacteria in preparing soymilk fermentation has received much attention [16]. Little attention has been given to bamabara groundnut milk fermentation by LAB [17] while no attention has been given to fermentation of Lima milk by LAB.
Growing awareness of the nutritional benefits of plant-based foods by health conscience consumers, religious reasons, vegetarianism, cholesterol free, lactose free and dairy free diets has led to the increased interest in production of vegetable milk [18]. A lot of attention has been given to soybean milk and its protein isolate beverage since they are considered to be nutritious and healthy [19]. Consequently, soybean and peanuts have been used in a variety of milk-based products including coffee creamers and chocolate milk drink [19]. Legumes and oil seeds have characteristics that make it convenient to combine two or more to obtain an acceptable product. Vegetable milk made from peanut and cowpea blends could be dehydrated to produce an inexpensive dry milk powder [19].
Lactose intolerance, milk allergies, cholesterol content and the increase in consumer vegetarianism are the major drawbacks related to the fermented dairy products [20]. Application of probiotic organisms is at present limited to dairy products especially to yoghurt which may contain residual lactose even after fermentation [20] and can only be afforded by the privileged few in the developing countries, therefore there is need to develop probiotic products from plant sources. The nondairy probiotic beverages may be made from a variety of raw materials, especially legumes like soybeans, lima beans and bambara groundnut which do not contain lactose.
This study was undertaken to produce legume yoghurts using Lactic Acid Bacteria (LAB) from soybeans, lima beans and bambara groundnut as well as to evaluate consumer acceptability of the different probiotic legume yoghurt. It is therefore important that new studies be carried out to test ingredients, explore more options of media that have not yet been industrially utilised, reengineer products and processes to meet the demands of people with lactose intolerance, milk allergies and vegetarian consumers for new nourishing and palatable cholesterol-free probiotic products [21].
Materials and Methods
Materials
Lima beans and bambara groundnut were obtained from a local market in Kaduna. The soybean was obtained from Oil mill market in Port-Harcourt. Commercial freeze-dried yoghurt LAB starter culture (Yogourmet) was obtained from Ariaria market, Aba, Abia State.
Isolation of Lactic Acid Bacteria
All microbiological processes were carried out in triplicates in a laminar flow to exclude all contaminations. All autoclavable materials were sterilised in an autoclave (Dixons, model ST 1GT UK).
Preparation of media and reagents
All media used were prepared according to specifications and procedures given by the manufacturers. The sterile diluent used was buffered peptone water. MRS agar was used for the selective cultivation of the LAB. The regents used were of analytic grade [10].
Isolation of organisms in the starter culture
The pure cultures of LAB were isolated by adding 0.5g of the commercial starter (Yogourmet) to 9.5ml of sterile Buffered Peptone Water (BPW) and homogenised [23]. Six 10-fold dilutions of the homogenates were then prepared and 1ml from each dilution was then subcultured in triplicate, into MRS agars (Biotech, India) used for isolating LAB, and incubated for 48h at 37oC. The isolates were purified by streak-plating on the same medium. Colonies with typical characteristics were randomly selected from plates and tested for Gram staining, cell morphology, catalase and oxidase reaction before further sugar fermentation tests. During the test the cultures were kept in MRS agar slant at refrigeration temperature.
Identification and Characterisation of Isolates
After obtaining pure culture, following tests were performed for identification purposes.
General Morphological Identification
Colonial identification
Features observed and recorded for colonial identification were shape (whether circular or irregular), size (whether large or small), colour and elevation (whether flat, raised or convex) on the solid medium.
Gram’s staining
A drop of distilled water was placed on a clean and grease-free microscope slide. A flamed and cooled loop was used to collect a 24h culture and used to make a smear on the water. The smear was airdried and heat-fixed and then flooded with crystal violet (primary stain). After 60 second, the stain was gently rinsed with water and Lugol’s iodine was applied on the smear for another 60 second. This was rinsed with a decolouriser (acetone-alcohol mixture) for 2-3 second, and rinsed with water. The smear was then covered with Safranin (secondary stain) for 60 second and then rinsed with water, carefully blotdried using filter paper. The smear was mounted on the microscope and viewed using x100 oil immersion lens. Observations were made regarding shape, arrangement of the cells and gram reaction (purple or blue for gram positive and pink or red for gram negative).
Presence of spores
A film of 24h old culture of the isolated organism was smeared on a clean slide. This was flooded with 10% aqueous malachite green solution and left to stand for 40-45 minutes. It was washed under running water and flooded with 0.5% aqueous Safranin solution. This was left to stand for 15 seconds and rinsed under running tap. The stained slide was gently blot dried and viewed. Bacteria bodies should stain red while spores should stain green.
Biochemical Identification
The isolated organisms were also subjected to the following biochemical tests [23].
Catalase test
A portion of the colony was transferred to a clean and grease-free glass slide and a single drop of 6% hydrogen peroxide solution was added. Instant effervescence due to the production of oxygen bubbles indicated the presence of the catalase enzyme. Absence of bubble was a negative reaction.
Oxidase test
A piece of filter paper in a petri-dish was moistened with three drops of oxidase reagent. A glass rod or a platinum loop was used to transfer a colony of the test culture to the filter paper and rubbed on the area moistened with oxidase reagent. The development of a dark purple colouration indicated the production of oxidase. Pink colour changes to purple immediately.
Indole test
The isolated culture was inoculated in a tube of peptone water containing 0.03% tryptophan and incubated at 30°C. About 0.2ml of Kovac’s reagent was added, shaken and allowed for 10min. A pink colouration at the surface indicated the presence of indole.
Temperature and salt tolerance
The growth of bacterial strains at 10°C and 45°C was visually confirmed by the changes in turbidity of MRS broth after 24, 48 and 72 h of incubation. The tolerance of microorganisms to the different levels of salt (4% and 6.5%) was also visually evaluated.
Carbohydrate Fermentation
Ten millilitre (10ml) of a 10% sugar solution and a 2ml of Andred indicator were poured into 90ml of peptone water and sterilised at 121°C for 10min. Five millilitre (5ml) was aseptically pipette into sterile Bijoux bottles with inverted Durham’s tubes incorporated to check for gas production and incubated overnight to check for sterility. A pure culture of the test organism was inoculated into the sterile bottles of peptone-sugar solution and incubated at 37°C for up to 7 days. The development of pink colouration indicated production of acid, and displacement of solution with gas in the Durham tube implied gas production. The identities of these isolated bacteria were crossmatched with those present in the standard manuals.
Preparation of the Different Legume Milks
Five hundred grams (500g) of whole soybeans, lima beans and bambara groundnut each, were sorted, washed, boiled separately for 30 minutes and then allowed to cool. They were then dehulled manually, separating the seed coat from the endosperm. The dehulled legumes were soaked separately overnight in clean tap water. After decanting the soak water, the seeds were thoroughly washed. The seeds were blended separately in a blender with the addition of hot water (seed: water ratio=1:5) till a very smooth consistency is achieved. The resultant slurry from each seed was filtered through cheese cloth to yield the milks of soybeans, lima beans and bambara groundnut respectively (Figure 1).
Production of the Different Legume Yoghurt Samples and Storage
A one hundred millilitres of the resultant legume milk from each seed were pasteurised at 90°C for 15 minutes and transferred into testtubes in triplicates. The first set had no glucose, the second set had 2% glucose while the third had 5% glucose added. The whole procedure was repeated so as to have two batches of all samples. According to manufacturer’s instruction, each test-tube was inoculated with 0.5g of the starter culture, mixed, corked and incubated at a temperature of 42°C for 48 hours in a water bath for fermentation to take place. After fermentation, the samples were stored for 10 days at refrigeration (4°C) and room temperature (28 ± 3°C) respectively (Figures 2 and 3).

Figure 1: Lima Beans.

Figure 2: Soybeans.

Figure 3: Bambara Groundnut.
Proximate Analysis of the Different Legume Milk Samples
The standard methods of AOAC [24] were used for the analysis of proximate composition of the legume milk samples. All analyses were done in triplicates.
Determination of moisture content
Two millilitres of each of the legume milk was transferred into dry and weighed crucibles respectively. The crucibles with the samples were transferred into moisture extraction oven at 105oC for 2h. The crucibles with the contents were cooled in a desiccator and weighed. The process was repeated until a constant weight was obtained. The loss in weight obtained for each sample represented the moisture content for each sample [25].

Determination of ash content
Two millilitres of the legume milk sample, each was transferred into a dry and weighed porcelain crucible. These were then charred over a Bunsen burner flame before igniting in the muffie furnace at 600oC for 6h until samples were completely ashed and whitish in colour. This was followed by cooling in a desiccator for one hour and reweighing the crucible with the ash [4]. The percentage loss of weight during combustion was calculated as ash content:

Determination of crude protein
The Total nitrogen and protein content of each sample were determined by Kjeldahl methods as described by Onwuka [26,27]. A 0.2ml of sample was transferred into a Kjeldahl digestion flask, into which 1g of copper sulphate, 1 tablet of Kjeldahl, 25ml of concentrated sulphuric acid and a few glass beads were added. The mixture was digested under a fume cupboard until a clear solution was obtained. All the digests were carefully transferred into a 100ml volumetric flask and made up to mark using distilled water. A 50ml portion of the digest was mixed with equal volume of 40% NaOH solution into a micro Kjeldahl (Markham distillation apparatus) unit and distilled. The distillate was collected into 10ml of 4% boric acid solution containing 3 drops of mixed indicator (bromocresol green-methyl red). A total of 50ml distillate was collected and titrated against 0.02N H2SO4 solution to a colour change from initial blueish-green colour to pink (end-point). Percentage total nitrogen and percentage crude proteins were calculated thus:
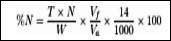
%Crude protein = % Nitrogen x C
Where N = Normality of the acid
Vf = Total volume of digest
Va = Volume of sample digested
W = Weight of digested sample
T = Volume of acid used to titrate the sample C = Correction factor (6.25)
Determination of crude fat content
The Soxhlet fat extraction method described by AOAC [24] was employed. A 250ml clean boiling flask was dried in the oven at 1050C for 30min after which it was transferred into a desiccator to cool and was labelled. A two millilitre (2ml) of legume extract sample was weighed into the labelled thimble. The corresponding labelled boiling flask was weighed (W1). The boiling flask was filled with 30ml of petroleum ether. The extraction thimble was plugged lightly with cotton wool and the Soxhlet apparatus was assembled and allowed to reflux for 6h. The thimble was then gently removed and the petroleum ether in the top of the container decanted into a conical flask for re-use. When the flask was almost free of petroleum ether, it was removed and dried at 1050C for 1h after which it was transferred from the oven to a desiccator and was allowed to cool and thereafter weighed (W2). The percentage of fat was calculated as follows (Chukwu et al., 2018b):

Determination of crude fibre content
A two millilitre (2ml) of the legume extract each was transferred into a 250ml beaker containing 200ml of 0.125M tetraoxosulphate (iv) acid. The mixture was heated in a steam bath at 70-90oC for 2h allowed to cool and then filtered using a muslin cloth over a Buckner funnel. The residue was washed three times with hot water to remove acid and then poured into a beaker containing 200ml of potassium hydroxide. The residue was heated again as before, filtered and the residue washed three times with hot water, then with alcohol and water. The final residue was put in a pre-weighed crucible and dried at 120oC to a constant weight. It was then incinerated in a muffie furnace at 550oC for 30min, cooled in a desiccator and weighed. The percentage crude fibre was calculated thus [4]:

Carbohydrate content determination
The carbohydrate content of the samples was determined by difference thus:
%CHO = (%MC + %fat + %protein + %fibre + %crude ash)
Where CHO = carbohydrate MC = moisture content
Sensory Evaluation
Sensory evaluations of the different legume yoghurt samples with 2% glucose were carried out with reference to dairy yoghurt by 20-member panellist consisting of staff and students of the Department of Food Science and Technology, Federal University of Technology, Owerri, Nigeria. The order of presentation of the samples to the panellist was randomized [28]. The panelists were instructed to evaluate the coded samples for appearance, taste, texture, aroma, mouthfeel and overall acceptability. A 9-point hedonic scale quality analysis as described by Peter-Ikechukwu et al. [10] was used with 1=dislike extremely, 5=neither like nor dislike, and 9=like extremely. The panelists were instructed to rinse their mouths with water after tasting every sample and not to make comments during evaluation to prevent influencing other panelists. They were also asked to comment freely on samples on the questionnaires given to them.
Statistical analysis
Triplicate data obtained were subjected to statistical analysis using SPSS software of version 21. Mean values were determined and OneWay ANOVA was done as well as Fisher’s Least Significant Difference [29,30] was used to determine for the separation of the means at (p≤ 0.05).
Results and Discussion
Proximate Composition of Legume Milk Samples
Table 1 shows the result of proximate composition of the samples. The protein content of all milk samples did not vary significantly, this shows that legume milk are excellent substitutes for cow milk. Soymilk had the highest amount of protein which was 3.4%. The protein of soymilk was higher than that of cow milk used as reference (3.29%), bambara groundnut milk (3.06%) and lima milk (3%). The level of protein in bambara groundnut milk was comparable to the protein content of melon seed milk (3.67%) and soybean milk (3.3%) as reported by Akubor [31] but higher than the protein content of benni-seed milk (2.86%) and soybean milk (2.71%) reported by Nnam [32]. Apparently, these differences in protein values reflect differences in seed variety, composition, method of extraction and pre-extraction treatments. Comparative levels of protein in all samples are nutritionally significant in terms of the potentials of these beverages to contribute to the increased protein intake of consumers. From previous reports, milk from legumes are good sources of proteins which are credited with significant lowering of body cholesterol levels [33, 34].
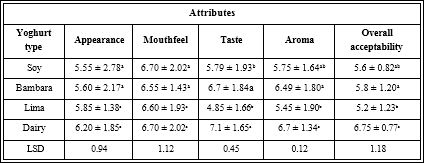
Table 1: Proximate composition/chemical analysis of the different milk samples.
Values with the same superscript in the same column are not significantly different at p ≤ 0.05.
There was no significant difference in the appearance and mouthfeel of all samples but significant difference existed for taste, aroma and overall acceptability.
The fat content of all samples varied significantly. Cow milk had the highest fat content of 3.61%. Soymilk as an oil seed had a fat content of 2.26%. Lima milk and bambara groundnut milk had lower fat content at 0.97% and 0.64% respectively. The low-fat contents recorded for lima milk and bambara groundnut milk may contribute to increased shelf life by decreasing the chances of rancidity. The fat content in cow milk which is slightly higher than other samples may easily contribute to the production of off flavor during storage [35]. The crude fat levels of the legume milk samples were considerably good. Legume lipids have been identified to be the healthy polyunsaturated types which reduce the risk of heart diseases and stroke with other associated health benefits, milk from legumes contain no cholesterol [36].
Total ash in the milk samples were low but comparable to the ash content of melon seed milk [31]. There was a significant difference between the ash content of the cow milk and that of the other legume milks. In all three legumes, soymilk had the highest ash content (0.53%) which did not vary significantly with that of lima milk (0.45%) and bambara groundnut (0.35%). The ash content is a reflection of the mineral content of the milk samples [31]. The low ash content of the legume milk samples may have resulted from the chelating effect of the anti-nutritive factors in the raw food materials [31]. The heat inactivation of these anti-nutrients may not reasonably assure the release and availability of the mineral ions [33, 34]. The implication is that legume milk would need to be fortified with some minerals, especially calcium, considering their critical role in intermediate metabolism [34].
The result showed there was no significant difference between the fibre content of lima milk (1.53%) and bambara groundnut milk (1.45%). Soymilk has the least fibre content (0.45%) and was significantly different from lima and bambara milk. Dietary fibre is essential for effective gastro-intestinal functions during digestion [37]. It could be effective in the treatment and prevention of many diseases including colon cancer, coronary heart diseases, obesity, diabetes, and gastrointestinal disorders [38]. High fibre content is one of the comparative advantages of legume milk over animal milk [36].
Moisture content of soymilk, lima milk and bambara groundnut milk were 92.05%, 91.25% and 90.40%. There was no significant difference (at p ≤ 0.05) between the moisture content of all legume milk samples. Cow milk which is the reference point had lower moisture content which was significantly different from the milk from the legumes.
The carbohydrate content of cow milk (4.34%) and bambara groundnut milk (4.10%) did not differ significantly and were higher than those of soymilk (1.31%) and lima milk (2.8%). The carbohydrate content of the samples showed that milk from legumes could serve as source of energy for the body. The sugar profile of legumes does not include lactose which makes them suitable and ideal for lactose intolerant individuals unlike cow milk. However, most of the studies on legume carbohydrates are mainly restricted to sugars such as sucrose, raffinose and starchyose [39]. The oligosaccharides in these legumes equally serve as prebiotics because they can be utilised by the LAB during fermentation [40].
Sensory Attributes of the Different Yoghurt Samples
The result of the test for sensory attributes (appearance, mouthfeel, taste, aroma and overall acceptability) of the different yoghurt samples is shown in Table 2. There was no significant difference (p ≤ 0.05) in appearance and mouthfeel of all samples. However, significant difference existed for taste, aroma and overall acceptability. In terms of taste, there was no significant difference between the dairy yoghurt and bambara groundnut yoghurt and both rated higher followed by soybeans and lima yoghurt. Significant difference existed between the aroma of the samples with dairy yoghurt, bambara yoghurt and soy yoghurt rating better than the lima yoghurt. Also, for the overall acceptability, dairy, bambara and soy yoghurt scored better than lima yoghurt.
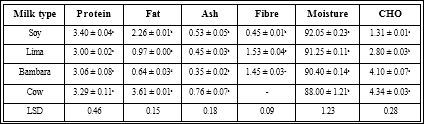
Table 2: Mean Values of sensory attributes of the different yogurt samples.
The high rating of the dairy Values with the same superscript in the same column are not significantly different at p ≤ 0.05. Table showing the nutritional composition of the milk of each legume. Significant differences existed in fat, ash, fibre, moisture and carbohydrate but not in protein of all samples in comparison with cow milk.
Yoghurt could be attributed to the fact that the sensory panelists were familiar with the diary yoghurt compared to the soy, Lima and bambara yoghurts. Also, the beany flavour of the soy, Lima and bambara yoghurts may have reduced their acceptability. Similar sensory results have been reported by Lee et al. [41], when milk-based yoghurt was compared with soymilk-based yoghurt. It was observed that low acceptability of soymilk yoghurt when evaluated with cow milk yoghurt by sensory panels. The lower rating could be attributed to the off-beany flavour in soymilk yoghurt. The lower the beany flavour, the higher the probability of panelists accepting the yoghurt. Bambara milk yoghurt in this study was more acceptable compared to soy and Lima yoghurt.
Conclusion and Recommendations
Conclusion
The findings of this research have shown that legumes like bambara groundnut and lima beans can be good raw materials for the production of probiotic yoghurt substitute. The proximate composition of lima beans and bambara groundnut legume milks showed that these legumes are nutritious, providing good quality protein and carbohydrates. Lactobacillus acidophilus, Lactobacillus bulgaricus and Streptococcus thermophilus showed good growth in the legume extracts. The addition of 2% glucose greatly enhanced the production of acid. The results showed that legume yoghurts could compete favourably against dairy yoghurt especially Bambara groundnut yoghurt which scored higher overall acceptability than other legume yoghurts. The successful application and consumer acceptability of legume yoghurt has the potential to increase the utilization of these crops and enhance their market value. The legume yoghurts also provide cheap, affordable and refreshing probiotic dairy yoghurt substitute. The use of these legumes as raw materials for probiotics will also create room for variety and sustainability.
Further increase in glucose concentration did not significantly increase the viable LAB number. The result of the sensory evaluation showed that probiotics from these legumes compared favourably with probiotic dairy yoghurt. Bambara groundnut yielded a more acceptable product than soybean. There is a genuine interest in the development of non-dairy based functional beverages with probiotics because they serve as a healthy alternative for dairy probiotics, are cholesterol free and also favour consumption by lactose intolerant consumers.
Recommendations
- Based on the results obtained from this research, the following recommendations are noteworthy:
- Revitalisation of the cultivation of these legumes and their use as raw materials for producing nutritious foods such as the production of probiotic products.
- To improve on the survival of the probiotics by the modification of the production process and exploring different storage
- Application of micro-encapsulation techniques (protective coating of microorganisms)
- Creation of more awareness as there has always been a low patronage of locally produced food products relative to imported ones.
Contributions to knowledge
The following are the contributions of this research to knowledge:
- LAB can grow and survive in good numbers in the milk extracted from soybeans, bambara groundnut and lima beans.
- Non-dairy probiotic yoghurt can be produced from other legumes aside This will reduce the overdependence on soybeans and increase the utilisation of other legumes.
- Addition of 2% glucose to legume extracts supports the growth and survival of probiotic bacteria, thus giving a better and more stable
- Fermentation of these legume extracts modified and improved the flavour and acceptability of these legumes.
- Legumes contain oligosaccharides which are prebiotics that can be utilised by the probiotic organisms during fermentation.
References
- Baloch MS, Zubair M (2010) Effect of nipping on growth and yield of J Anim Plant Sci 20: 208-210.
- Chukwu MN, Kabuo NO, Onyeka EU, Odom TC, Nwogu O, et al. (2017) Production and Organoleptic Attributes of Ogiri-ahuekere Produced from Groundnut (Arachis hypogaea Linn) Seeds Res J Food Sci Qual.Cont 3: 63-72.
- Chukwu MN, Nwakodo CS, Alozie Q Ndulaka JC (2018) Compara- tive Studies on Organoleptic Properties of Ogiri-Ahuekere and Ogi- ri-Egusi Res J Food Sci Qual Cont 4: 11-19.
- Chukwu MN, Nwakodo CS, Ndulaka JC, Nwokocha NJ (2018) Production and Proximate Composition of Ogiri-Ahuekere (Arachis hypogaea Linn) Seed Condiment. Res J Agric Environ Manage 7: 7-17.
- Anjum FMI, Ahmad MS, Butt MA, Sheikh A, Pasha I (2005) Amino acid composition of spring wheats and losses of lysine during cha- patti baking. J Food Comp Anal 18:523-532.
- Sai-Ut S, Ketnawa S, Chaiwut P, Rawdkuen S (2009) Biochemical and functional properties of proteins from red kidney, navy and ad- zuki beans. As J Food Ag Ind 2: 493-504.
- Khattab RY, Arntfield SD (2009) Nutritional quality of legume seeds as affected by some physical treatments. Antinutritional factors. Food Sci Technol 42:1113-1118.
- Maneepun G (2000) Traditional processing and utilization of le- gumes. Report of the APO seminar on processing and utilization of legumes held in Japan October.
- Ojinnaka MC, Nnorom CC (2015) Quality Evaluation of Wheat-Co- coyam-Soybean Nigerian J. of Agriculture Food and Envi- ronment 11: 123-129.
- Peter-Ikechukwu A, Osuji CM, Ihediohamma NC, Okafor DC, Chuk- wu MN (2019) Proximate composition and organoleptic characteris- tics of sausage rolls made from cocoyam and wheat flour enriched with soybean Research Journal of Food Science and Nutrition 4: 1-11.
- Fadahunsi IF Sanni AI (2010) Chemical and biochemical changes in Bambara nut (Voandzeia subterranean (L) thours) during fermenta- tion to tempeh. Elect J Environ Agric Food Chem 9: 275-283.
- Adu-Dapaah HK, Sangwan RS (2004) Improving bambara groundnut productivity using gamma irradiation and in vitro tech- Afr J Biotechnol 3: 260-265.
- Minka SR, Bruneleau M (2000) Partial chemical composition of Bambara pea (Vigna subterranean Verde). Food Chem 68: 273- 276.
- Granato D, Branco G, Nazzaro F, Cruz AG, Faria AF (2010) Func- tional Foods and Non-dairy Probiotic Food Developments: Trends, Concepts and Products. Wiley Online Library 9: 292-302.
- Prado FC, Parada JL, Pandey A, Soccol CR (2008) Trends in non- dairy probiotic beverages. Food Research International 41: 111-
- Quasem JM, Mazahreh AS Abu-Alruz K (2009) Development of vegetable-based milk from decorticated American Journal of Applied Science 5: 888-896.
- Wang FN, Shi YH, Sun J, Le GW (2007) Evaluation of peanut flour fermented with lactic acid bacteria as a probiotic food. Food Sci Technol Int 13: 469-475.
- Lawal SO, Adebowale KO, Adebowale YA (2007) Functional prop- erties of native and chemically modified proteins concentrate from bambara groundnut. Food Research International 40: 1003-1011.
- Shimakawa Y, Matsubara S, Yaki N, Ikeda M, Ishikawa F (2003) Evaluation of Bifidobacterium breve strain Yakult-fermented soymilk as a probiotic food. International Journal of Food Microbiology 81: 131-136.
- Aidoo H, Sakyi-Dawson E, Tano-Debrah K, Saali FK (2010) Development and characterisation of dehydrated peanut-cowpea milk powder for use a dairy milk substitute in chocolate manufacture. Journal of Food Research International 43: 79-85.
- Blandino A, Al-Aseeri ME, Pandiella SS, Cantero D, Wedd C (2003) Cereal-based fermented food and beverages. Food Research International 36: 527-543.
- Isanga J, Zhang G (2009) Production and evaluation of some physicochemical parameters of peanut milk Food Science and Technology 42: 1132-1138.
- Uzuegbu JO, Kabuo NO, Ezeji C (2001) Food microbiology laboratory manual. Department of food science and technology, FUTO Owerri, Osprey Publication Centre 21-22.
- AOAC (2000), “Official Method of Analysis (17th )” Association of Analytical Chemist. Washington, DC, USA.
- Obiegbuna JE, Ishiwu CN, Akubor PI, Igwe EC (2014) Effect of Processing and Storage Relative Humidity on Selected Functional Properties of Cocoyam (Colocasia Esculenta) Corm Flour. Food Science and Quality Management 28: 123-136.
- Onwuka GI (2005) Food Analysis and Instrumentation: Theory and Naphthali Prints Lagos 39: 122-128.
- Onwuka GI (2018) Food Analysis and Instrumentation: Theory and Practice. 2nd Edn. Naphtali Prints Lagos 179-228.
- Kabuo NO, Alagbaoso OS, Omeire GC, Peter-Ikechukwu AI, Akajiaku LO, et al. (2018) Production and Evaluation of Biscuits from Cocoyam (Xanthosoma sagittifolium Cv Okoriko)-Wheat Composite Research Journal of Food and Nutrition 2: 53-61.
- Pallant J (2004) SPSS Survival Manual. Open University Press,
- Chukwu M N, Ezeagwula C, Oti W, Nwakaudu AA (2019) Microbial Loads of Ogiri-Ahuekere Condiment Produced from Groundnut Seed (Arachis hypogaea Linn). Agriculture and Food Sciences Research 6: 114-119.
- Akubor PI (1998) Physico-chemcial and sensory characteristics of melon seedmilk. J Food Sci Technol 35: 93-95.
- Nnam MN (2003) Chemical and sensory evaluation of vegetable milks from Africa yam bean Sphenostylis stenocarpa (Hochst ex A Rich) Harms and maize (Zea mays L.). Plant Foods for Human Nutrition 51: 265-275.
- Farinde EO, Obatolu VA, Fasoyiro SB, Adeniran SH, Agboola ER (2008) Use of alternative raw materials for yoghurt African Journal of Biotechnology 7: 3339-3345.
- Hogervorst E, Sadjimim T, Yesufu A, Kreager P, Rahardjo TB (2008) High tofu intake is associated with worse memory in elderly Indonesian men and Dementia and Geriatric Cognitive Disorders 26: 50-57.
- Yoon KY, Woodams EE, Hang YD (2006) Production of probiotic cabbage juice by lactic acid bacteria. Bioresource Technology 97: 1427–1430.
- Henkel J (2000) Soy: Health claims for soy protein, question about other components. FDA Consumer 34: 18-20.
- William SM, Akiko AS (2000) Tofu and Soymilk Production. 3rd Laffegette California 2: 314-324.
- Anderson JM, Smith BM, Guftafson NJ (1994) Health benefits and practical aspects of high fibre diets. American Journal of Clinical Nutrition 59: 1242-1247.
- Fukushima D (2001) Recent progress in research and technology on soybeans. Food Science and Technology Research 7: 8-16.
- Parvez SKA, Malik S, Kang TAH Kim HY (2006) Probiotics and their fermented food products are beneficial for health. J Appl Microbiol 100: 1171-1185.
- Lee WJ, LuceyJA (2000) Formation and physical property of yoghurt. Asian Australia Journal of Animal Science 23: 1127-1136.
Citation:Agim-Ezenwaka OA, Anyaogu I, Onuh EF, Onwusiribe UD, and Chukwu MN (2020) Proximate Composition and Organoleptic Attributes of Legume-Yoghurt Samples Fermented By Lactic Acid Bacteria. J Nutr Food Sci 2: 016.
Copyright: © 2020 Agim-Ezenwaka OA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


