*Corresponding Author:
Adama Isah Ladu,
Department of Haematology and Blood Transfusion, University of Maiduguri Teaching Hospital, Borno state, Nigeria
Tel: +234 803772013
E-mail: adamaisahladu@gmail.com
Abstract
Background: Advancement in medical management and diagnosis has seen a rise in the population of Sickle Cell Anaemia (SCA) patient reaching adulthood, albeit increased prevalence of chronic organ failure. Nigeria has the largest cohort of SCA worldwide; however, there is paucity of studies describing the morbidity associated with this disorder. Herein, we described the spectrum of chronic complications encountered in a population of adult patients attending for medical care in a tertiary hospital in North Eastern Nigeria.
Methods: This was a cross sectional study involving 232 patients aged 16 years and above with sickle cell anaemia that attended the haematology unit from October 2017 to July 2018. Data on socio-demographic and anthropometric parameters, history of hospitalization, frequency of painful crisis, blood transfusion therapy and drug history were obtained. Additional information regarding chronic complications was obtained from the records where necessary.
Results: The median [interquartile range] age of the patients was 22.5[18] years and consisted of 119 (51.3%) males. The most prevalent chronic complications were chronic kidney disease (43.3%) and auto-splenectomy (42%). Pulmonary hypertension and cholelithiasis were present in 25.3% and 21% respectively. The least common complication was stroke (3%). Pulmonary hypertension and leg ulcer were commoner in males than females (p=0.011and p =0.003, respectively). Regression analysis revealed that male gender was significantly associated with the development of pulmonary hypertension (p=0.038) and leg ulcer (p=0.019); chronic kidney disease was associated with age (p=0.003) and haematocrit (p=0.000). Haematocrit was the only determinant of a vascular necrosis (p=0.027)
Conclusion: Patients with sickle cell disease receiving care in our hospital are diagnosed early, hospitalized and transfused frequently, and have multiple chronic complications. While most are regular on folic acid and antimalaria prophylaxis, very few are on hydroxyurea.
Keywords
Chronic, Complications, Sickle cell anaemia
Introduction
Sickle Cell Anaemia (SCA) is an inherited haematological disorder due to a point mutation in exon 1 of the β globin gene (GAG → GTG). This results in the substitution of glutamic acid with valine at position 6 of the βglobin polypeptide chain. The mutant haemoglobin (HbS) undergoes polymerization under hypoxic condition resulting in sickling of the erythrocytes. Inflammation, haemolysis, microvascular occlusion and chronic organ damage characterizes the clinical expression of SCA [1,2]. Despite the presumed high childhood mortality, the prevalence of SCA in adults African is still high [3]. This is attributed to improvement in knowledge and public health prevention strategies that have improved the life of patients with this condition, transforming it into a chronic disease. Nevertheless, progressive deterioration of organ function occurs, and end-organ damage is irreversible. It is estimated that 48% of patients with SCA will develop multiple organ failure by the fourth decade of life [3,4].
Nigeria has the highest burden of SCA in the world [5]; however, there is still no clear baseline data on survivorship, or the major causes of morbidity and mortality amongst cases with SCA. Few studies exist that describe the severity of complications and treatments available. We therefore conducted a cross sectional studies of adult SCA patients attending a tertiary center in North eastern Nigeria. Our primary aim was to determine the prevalence of the various complications encountered in this population. Our secondary aim was to determine factors associated with the development of some of these complications.
Methods
This was a cross sectional study involving 232 patients aged 16 years and above with homozygous (HbSS) Sickle Cell Anaemia (SCA), that attended the haematology unit of the University of Maiduguri teaching Hospital, a major referral center in north eastern part of Nigeria. The study was conducted from October 2017 to July 2018. Data on socio-demographic and anthropometric parameters, history of hospitalization, frequency of painful crisis, blood transfusion therapy and drug history were obtained. Additional information regarding chronic complications was self-reported or obtained from the records where necessary.
Statistical analysis
The data obtained was entered into excel spreadsheet for data management and transferred to SPSS (version 20) for analysis. The data was analyzed for normality amongst continuous variables and log transformed when applicable prior to analysis, otherwise, continuous variables were presented as median [Inter Quartile Range IQR]. Categorical variables were presented as proportions (percentages). Differences between proportions and continuous variables were determined using Chi square and Student T test respectively. The p value of < 0.05 was considered significant. Univariable and multivariable logistic regressions were used to determine factors associated with the development some of the chronic complications.
Results
General profile of patients: The median [interquartile range] age of the patients was 22.5[18] years and consisted of 119 (51.3%) males. The median age at diagnosis of sickle cell anaemia was 1[4] year; more than half of the patients (52.2%) were diagnosed during infancy, and only 19 (9.5%) of the participants were diagnosed after the age of 18 years (Table 1). Majority of the patients were previously hospitalized (70.2%) more than three times. Of the 149 (71.3%) that have received transfusion, 38 (25.5%) have been transfused more than ten times, while two patients have received more than 50 units of lifetime trans- fusion. A good number of the patients were taking folic acid (61%) and anti-malaria prophylaxis (61%) regularly, but only 4 (1.7%) were on hydroxyurea.
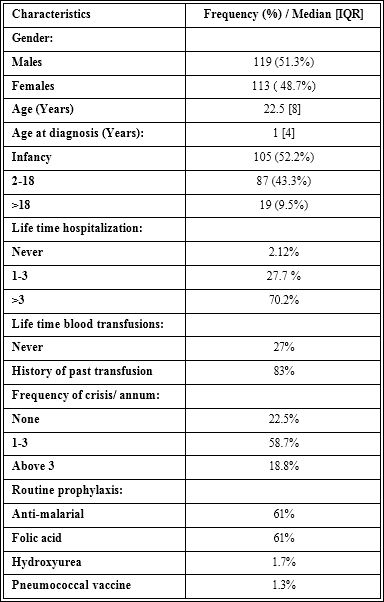
Table 1: General characteristics of study participants.
Prevalence of chronic complications
The most prevalent chronic complications were chronic kidney disease (43.3%) and auto-splenectomy (42%) (Figure 1). Pulmonary hypertension and cholelithiasis were present in 25.3% and 21% re- spectively. Leg ulcers (8.4%) and stroke (3%) were the least reported chronic complications. The life time prevalence of CKD (p=0.123), AVN (p=0.209) and stroke (p=0.52) was similar amongst gender (Ta- ble 2); whereas, pulmonary hypertension and leg ulcers were com- moner in males than females (p=0.011and p =0.003) respectively.

Figure 1: Spectrum of sickle-cell related chronic complications. AVN, avascular ne- crosis; CKD, chronic kidney disease; PH, pulmonary hypertension.
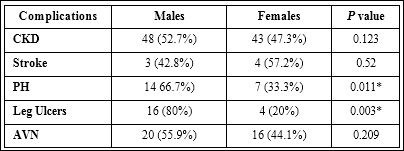
Table 2: Prevalence of chronic complications based on gender.
AVN: Avascular Necrosis; CKD: Chronic Kidney Disease; PH: Pulmonary Hyperten- sion; PCV: Packed Cell Volume; *p < 0.05.
Factors associated with CKD, AVN, leg ulcers
Chronic kidney disease was associated with age (p=0.003) and haematocrit (p=0.000) (Table 3). Male gender wasassociated with pul- monary hypertension (p=0.038) and leg ulcer (p=0.019). Haematocrit was the only determinant of avascular necrosis (p=0.027).
Discussion
This study describes the general profile and spectrum of chronic complications encountered in adult patients with SCA attending a tertiary hospital in north eastern Nigeria. To the best of our knowledge, this is the first comprehensive study documenting the pattern of morbidity encountered in adult patients with SCA from this region. Despite the relatively young age of our patient group, several chronic complications had already developed. Chronic kidney disease and autosplenectomy were the most frequent chronic complications. Pulmonary hypertension and cholelithiasis were also encountered with relative frequency. Priapism and leg ulcer occurred not so often. Stroke was the least encountered chronic complication in our study population.
The median age of the study population was 22.3 years; an overwhelming majority of the patients were less than 40 years old. This may be due to the reduced life expectancy in patients with SCA in Sub-Saharan Africa (SSA) as previously documented [6], in sharp contrast to the average age of SCA patients in developed countries of above 40years [7]. The low life expectancy could be attributed to lack of policy for Newborn Screening (NBS) [8], increased prevalence of communicable disease [9], poverty and limited access to disease modifying drugs such as hydroxyurea (HU).

Table 3: Logistic regression analysis of some complications with clinical variables
AVN: Avascular Necrosis; CKD: Chronic Kidney Disease; PH: Pulmonary Hypertension; PCV: Packed Cell Volume; *p< 0.05.
In the current study, majority of the patients were on regular prophylaxis with folate and antimalaria, 1.7% were on HU and only 1.3% have had pneumococcal vaccination. Although, there is no current policy for NBS for SCA in Nigeria, the median age of diagnosis of SCA in our patients was 1year [IQR 4], and more than half of the patients were diagnosed at infancy. This may reflect presence of severe disease phenotype, necessitating patients to seek medical care as early as infancy. Majority of the patients have been hospitalized for painful crisis and most reported more than three episodes of crisis per annum. In addition, most have received blood transfusion, these features indicate severe disease.
Chronic Kidney Disease (CKD) was the leading chronic complication encountered in our patients’ population, at a prevalence rate of 43.3%. The reported prevalence varies between 20-50% from different studies in Nigeria [10-13].Involvement of the kidney is a well-known complication of SCA [14,15]; however not all patients develop nephropathy, it is not clear what factors predict or promote progression of nephropathy in susceptible patients. Some studies have shown increasing age, low haematocrit, high blood pressure, history of blood transfusion to be associated with increased risk of developing sickle cell kidney disease [11,16]. In the current study, age was directly associated with the development of CKD (p=0.001), while haematocrit showed an inverse relationship with CKD (p=0.000).
Pulmonary hypertension is associated with increased morbidity and mortality in patients with SCA [17,18]. In the current study, the prevalence rate of elevated Tricuspid Regurgitation Velocity (TRV) on transthoracic echocardiography was 25.6%, similar to the reported prevalence rates amongst adult population of SCA in Nigeria of 3-25% [19-21]. The gold standard for determining PH is Right Heart Catheterization (RHC) [22], however, this is not readily available in resource poor centers. In addition, report of Gladwin et al showed that an elevated TRV, irrespective of the presence of RHC-proven PH is associated with poor outcome in adults with SCA [23]. It is possible that the elevated TRV indicates presence of underlying cardiac pathologies, such as, sickle cell cardiomyopathy, diastolic dysfunction or a vasculopathy [24-27].
Avascular Necrosis (AVN) is the leading cause of disability in patients with SCA and has been shown to interfere with their quality of life [28,29]. In the current study, we found AVN of at least one hip in thirty-six patients (16.1%), in corroboration with previous reports [29-31]. In sharp contrast, earlier studies showed a higher prevalence rate of 42%48% in adults with SCA, with a four-years progression rate of 67% [28,32,33]. Several factors have been implicated as risk factors for AVN including age and male gender [30,31,34,35]; an earlier report showed that the likelihood of developing AVN between the ages of 10-29 years was 89% [36]. Our study also found a positive association between haematocrit and the development of AVN, as previously reported [35]. It is thought that a high hematocrit results in hyperviscosity within the sinusoids, which promotes sickling of the red cells resulting in ischemia and subsequent infarction. Early diagnosis of AVN is very important since outcome of conservative management depends on the stage of the disease.
The prevalence of leg ulcer was 8.4% in the current study; reported prevalence from studies conducted in Nigeria ranges between 3-16%, [29,30,37-39]. In contrast, a significantly higher rate of > 40% has been reported from Jamaica [40] and a lower rate of 2.5% from the United State [41]. The discrepancy in the results could be due to the difference in geographical location, variation in the study population and haemoglobin genotype and haplotype. Presence of chronic leg ulcers have been associated with a more severe clinical course and is a sign of end organ damage in patients with SCA [42,43]. Factors associated with development of leg ulcers include trauma, age greater than 20, male gender, low haemoglobin and low haemoglobin F level [41,44]. The current study found significant gender variation in the prevalence of leg ulcers amongst the study population (p=0.003). Also, male gender was significantly associated with the development of leg ulcers (p= 0.019) as previously reported [39,41,45]. It is possible that the male patients due to their active nature are more prone to traumatic injury.
The hepatobiliary system can be frequently involved in SCA [4648]. In the current study, hepatomegaly was found in 7.2% of the patients, while combined hepatomegaly and cholelithiasis was documented in 2.6% of the patients (data not shown). The prevalence of cholelithiasis was 21%, in contrast to a previous report of 4.4% from the southern part of the country [49]. The incidence and prevalence of cholelithiasis is influenced by local diet and genetic factors [50]. Crystallization of gallstone by ceftriaxone and other third generation cephalosporin have also been implicated in the pathogenesis [51]. Cholelithiasis develops as early as the first two years of life, however,it is usually asymptomatic. Cholecystectomy should only be considered when there is evidence of cholecystitis [52].
The spleen is one of the earliest organs affected by SCA [53]. The loss of splenic function increases the risk of death from infection with encapsulated bacteria or parasitized red cells [54]. Autosplenectomy was documented in 42% of the current study population similar toreports from this region [55-57]. On the other hand, 4.3% of our patients had splenomegaly (data not shown).The persistent splenomegaly has been attributed to malaria infection [58,59].
Priapism is one of the medical emergencies encountered by male patients with SCA. The prevalence rates for the occurrence of stuttering and ischemic priapism amongst patients with SCA ranges between 2.5% to 44.9% [60-63]. The prevalence of priapism in our study was 11.9%. Frequent episodes of stuttering priapism or prolonged episode of acute priapism may result in scarring and damage to the tissues within the penis which can disrupt normal blood flow into the penis and results in erectile dysfunction [64,65].
One of the most critical complication of SCA is stroke, the longterm consequence includes neuro-cognitive and neuro-psychological dysfunction [66]. The primary prevention of stroke in SCA patients using transcranial doppler scan to identify high risk individual followed up by chronic transfusion treatment has resulted in a significant decline in the incidence of stroke in children and its prevalence in the adult population [67,68]. It was the least encountered complication in our study, documented in only 3% of our patients’ population. There is currently no recommended test to predict stroke risk in adults with SCA; hydroxyurea or chronic transfusion may be considered to prevent recurrent stroke [68].
General Conclusion and Perspective
Sickle cell anaemia can be associated with a spectrum of chronic complications in the adult population as shown in the current study. Patients receiving care in our hospital are diagnosed early, hospitalized and transfused frequently, and most have developed chronic complications including CKD, PH, AVN, autosplenectomy and cholelithiasis. Therefore, this study calls for a review of the existing care program provided to these patients with the view of reducing morbidity. Also, given the multi-organ involvement of the disease process, improving care with a multidisciplinary teams’ approach is strongly advised [66,69]. Early-stage diagnosis of SCD patients through NBS is recommended, this can prevent the progression of the disease through the adoption of simple and cost-effective interventions and preventive measures [70]. While most of our patients were on regular folic acid and antimalaria prophylaxis, very few were on the disease modifying agent hydroxyurea.
Acknowledgment
The authors are grateful to all participants of this study.
References
- Ballas SK, Kesen MR, Goldberg MF, Lutty GA, Dampier C, et al. (2012) Be- yond the definition of the phenotypic complications of Sickle Cell Disease: An Update on Management. Sci World J 949535.
- Menaa F (2014) Sickle Cell Disease - Related Pathophysiology of Vaso-oc- clusion and Hemolysis: Remaining Challenges. J Blood Disord 1: 2.
- Makani J, Williams TN, Marsh K (2007) Sickle cell disease in Africa: Burden and research priorities. Ann Trop Med Parasitol 101: 3-14.
- van Beers EJ, van Tuijn CF, Mac Gillavry MR, van der Glessen A, Schnog JJB, et (2008) Sickle cell disease-related organ damage occurs irrespec- tive of pain rate: implications for clinical practice. Haematologica 93: 757-760.
- Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, et (2013) Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatisti- cal model-based map and population estimates. Lancet 381: 142-151.
- Diop S, Mokono SO, Ndiaye M, Toure Fall AO, Thiam D, et al. (2003) Ho- mozygous sickle cell disease in patients above 20 years of age: follow-up of patients in Dakar. Rev Med Interne 24: 711-715.
- Muoghalu CO (2018) Sickle Cell Disease Child Mortality - A Silent Epidemic in Nigeria: Issues in Political Economy. Blood Res Transfus J 2: 555-584.
- Platt OS, Brambilla DJ, Rosse WF (1994) Mortality in sickle cell disease, life expectancy and risk factors for early death. N Engl J Med 330: 1639- 1644.
- Ogun G, Ebili H, Kotila T (2014) Autopsy findings and pattern of mortality in Nigeria sickle cell disease patients. Pan Afr Med J 18: 30.
- Yusuf R, Hassan A, Ibrahim IN, Babadoko AA, Ibinaiye PO (2017) Assess- ment of kidney function in sickle cell anemia patients in Zaria, Sahel Med J 20: 21-25.
- Arogundade FA, Sanusi AA, Hassan MO, Salawu L, Durosinmi MA, et al. (2010) An appraisal of renal dysfunction and its risk factors in patients with sickle cell disease. Nephron Clin Pract 118: 225- 231.
- Bolarinwa RA, Akinlade KS, Kuti M, Olawale OO, Akinola NO (2012) Renal disease in adult Nigerians with sickle cell anaemia: a report of prevalence, clinical features and risk factors. Saudi J Kidney Dis Transpl 23: 171-175.
- Aneke JC, Adegoke AO, Oyekunle AA, Osho PO, Sanusi AA, et al. (2014) Degrees of Kidney Disease in Nigerian Adults with Sickle-Cell Disease. Med Princ Pract 23: 271-274.
- Pham PT, Pham PC, Wilkinson AH, Lew SQ (2000) Renal abnormalities in sickle cell disease. Kidney Int 57: 61.
- Saborio P, Scheinman JI (1999) Sickle cell Nephropathy. J Am Soc Nephrol 10: 187- 192.
- Geard A, Pule GD, Chemegni BC, Bitoungui VJN, Kengne AP, et al. (2017) Clinical and genetic predictors of renal dysfunction in sickle cell anaemia in BJH 178: 629-639.
- Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ (2012) Mortality in adults with sickle cell disease and pulmonary JAMA 307: 1254-1256.
- Castro O, Hoque M, Brown BD (2003) Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood 101:1257-1261.
- Aliyu ZY, GordeukVR, Babadoko A, Mamman AI, Akpanpe P, et al. (2008) Prevalence and risk factors for pulmonary artery systolic hypertension among sickle cell disease patients in Nigeria. Am J Hematol 83: 485-490.
- Dosunmu AO, Balogun TM, Adeyeye OO (2014) Prevalence of pulmonary hypertension in Sickle cell anaemia patients of a tertiary hospital in Nigeria. Niger Med J 55: 161-165.
- Amadi VN, Balogun MO, Akinola NO, Adebayo RA, Akintomide AO (2017) Pulmonary hypertension in Nigerian adults with sickle cell anemia. Vasc Health Risk Manag 13: 153-160.
- Hayes MM, Vedamurthy A, George G, R Dweik, ES Klings, et (2014) Pul- monary hypertension in sickle cell disease. Ann Am Thorac Soc 11: 1488- 1489.
- MT Gladwin, V Sachdev, ML Jison, Shizukuda Y, JF Plehn, et (2004) Pul- monary hypertension as a risk factor for death in patients with sickle cell disease. NEngl J Med 350: 886-895.
- Fonseca GH, Souza R, Salemi VM, Jardim CV, Gualandro SF (2012) Pul- monary hypertension diagnosed by right heart catheterization in sickle cell Eur Respir J 39: 112-118.
- Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, et al. (2010) Pulmo- nary hypertension and nitric oxide depletion in sickle cell Blood 116: 687-692.
- Knight-Perry JE, L de Las Fuentes, Waggoner AD, Hoffman RG, Blinder MA, et al. (2011) Abnormalities in cardiac structure and function in adults with sickle cell disease are not associated with pulmonary J Am Soc Echocardiogr 24: 1285-1290.
- Johnson MC, Kirkham FJ, Redline S, Rosen CL, Yan Y, et al. (2010) Left ventricular hypertrophy and diastolic dysfunction in children with sickle cell disease are related to asleep and waking oxygen desaturation. Blood 116: 16-21.
- Ware HE, Brooks AP, Toye R, Berney SI (2991) Sickle cell disease and silent avascular necrosis of the hip. J Bone Joint Surg (Br) 73: 947-949.
- Mosaku SK, Oyekunle AA, Aneke JC, Bolarinwa RA, Osho PO, et al. (2015) Avascular necrosis significantly impairs quality of life in sickle cell disease. J Clin Sci 12: 41-47.
- Madu AJ, Madu AK, Umar GK, Ibekwe K, Duru A, et al. (2014) Avascular necrosis in sickle cell (homozygous S) patients: Predictive clinical and labora- tory indices. Niger J Clin Pract 17: 86-89.
- Balogun RA, Obalum DC, Giwa SO, Adekoya-Cole TO, Ogo CN et (2010) Spectrum of musculo-skeletal disorders in sickle cell disease in Lagos, Nige- ria. J Orthop Surg Re 5: 2.
- Adekile AD, Gupta R, Yacoub F, Sinan T, Al-Bloushi M, et al. (2001) Avascular necrosis of the hip in children with sickle cell disease and high Hb F: Mag- netic resonance imaging findings and influence of α-thalassemia trait. Acta Haematol 105: 27-31.
- Marouf R, Gupta R, Haider MZ, Al-Wazzan H, Adekile AD (2003) Avascular necrosis of the femoral head in adult Kuwaiti sickle cell disease Acta Haematol 110: 11-15.
- Man S, Koren A (1993) Avascular Necrosis of Bones in Children with Sickle Cell Anemia, Pediatric Hematology and Oncology 10: 385-387.
- Mukisi-Mukaza M, Elbaz A, Samuel-Leborgne Y, Keclad L, Turdu-Chicot, et (2000) Prevalence, clinical features, and risk factors of osteonecrosis of the femoral headamong adults with sickle cell disease. Orthopedics 23: 357- 363.
- Lee RE, Golding JS, Serjeant GR (1981) The radiological features of avas- cular necrosis of the femoral head in homozygous sickle cell disease. Clin Radiol 32: 205-214.
- Bazuaye GN, Nwannadi AI, Olayemi EE (2010) Leg ulcers in adult sickle cell disease patients in Benin City. Nigeria. Gomal J Med Sci 8: 190-194.
- Durosinmi MA, Gevao SM, Esan GJ (1991) Chronic leg ulcers in sickle cell disease: Experience in Ibadan, Nigeria. Afr J Med Med Sci 20:11-14.
- Hassan A, Gayus DL, Abdulrasheed I, Umar MA, Ismail DL, et al. (2014) Chronic leg ulcers in sickle cell disease patients in Zaria, Nigeria. Arch Int Surg 4: 141-145.
- Serjeant GR, Serjeant BE, Mohan JS, Clare A (2005). Leg ulceration in sickle cell disease: Medieval medicine in a modern Hematol Oncol Clin North Am 19: 943-956.
- Koshy M, Entsuah R, Koranda A, Kraus AP, Johnson R, et al. (1989) Leg ulcers in patients with sickle cell disease. Blood 74: 1403-1448.
- Minniti CP, Eckman J, Sebastiani P, Steinberg MH, Ballas SK (2010) Leg ulcers in sickle cell disease. Am J Hematol 85: 831-833.
- Dampier C, LeBeau P, Rhee S, Lieff S, Kesler K, et (2011) Comprehensive Sickle Cell Centers (CSCC) Clinical Trial Consortium (CTC) Site Investiga- tors. Health-related quality of life in adults with sickle cell disease (SCD): A report from the comprehensive sickle cell centers clinical trial consortium. Am J Hematol 86: 203-205.
- Trent JT, Kirsner RS (2004) Leg ulcers in sickle cell Adv Skin Wound Care 17: 410-416.
- Ankra-Badu GA (1992) Sickle cell leg ulcers in Ghana. East Afr Med J 69: 366-369.
- Koskinas J, Manesis EK, Zacharakis GH, Galiatsatos N, Sevastos N, et al. (2007) Liver involvement in acute vaso-occlusive crisis of sickle cell disease: prevalence and predisposing factors.Scand J Gastroenterol 42: 499-507.
- Rennels MB, Dunne MG, Grossman NJ, Schwartz AD (1984) Cholelithiasis in patients with major sickle Am J Dis Child 138: 66-67.
- Dalia SaiedMona, S.El-Raziky, Mona K.El-Ghamrawy, MarwaA.Mahmoud (2017) The pattern of hepatobiliary complications among Egyptian sickle cell disease children. Egyptianpaediatric association gazette 65: 54-59.
- Madu AJ, Ubesie AC, Ughasoro M, Ugwu AO, Madu KA, et al. (2017) Cholelithiasis and Hepatic derangement in Cohort of Homozygous Sickle cell disease patients in Nigeria. Nigeria Journal of Haematology 1: 21-27.
- MZ Haider, SAshebu, P Aduh, AD Adekile (1998) Influence of α-thalassemia on cholelithiasis in SS patients with elevated Hb F. ActaHaematologica 100: 147-150.
- JLopez, PO’Keefe, M Morrissey, J Pickleman (1991) Ceftriaxone-induced Annals of Internal Medicine 115: 712-714.
- Haberkern CM, Neumayr LD, Orringer EP, Earles AN, Robertson SM, et al. (1997) Cholecystectomy in sickle cell anemia patients: perioperative outcome of 364 cases from the National Preoperative Transfusion Preoperative Transfusion in Sickle Cell Disease Study Group. Blood 89: 1533-1542.
- William BM, Corazza GR (2007) Hyposplenism: a comprehensive review. Part I: basic concepts and causes. Hematology 12: 1-13.
- Tubman VN, Makani J (2017) Turf Wars: Exploring Splenomegaly in Sickle Cell Disease in Malaria-Endemic Regions.Br J Haematol 177: 938-946.
- Babadoko AA, Ibinaye PO, Hassan A, Yusuf R, Ijei IP (2012) Autosplenectomy of sickle cell disease in zaria, Nigeria: an ultrasonographic assessment. Oman Med J 27: 121-123.
- B Attalla (2010) Abdominal Sonographic Findings in Children with Sickle Cell Journal of diagnostic medical sonography 26: 281-285.
- Balcı A, Karazincir S, Sangun O, Gali E, Daplan T, et (2008) Prevalence of abdominal ultrasonographic abnormalities in patients with sickle cell disease. Diagn Interv Radiol 14: 133-137.
- Awotua-Efebo O, Alikor EA, Nkanginieme KE (2004) Malaria parasite density and splenic status by ultrasonography in stable sickle-cell anaemia (HbSS) Niger J Med 13:40-43.
- Adekile A, Adedodu O, Jeje A, Odesanmi W (1988) Persistent gross splenomegaly in Nigerian patients with sickle cell anaemia: relationship to malaria. Ann Trop Paediatr8:103-107.
- AB Adeyoju, ABK. Olujohungbe, J Morris, A Yardumian, D Bareford, et al. (2002) Priapism in sickle-cell disease; incidence, risk factors and complications – an international multicentre study. BJU International 90: 898-902.
- BF Morrison, UA Anele, ME Reid, WA Madden, Z Feng, et al. (2015) Is Testosterone Deficiency A Possible Risk Factor For Priapism Associated With Sickle Cell Disease? Int Urol Nephrol 47: 47-52
- Aghaji AE (2000) Priapism in adult BJU Int 85: 493-495.
- B Nwogoh, AAdewoyin, GN Bazuaye, IA Nwannadi (2014) Prevalence of priapism among male sickle cell disease patients at the University of Benin Teaching Hospital, Benin City. Nigerian Medical Practitioner 65: 3-7.
- Surafel G, Matthew NS, Drogo KM (2015) Genitourinary manifestations of sickle cell disease. Cleveland clinic journal of medicine 82: 679-683.
- Emond AM, Holman R, Hayes RJ, Serjeant GR (1980) Priapism and impotence in homozygous sickle cell disease. Arch Intern Med 140: 1434-1437.
- Menaa F (2013) Stroke in sickle cell anemia patients: a need for multidisciplinary approaches. Atherosclerosis 229: 496-503.
- Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, et al. (1998) Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranialdoppler New England Journal of Medicine 339: 5-11.
- Ware RE, Davis BR, Schultz WH, Brown RC, Aygun B, et (2016) Hydroxycarbamide versus chronic transfusion for maintenance of transcranial Doppler flow velocities in children with sickle cell anaemia tcd with transfusions changing to hydroxyurea (twitch): A multicenter, open-label, phase 3, non-inferiority trial. TheLancet 387: 661-670.
- Menaa F, Khan BA, Uzair B, Menaa A (2017) Sickle cell retinopathy: improving care with a multidisciplinary J Multidiscip Healthc 10: 335-346.
- Akinyanju OO, Otaigbe AI, Ibidapo MOO (2005) Outcome of holistic care in Nigerian patients with sickle cell anaemia. Clin Lab Haematol 27: 195-199.
Citation: Ladu AI, Abba AM, Ogunfemi MK, Sulaiman MM, Abulfathi FA, et al. (2020) Prevalence of Chronic Complications among Adults With Sickle Cell Anaemia Attending a Tertiary Hospital in North Eastern Nigeria. J Hematol Hemother 5: 007.
Copyright: © 2020 Ladu AI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.