*Corresponding Author:
Macêdo RO,
Department of Pharmaceuti- cal Sciences, Unified Development Laboratories and Essays of Drugs Federal University of Paraíba, 58059-900, João Pessoa, PB, Brazil
Tel: 8332167694
Fax: 8332120131
E-mail: ruimacedo@ltf.ufpb.br
Abstract
Polymyxins subjected to different storage conditions were assessed by the Hen’s egg chorioallantoic membrane at 10th. Samples of polymyxins were stored for 6 months under the following conditions: (P1) polymyxins stored in a refrigerator at temperature between 2 to 8°C, (P2) polymyxins stored in temperature of 25ºC and refrigerated between 2 to 8°C; (P3). Polymyxin was storage in temperatures around 25°C for 6 months. The polymyxin sulphate presented more pharmacological potency in induction of angiogenesis and blood flow hair stored in refrigeration at 2 to 8°C than samples P2 and P3. The Hen’s egg chorioallantoic membrane method allowed to differentiate pharmacological potent of polymyxin sulphate stored in different conditions.
Keywords
Chorioallantoic membrane; Polymyxins; Storage conditions; Toxicity
Introduction
Quality of Pharmaceutical products is essential to assure the efficacy and safety use for the patients. Impurities and potential degradation products can affect chemical, pharmacological and toxicological properties of drugs with significant impact on product quality and safety. Stable drugs and formulations are important to ensure deliver of therapeutic values to the patients [1].
Polymyxins are cyclic peptides which exhibit strong antimicrobial activity against Gram-negative bacteria [2]. Polymyxins can bind to the lipopolysaccharide of the outer membrane of Gram-negative [3]. Polymyxin B is labile in gastrointestinal environment and negligibly absorbed by the intestinal epithelium, used extensively for parenteral and topical treatment of gram-negative infections although the therapy is associated with severe toxicity, mainly nephrotoxicity and neurotoxicity [4]. Although polymyxins have fallen out of favor since their use in the 1960s due to nephrotoxicity and neurotoxicity, they have re-emerged as a promising antibiotic against multidrug-resistant pathogens [3].
The Hem egg chorioallantoic membrane specifically can be used to evaluate the activity or toxicity of a drug [5]. Zwadlo-Klarwasser et al. and Valdes et al. demonstrated that the chick Chorioallantoic Membrane (CAM) model can be used as an alternative for studying the angiogenic and inflammatory response to biomaterials [6,7]. Several in vitro assays have been developed as alternatives test to the in vivo toxicity test. In vitro tests are generally rapid, sensitive, amenable to automation and economical when compared to the use of animals. Different endpoints have been used to assess cytotoxicity in vitro. [8,9] These endpoints generally assess different aspects of cellular functions. The assays neutral red uptake is a measure of lysosomal integrity since it reflects the capacity of viable cells to incorporate vital dye into these organelles [10,11]. The aim of this study was to assess the toxicity of Polymyxins stored under different conditions by the Hem egg chorioallantoic membrane.
Materials and methods
Polymyxins B stored in different conditions
Samples of Polymyxins B were stored under the following three conditions: temperature between 2-8ºC (P1) for a period of nine months; temperature of 25ºC ± 2ºC for a period of six months and refrigerated between 2-8°C (P2) until nine months; and stored at 25ºC ± 2ºC for a period of six months and kept at 25ºC ± 1ºC (P3) until nine months.
Preparation of Polymyxins solution
Pharmaceutical products of Polymyxin B sulfate , containing polymyxin B 500.000 IU, approximately 50 mg of polymyxin B, per vial were obtained from the same manufacturer (IBF Pharma, Pavia - Italia), and were reconstituted with 12 mL of sterile water for injection, immediately before use.
Hen’s egg-chorioallantoid membrane
The 10-day-old fertilized eggs were obtained from Guaraves Guarabira Aves Ltda (Sertãozinho, Paraíba - Brazil) and maintained at 37ºC. The test was performed using five eggs for each test substance, and the egg shell removed above the air space. The Hen’s egg-chorio- allantoic membrane was accessed by removal of the shell membrane above the air cell using a dental drill say blade and forceps. The solutions of the storage conditions P1, P2 and P3 were applied in Hen’s egg chorioallantoic membrane at concentrations of 1000, 2000.and 4000 IU. Vascular changes in the chorioallantoic membrane were observed with time for appearance of angiogenesis and increased blood flow.
Data analysis
The results tests in the CAM were expressed as mean ± S.D., the mean values represent the values five repetitions. For statistical analysis was used the t test, with confidence interval 0.95.
Results
The polymyxin sulfate samples submitted to the studies in different storage conditions were evaluated in chorioallantoic membrane of the egg. Studies with egg chorioallantoic membrane were performed by measuring responses on the initial time to increase blood flow and angiogenesis. The samples P1, P2 and P3 evaluated in the chorioal- lantoic membrane showed increasing in blood flow and angiogenesis which were initiated at the times reported in seconds (s).
(Figures 1A & 1B ) show that polymyxin stored in refrigerator at 2 to 8°C presented increasing of blood flow with 55 s using 1000 IU of antibiotic and was declining up to 26 s at a dose 4000 IU. The start times for onset of angiogenesis have been found 100s for polymyxin dose of 1000 IU and 56s for dose of 4000 IU.
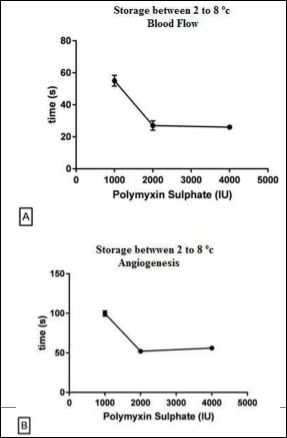
Figure 1: Onset of Blood Flow and Angiogenesis by Polymyxin Sulphate Stored at Temperature between 2 - 8oC and kept at refrigeration until analyses. Blood Flow (A), Angiogenesis (B).
(Figures 2A & 2B) show that polymyxin stored in 25°C for 6 months followed by refrigerated storage showed the onset of blood flow increasing at the 82s for the dose of 1000 IU and 36s in the dose of 4000 IU. In relation to the start time of onset of angiogenesis has been found that at a dose of 1000 IU the time was 112s and 66s at a dose of 4000 IU.
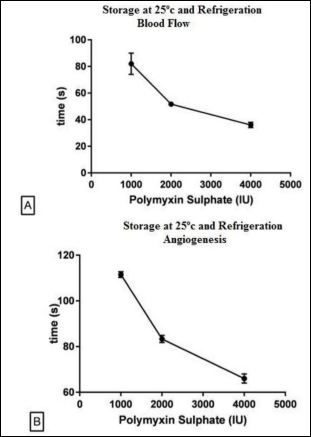
Figure 2: Onset of Blood Flow and Angiogenesis by Polymyxin Sulphate Stored at Temperature 25o C for 6 months and kept in Refrigeration until analyses. Blood Flow (A), Angiogenesis (B).
(Figures 3A & 3B) show that polymyxin stored at 25°C presented an increase in blood flow at time of 75s for the dose of 1000 IU and 36s at a concentration of 4000 IU. The angiogenesis started its onset at 110s for dose of 1000 IU and 66s in the dose of 4000 IU. The curve of the onset of flow was adjusted to a e inverse linear dependence
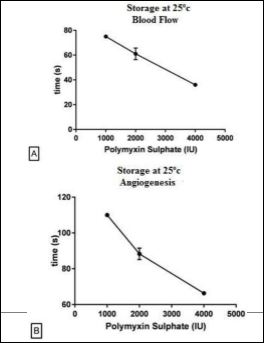
Figure 3: Onset of Blood Flow and Angiogenesis by Polymyxin Sulphate Stored at Temperature 25o C for 6 months and kept at 25o C until analyses. Blood Flow (A), Angiogenesis (B).
The (Table 1 & 2) data showed standard deviation between experiments intraday and different days evidencing the method precision. The samples P1, P2 and P3 evaluated in the Hen’s egg-chorioallantoic membrane promoted increased blood flow and angiogenesis. When analyzed the samples P2 and P3 showed a significant increase (α ≤ 0.05) at the initial time (in seconds) of the sample responses to P1, for both parameters. When P2 and P3 were analyzed samples showed that there was no significant difference between these samples.
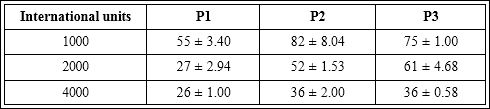
Table 1: Increase blood flow promoted by samples P1, P2 and P3 in chorioallantoic membrane, valued initial time (s).
Increase blood flow which was initiated at the following time (seconds), (n=5).

Table 2: Angiogenesis promoted by samples P1, P2 and P3 in chorioallantoic membrane, valued initial time (s).
Angiogenesis which was initiated at the following time (seconds), (n=5).
Discussion
The biological assays for safety assessment, always been performed in vivo, in animals, since they can be used to evaluate most of the potential risks involved in toxicity. However, some research centers currently are adopting in vitro alternatives to animal testing [12]. The potential use of the Hen’s egg-chorioallantoic membrane represents an alternative to mammalian models can be used to evaluate the activity or toxicity of a drug on both. Toxicity of drugs can be evaluated in terms of embryo death or adverse effects on the CAM including inflammation and neovascularization [5]. The samples P1, P2 and P3 evaluated in the chorioallantoic membrane promoted increased blood flow and angiogenesis. The samples P2 and P3 were similar regarding the initial time to onset of increased blood flow and angiogenesis compared to sample P1. Samples P2 and P3 were previously stored at a temperature of 25 ± 2°C for a period of six months, showing changes in pharmacological responses of polymyxin B in the vascular system. Due to their possible composition, pharmaceuticals are especially sensitive to environmental factors. Strict storage conditions are necessary for the maintenance of integrity and product activity. The data showed that the sample P1, stored under refrigeration for 06 months, showed higher pharmacological activity in increasing the blood flow and the ability to enhance angiogenesis in relation P2 and P3.
Conclusion
The polymyxin B sulfate had an increase of blood flow and angiogenesis in the product stored under refrigeration at 2 to 8°C. Products stored at a temperature of 25 ± 2°C decrease in pharmacological activity increasing the time of onset blood flow and angiogenesis.
The Hen’s egg chorioallantoic membrane method allowed differentiating pharmacological potency of polymyxin sulphate stored in different conditions.
References
- Ivana I, Ljiljana Z, Mira Z (2006) A stability indicating assay method for cefuroxime axetil and its application to analysis of tablets exposed to accelerated stability test conditions. J Chromatogr A 1119: 209-215.
- Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K (2005) Evaluation of colistin as an agent against multi-resistant Gram-negative Int J Antimicrob Agents 25: 11-25.
- Urakawa H, Yamada K, Komagoe K, Ando S, Oku H, et al. (2010) Structure-activity relationships of bacterial outer-membrane permeabilizers based on polymyxin B heptapeptides, Bioorg Med Chem Let. 20: 1771-1775.
- Falagas ME, Kasiakou SK (2006) Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 10: 27.
- Vargas A, Zeisser-Labouèbe M, Lange N, Gurny R, Delie F (2007) The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv Drug Deliv Rev 59: 1162-1176.
- Zwadlo-Klarwasser G, Gorlitz K, Hafemann B, Klee D, Klosterhalfen B (2001) The chorioallantoic membrane of the chick embryo as a simple model for the study of the angiogenic and inflammatory response to biomaterials, J Mater Sci Mater Med 12: 195-199.
- Valdes TI, Kreutzer D, Moussy F (2002) The chick chorioallantoic membrane as a novel in vivo model for the testing of J Biomed Mater Res 62: 273-282.
- De Conti R, Oliveira DA, Fernandes AMAP, Melo PS, Rodriguez JA, et al., (1998) Application of A Multi-Endpoint Cytotoxicity Assay to the Trypanocidal Compounds 2-Propen-1-Amine Derivatives and Determination of their Acute Toxicity. Mary Ann Liebert Inc Publ 11: 153-160.
- Melo PS, Durán N, Haun M (2002) Derivatives of dehydrocrotonin, a diterpene lactone isolated from Croton cajucara: Cytotoxicity in rat cultured hepatocytes and in V79 cells, Hum Exp Toxicol 21: 281-288.
- Renzi D, Valtolina M, Forster R (1993) Evaluation of a multi-endpoint cytotox- icity assay system, ATLA-Altern Lab Anim 21: 89-96.
- Chorilli M, Tamascia P, Rossim C, Salgado HRN (2009) Ensaios Biológicos para avaliação de segurança de produtos cosméticos. Rev Ciênc Farm Básica Apl 30: 19-30.
- Ahuja S, Alsante K (2003) Handbook of Isolation and Characterization of Impurities in Pharmaceuticals (5th edn). San Diego, USA Pg no: 430.
Citation: Cavalcante HMM, Macêdo RO (2016) Pharmacological Ac- tivities of Angiogenesis and Increasing Blood Flow by Polymyxin Sul- fate Stored In Different Conditions. J Hematol Hemother 1: 002.
Copyright: © 2016 Macêdo RO. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.