*Corresponding Author:
Emam MAR Kheder,
Department of Orthopedics, King Fahd Military Medical City, Dhahran, Saudi Arabia
Email: emamkheder@gmail.com
Abstract
Background: Lumbo-Pelvic (LPP) pain is common and non spe- cific problem during pregnancy and post partum. Despite the fact that Perinatal Pyogenic Sacroiliitis (PSI) during this period is rare, it should be considered as a vital differential diagnosis in women who have debilitating lower back and pelvic girdle pain.
Case: A 34 years old primigravida presented to the emergency de- partment with extreme right sided lower back pain radiating to the right gluteal region and down to the back of the right thigh. This pain began twelve days prior to her presentation and eventually wors- ened to the point that she couldn’t stand or walk. Her vital signs were within normal limits, and she was febrile. Apart from a slight widening of the symphysis pubis, her pelvic and lumbo-sacral plain x-rays revealed no important findings. With the clinical impression of right LPP, the patient was admitted for pain management and further inquiries.
Conclusion: Despite the fact that lower back and pelvic girdle pain are normal throughout pregnancy and the postpartum period, peri- natal PSI is uncommon. It’s a tough diagnosis to make because the symptoms and signs aren’t clear, and the tests aren’t definitive. When pathognomonic clinical and radiologic signs indicate an infec- tious process and isolation of pathogenic bacteria is not possible, medical management with empirical antibiotics should not be de- layed.
Keywords
Infection; Magnetic resonance imaging and pregnancy; Pelvic pain; Sacroiliac joint
Introduction
Case Presentation
A 26-year-old woman, morbidly obese with a BMI of 43, gravida-1, para-1+0, type 2 diabetes mellitus and hypothyroidism. She came to the causality with twelve days history of severe right sided lower back pain, right side pelvic pain increased with ambulation. Her pain started in a mild way prior to her recent admission for elective cesarean section for twin babies, using spinal epidural anesthesia, got worse and began to radiate to the back of the right thigh, increased with ambulation. There was no history of trauma, fever, chills, urinary, gynecological or other systemic symptoms. Her physical examination revealed a temperature of 37.1ºC, severs tenderness over the lower back, and right side of the pelvic region with intact neurology. Her cesarean section wound in the abdomen has healed. The straight leg rising test was positive (30-40 degrees), and the Faber Patrick’s test was difficult to evaluate due to intense pain despite the strong analgesia.
The lumbo-sacral spine X-ray was unremarkable, but the pelvis X-ray revealed a 12 mm widening of the symphysis pubis (Figure 1).
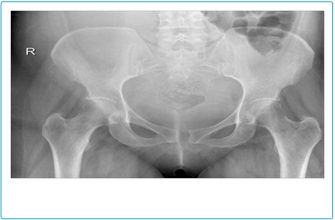
Figure 1: AP plain radiographic of the pelvis and sacroiliac joints showing little wid- ening of the symphysis pubis.
She was hospitalized for further examinations and pain control after a clinical diagnosis of perinatal right-sided LPP. Laboratory testing found 6.85 white blood cells (WCC) G/l with 69.2% neutrophils, a 48 mm/h elevated erythrocyte Sedimentation Rate (ESR) (Table 1), normal procalcitonin (0.02), and a 68.7 mg/l elevated C- reactive protein (Table 2).
The lumbar spine Magnetic Resonance Imaging (MRI) was negative, but the pelvic MRI found minor fluid signal amplitude in the right sacroiliac joint, which was associated with myositis (Figure 2).
The patient was accused of developing unilateral sacroiliac arthritis and piriformis muscle syndrome. In the context of pain modality and incremental mobilization, we began her medical care with narcotic and non-narcotic analgesics, non-steroidal anti-inflammatory drugs and physiotherapy. Her pain could not be controlled, and she became worse, unable to ambulate or step in or out of bed. Neuro- surgery, neurology, rheumatology, anesthesia, and obstetrics and gy- necology teams were consulted, but nothing was added to the pre- liminary diagnosis or the treatment plan. Despite the absence of any symptoms or signs indicating an acute or chronic infection, urine and blood cultures, PPD (protein purified derivative) for tuberculosis, and serology for brucella were required on day 8. Because it was impos- sible to place the patient in the prone position on the same day, CT guided aspiration of the right Sacroiliac Joint (SIJ) was performed in the left lateral position (Figure 3), but no aspirated fluid was obtained, however SIJ block was performed using 20 ml of 0.25% bupivacaine after which she briefly noticed some relief of her shooting pain.
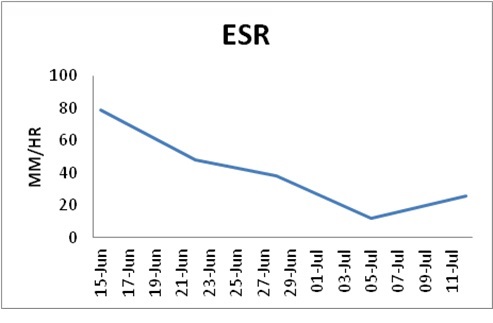
Table 1: ESR mm/hr.
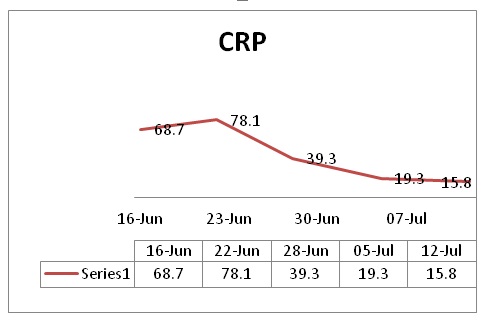
Table 2: CRP mg/l.
Although the blood culture, brucella serology, and PPD all came back negative, the urine culture revealed asymptomatic E. coli bac- teriuria. The patient was started on IV ceftriaxone 1 gram every 12 hours and gentamycin 400 mg once a day by the ID team. She showed steady and progressive clinical improvement just 48 hours after IVAB (Intravenous Antibiotics). Ceftrixone was given for another two weeks, and gentamycin was given for another ten days. Her pain score (VAS 2 out of 10) improved significantly, as did her walking capacity, bathroom privileges, and blood parameters. The patient was released at home on oral ciprofloxacin 750 mg twice daily for 6 weeks after discharge on day 26. Nevertheless, her MRI on institutional discharge showed no substantial improvement compared to the initial one. She did well with regular ambulation and normal blood parameters at follow-up visits, 6 weeks, 3 months and 6 months. Her one-year fol- low-up MRI was uneventful (Figure 4), while she did have periodic minor right-sided lumbo-pelvic pain that did not interfere with her daily activities.
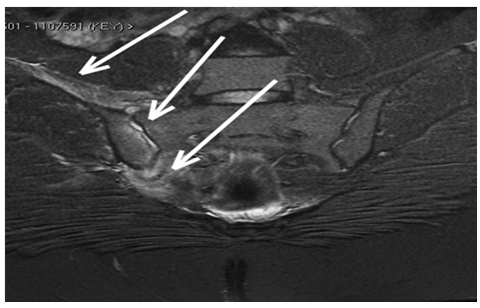
Figure 2: Pelvic MRI on admission showing Bone marrow edema, small intraarticular fluid and muscle edema-like change (myositis).
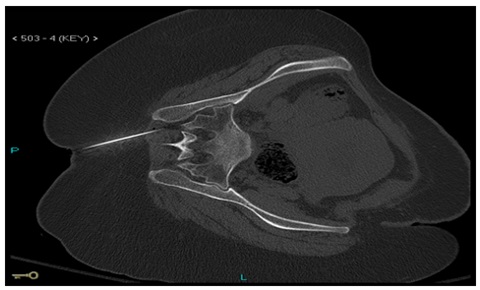
Figure 3: CT guided right SI joint trial of aspiration in left lateral position.
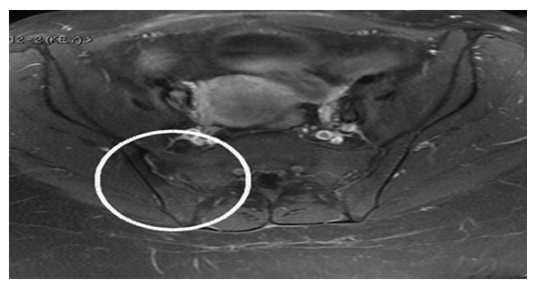
Figure 4: A year later, a pelvic MRI reveals normal bone marrow signal and normal surrounding muscle. There are no intraarticular fluids.
Discussion
Pain in the lower back and buttocks is normal and sometimes non- specific in 20% of pregnant women which can render diagnosing sac- roiliac joint disorders difficult [6]. Despite the fact that PSI accounts for just 1 to 1.5 percent of all septic arthritis cases, approximately 10% or more of these infections occur in women during pregnancy, postpartum, or after an abortion [7,8].
The involvement of the Sacroiliac Joint (SIJ) in pyogenic sacroilii- tis is always unilateral, with left side predominance in 59% of cases. The diagnosis is rarely (12.85%) suspected on admission and the clin- ical picture may be misinterpreted as sciatica or spondylodiscitis. This atypical clinical presentation appeared to explain the lengthy time to diagnosis [9].
Owing to the increased weight and hormone-induced changes in the pelvis, the pelvic ligaments relax with increased pelvic move- ments, which can influence the microvasculature of joint surfaces, rendering the periosteum more susceptible to transient bacteraemia and bacterial invasion [10-12]. Furthermore, the venous plexus sys- tem, which drains the paravertebral and pelvic areas and the sub- chondral circulation in the ilium have a sluggish blood flow, which is thought to increase the risk of blood-borne bacteria forming a host site in the Sacroiliac Joints (SIJ) [13,14].
ISI can be diagnosed if bacteriological confirmation of sacroilii- tis was obtained, or if, in the absence of pathogenic agents, clinical, biological, and radiological evidence is consistent with this diagnosis and evolution was favorable on antibiotic therapy [9].
Despite the fact that urinary tract infection is one of the most com- mon causes of reactive arthritis and may be a risk factor for ISI, yet it was extremely difficult to distinguish between reactive and infectious arthritis in our case [15]. The most frequent manifestations of pyogen- ic sacroiliitis are localized pain, sacroiliac joint tenderness and am- bulatory impairment. The endurance capacity for standing, walking, and sitting is diminished. Fever is absent in a substantial number of cases [3]. The physical examination and special tests like pain prov- ocation tests, P4/thigh thrust, Patrick’s Faber and the Active Straight Leg Raise (ASLR) test are recommended but are not conclusive [16].
The laboratory tests initially increased the difficulty of diagnosis as they are non-specific. Leucocyte count has not been found to be a sensitive marker of pyogenic sacroiliitis and one third of the patients have a normal white cell count, however the level of C-Reactive Pro- tein (CRP) and erythrocyte sedimentation Rate (ESR), may be a rela- tively sensitive markers of ISI [3,8,9]. Blood cultures are positive in only one-third to two thirds of the patients [17]. Gram-positive cocci, predominantly S aureus, have been reported to be the most frequently cultured organisms and less than 20% of previously reported cases were caused by Gram-negative bacillus, of which Pseudomonas aeru- ginosa and Escherichia coli were the most commonly isolated [18]. Invasive diagnostic procedures in patients whose blood culture results fail to disclose a causative pathogen are a last resort, as collection of synovial fluid from the sacroiliac joint is difficult process in such patients who are in agony during positioning and during the technique itself. Confirmation frequently requires a CT-guided needle aspiration, fluoroscopic guided fine-needle aspiration or open biopsy [19].
Plain roentgenogram of the pelvis are usually normal at presen- tation [1,8]. The earliest changes on plain films are blurring of joint margins, an expanded joint space, or periarticular erosion, which ap- pear 2 weeks after the onset of symptoms [7] since major bone de- struction is needed before changes can be seen on plain films. As a result, false negative radiographs are usual in acute cases, and caution should be exercised when ruling out a recent infection based on ini- tially normal-looking photos [20-22]. In the perinatal phase, MRI is most likely the imaging diagnostic tool of choice for detecting sacroi- liitis. It allows for a thorough examination of the joint and underlying soft tissue in pregnancy without exposing the fetus to ionizing radiation [2]. When low signal intensity on T1 and high signal intensity on T2 were observed on oriented MRI slices, Pyrogenic Sacroiliitis (PSI) should be suspected [9]. This test also allows physicians to see whether the infection has spread to nearby muscle tissues, which was seen in 48.1 percent of cases [23].
Because MRI signal anomalies can last for months, even though there is no fever or clinical and biological progress appears to be on the way, it should be done in a systematic manner [24]. The most sen- sitive imaging modality for infection is 99mTc radionuclide scanning. Increased radionuclide uptake in the sacroiliac region can happen as early as two to seven days after the illness begins. 6’22” As a result, a positive bone scan will help to avoid delays in diagnosis and care. Al- though highly sensitive for infection, the specificity of bone scanning is low. A Technetium scan should be accompanied by a Gallium-67 citrate (67Ga) scan in cases of suspected sacroiliac infection. This radiopharmaceutical is useful for detecting infections because it has a preference for polymorphonuclear leucocytes (PMNs). 23. Although radionuclide scans can be useful for monitoring post-delivery care, they should not be used during pregnancy [25-27].
In suspected pyogenic sacroiliitis, CT can reliably direct joint as- piration of joint fluid. If an aspirate is not obtained, flushing the joint with normal saline and aspirating the saline will increase the likeli- hood of obtaining a positive culture. A para-articular bone biopsy may be used to confirm or rule out infection if sacroiliac joint fluid cannot be collected despite saline flushing, as in tuberculous sacroiliitis. CT can also be used to guide pigtail insertion into pyogenic sacroiliitis abscesses [28].
The treatment for pregnancy-related pyogenic sacroiliitis is close to that for non-pregnancy-related cases [4]. Medical care should not be deferred because delayed diagnosis and treatment can lead to not only joint and bone damage, but also maternal and neonatal septi- cemia [5]. There is no consensus about how long antibiotic therapy should last in PSI, but it seems fair to suggest parenteral treatment for two weeks followed by oral treatment for six weeks. Prolonging care beyond 6 weeks does not seem to be justified, as it does not re- duce the likelihood of relapse [29] In suspected PSI or in the absence of any known microorganism, empirical antibiotic treatment active against Staphylococcus [30] should be considered before the specific organism(s) and antimicrobial sensitivities have been established and, in the event of failure, should be extended to include Gram-negative bacilli [9,31].
Surgical intervention, such as incision and drainage, has been shown to aid clinical recovery when used in conjunction with anti- biotic therapy [32]. Long-term follow up might be needed as lumbo- gluteal pain that worsened during daytime activities was reported to persist in more than one-third of cases in the literature [33].
Conclusion
Perinatal sacroiliitis is a difficult diagnosis to make since the symp- toms and signs are nonspecific, and investigations are inconclusive, thus delaying proper care. The high intensity of patient’s lumbo-glu- teal pain on presentation, which is exacerbated by weight bearing or some effort to shift the sacroiliac joint, may help doctors differentiate PSI from other causes of musculoskeletal pain. Pain aversion to opi- oids and/or non-narcotic analgesics, a dramatic clinical reaction to parentral antibiotics, and the existence of a defined source of infec- tion are all additional diagnostic clues. In the absence of a fever or a positive blood culture, as well as standard biological parameters like ESR, CRP, and WCC, MRI is the most effective imaging technique for determining early and subsequent joint changes
References
- 1. Mens JMA, Vleeming A, Snijders CJ (2001) Reliability and validity of the active straight leg raise test in posterior pelvic pain since Spine 26: 1167-1171.
- 2. Jedwab M, Ovadia S, Dan M (1999) Pyogenic sacroiliitis in pregnancy. International Journal of Gynecology & Obstetrics 65: 303-304.
- 3. Almoujahed MO, Khatib R, Baran J (2003) Pregnancyassociated pyogenic sacroiliitis: Case report and Infect Dis Obstet Gynecol 11: 53- 57.
- 4. Egerman RS, Mabie WC, Eifrid, Whitnack E, Sibai BM (1995) Sacroiliitis associated with pyelonephritis in Obstet Gynecol 85: 834-835.
- 5. Cekmez Y, Göçmen A, Arslan O, Şanlıkan F, Türkmen SB (2015) A rare reason for pelvic pain in pregnancy: Infectious sacroiliitis. Case Reports in Medicine
- 6. Gordon G, Kabins SA (1980) Pyogenic Am J Med 69: 50-56.
- 7. Millwala F, Chen S, Tsaltskan V, Simon G (2015) Acupuncture and post- partum pyogenic sacroiliitis: A case report. Journal of Medical Case Re- ports 9.
- 8. Vyskocil J, McIlroy M, Brennan T, Wilson FM (1991) Pyogenic infection of the sacroiliac joint: Case report and review of the literature. Medicine 70: 188-197.
- 9. Hermet M, Minichiello E, Flipo RM (2012) Infectious sacroiliitis: A retro- spective, multicentre study of 39 adults. BMC Infect Dis 12: 305.
- 10. Haq I and Morris V (2001) Post-partum septic sacroiliitis. Rheumatology 40: 1191-1192.
- 11. Moros ML, Rodrigo C, Villacampa A, Ruiz J, Lapresta C (2009) Septic shock in pregnancy due to pyogenic sacroiliitis: A case report. Journal of Medical Case Report.
- 12. Kanakaris NK, Psarakis S, Chalidis B, Kontakis, Giannoudis PV (2009) Management of pelvic instability secondary to chronic pyogenic sacroilii- tis: Case report. Surg Infect (Larchmt)10: 353-358.
- 13. Batson OV (1940) The function of the vertebral veins and their role in the spread of metastases. Ann Surg 112: 138-149.
- 14. Resnick D, Niwayama G (1981) Diagnosis of bone and joint disorders (1st edn). Saunders, Philadelphia,
- 15. Butrimiene I, Ranceva J, Griskevicius A (2006) Potential triggering infec- tions of reactive arthritis. Scand J Rheumatol 35: 459-462.
- 16. Vleeming A, Albert HB, Ostgaard HC, Sturesson, Stuge B (2008) Euro- pean guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J 17: 794-819.
- 17. Kerr R (1985) Pyogenic Orthopedics 8: 1028-1034.
- 18. Bindal M, Krabak B (2007) Acute bacterial sacroiliitis in an adult: A case report and review of the Arch Phys Med Rehabil 88: 1357-1359.
- 19. Murphey MD, Wetzel LH, Bramble JM, Levine E, Simpson KM, et al. (1991) Sacroiliitis: MR imaging Radiology 180: 239-244.
- 20. Dunn E, Bryan D, Nugent J, Robinson RA (1976) Pyogenic infections of the sacro-iliac Clin Orthop Relat Res 118: 113-117.
- 21. Cohn SM, Schoetz DJ (1986) Pyogenic sacroiliitis: Another imitator of the acute Surgery 100: 95-98.
- 22. Linnet KM, Gammelgaard L, Johansen M, Krarup N, Rasmussen KL (1996) Bilateral pyogenic sacroiliitis following uncomplicated pregnancy and Acta Obstet Gynecol Scand 75: 950-951.
- 23. Braun J, Sieper J, Bollow M (2000) Imaging of sacroiliitis. Clin Rheuma- tol 19: 51-57.
- 24. Stürzenbecher A, Braun J, Paris S, Biedermann T, Hamm B, et al. (2000) Imaging of septic Skeletal Radiol 29: 439-446.
- 25. Shapiro SK, See CE (1986) Pyogenic Minn Med 69: 201-204.
- 26. Gupta S, Herrera N, Chen C (1982) Scintigraphic demonstration of pyo- genic Clin Nucl Med 7: 295-296.
- 27. Kirchner P, Simon M (1981) Current concepts review: Radioisotopic eval- uation of skeletal J Bone Joint Surg Am 63: 673-681.
- 28. Braun J, Bollow M, Seyrekbasan F, Häberle HJ, Eggens U, et al. (1996) Computed tomography guided corticosteroid injection of the sacroiliac joint in patients with spondyloarthropathy with sacroiliitis: Clinical out- come and followup by dynamic magnetic resonance J Rheumatol 23: 659-664.
- 29. Roblot F, Besnier JM, Juhel L, Vidal C, Ragot S, et al. (2007) Optimal duration of antibiotic therapy in vertebral osteomyelitis. Semin Arthritis Rheum 36: 269-277.
- 30. Doita M, Yoshiya S, Nabeshima Y, Tanase Y, Nishida K, et (2003) Acute pyogenic sacroiliitis without predisposing conditions. Spine 28: 384-389.
- 31. Wu MS, Chang SS, Lee SH, Lee CC (2007) Pyogenic sacroiliitis-a com- parison between paediatric and adult Rheumatology (Oxford) 46: 1684-1687.
- 32. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, et (2006) Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 355: 666-674.
- 33. Mancarella L, De Santis M, Magarelli N, Ieradi AM, Bonomo L, et al. (2009) Septic sacroiliitis: An uncommon septic arthritis. Clin Exp Rheu- matol 27:1004-1008.
Citation:Khede EMAR (2021) Perinatal Sacroiliitis Diagnostic Challenges: A Case Report. J Case Repo Imag 5: 040.
Copyright: © 2021 Khede EMAR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


