*Corresponding Author:
Lisa L Dean,
Food Science and Market Quality and Handling and Research Unit, USDA, Agricultural Research Service, Raleigh, US, NC 27695-7624
Tel: 919-515-9110
E-mail: Lisa.Dean@usda.gov
Abstract
Peanuts are a source of vegetable protein and interest in applications and uses have increased greatly in the past few years. The use of peanuts as a source of edible oil produces millions of tons of high-protein processing byproduct material that is available for food applications. A review of the literature for the past five years has produced a range of studies for the high protein meal and for protein isolates that adds value to the peanut crop. Food uses include flours in baked goods, meat and dairy substitutes, and extruded snacks. Peanut protein isolates have been used as gels, emulsifiers, salt substitutes, nanoparticles, and bioactive peptides. Issues of using peanut protein include solubility, oxidation, degradation and allergenicity which researchers are working to address. Peanuts have proven to be a valuable source of protein for humans beyond conventional consumption of the whole seed.
Keywords
Emulsifiers; Protein isolates; Peanut protein; Vegetable protein
Introduction
The current consumer interest in plant-based sources of protein has increase exponentially over the past few years. One of the drivers is the carbon footprint which is an important measure of food production sustainability. Meat animals for protein range from 2 to 150 kilograms CO2 per kilogram of the finished meat product, while vegetable sources range from only 1 to 2 [1]. Non animal protein ingredients have traditionally been sourced from soybean and wheat gluten. Newer ones have included pea, tree nuts, rice, quinoa, and lentils among others [2]. The widening of the scope of the sources of these alternative proteins can provide opportunities for the use of many other foods. The inclusion of peanut as such a protein source should be considered as the protein content is higher than that of other plant sources [3]. In addition, peanut protein is the third largest source of nonmeat protein available globally [4]. Peas and beans contain between 5 and 13 grams per 100 grams with soybean being the highest. Leafy green foods such as spinach and grains such as rice contain less than 3 grams per 100 grams. Peanut contains between 20 and 25 grams per 100grams compared to trees nuts with values between 9 and 22 grams per 100 grams [5].
Peanuts (Arachis hypogaea L.) are a legume crop with the unusual growth characteristic of producing their seeds in pods underground. As a commodity crop, peanuts are grown in many parts of the world with tropical or subtropical climates. The leading countries in peanut production are China with 17.5 million metric tons, India with 6.5 million metric tons followed by Nigeria with 3.9 million metric tons and the USA with 2.8 million metric tons [6]. The availability of peanuts throughout the world positions them well to be a source of plant-based protein. In the past few years, approximately 50 % of the global peanut crop of 40 to 50 million metric tons of peanuts has been crushed for oil, leaving 20 to 25 million metric tons of defatted peanut meal containing approximately 50% protein. This high-quality protein is available for further value-added applications [7]. Peanut is an incomplete protein. Several of the essential amino acids are below the level for optimum human nutrition. Transgenetic lines have been developed with increases in the limiting amino acids, valine, isoleucine, leucine, methionine, and threonine with corresponding decreases in tyrosine, phenylalanine, and cysteine [8]. Not only amino acid content, but the protein digestibility is important when evaluating protein nutrition. Peanut holds the advantage in that compared to soy, the in vitro digestion is higher (81% for peanut and 72% for soy) indicating that peanut protein is more bioavailable [9]. When measured in the raw peanut, the value is even higher at 92.65%.
The use of peanut protein in foods in the past have been readily accepted [10]. As one of the world’s most widely grown oilseeds, the protein containing portion remaining after crushing is prized for its low processing cost, lack of intense flavor and low color impact. A review of using peanuts as an ingredient in food products included a description of the influence of protein on human health as part of the whole seed [11]. This review reports on studies in the past decade that used peanut protein as a functional ingredient.
Peanut Processing
When roasted either under dry conditions with an oven or in oil, peanuts have a texture and flavor that is similar in character to tree nuts, so they are often classified with them as “nuts”. When boiled, their flavor and texture is similar to other legumes such as soy and chickpeas. Further processing of roasted peanuts includes peanut butter, peanut oil and peanut flour. Roasting produces Maillard reaction products from the thermally catalyzed polymerization of certain amino acids with reducing sugars. Many of these reaction products have proven to have antioxidant capacities [12].
The crushing of peanuts for the production of peanut oil results in a large amount of solid material containing high levels of protein. Of the global production of peanuts in 2020 of 47 million metric tons, over 50% are grown in China and India [13]. From these crops, 90% are used for oil production [14]. Finding applications for the defatted peanut material has driven much of the research into peanut protein. Traditionally, oilseed cakes have found use as animal feed, but the quality of peanut protein lends itself to more value-added products. Peanut flour, which is the partially defatted solid material, usually from roasted peanuts is used in baked goods in combination with wheat flour and increases the protein content over products made with wheat flour alone [15,16].
The high-quality protein content of peanut flour compared to other plant sources is the driver for its use in novel food products for health [17]. The treatment used to remove the oil denatures the peanut protein which reduces the solubility and negatively affects the functionality [18]. Recovery of protein from peanut is only 40 to 50 % due to the low solubility in buffer systems [19]. Subsequent treatments have proven to improve the functionality. Reverse micelles are created from surfactants such as sodium sulfosuccinate. These compounds form aggregates with polar cores when in an organic solvent. The cores solubilize the proteins which are hydrophilic and remove them from the oil matrix [20,21]. This technique serves to allow for isolation of the protein without decreasing functionality.
Peanut “milks” have been produced which are analogous to other types of plant “milks” [22]. The product is made by soaking the roasted kernels in water, then blending and straining away the remaining solid material. These products are emulsified systems with the protein suspended in an aqueous phase along with the starch, oil bodies and other solids. Using roasted peanuts rather than raw ones suppresses the unfavorable beany and green flavors as well as giving a more appealing color and appearance and improving the shelf life [23]. Products containing peanut protein in combination with melon and coconut milk were found to be acceptable to consumers with protein contents of 2.16 g of protein per 100 mL with a moderate liking score of 6.55 ± 1.88 on a 9 point hedonic score [24]. These beverage products are ideal for delivery systems for nutrition in parts of the world that are at risk for severe malnutrition. The high-quality protein combined with the positive consumer acceptance result in a readily accepted product [25].
The product can often be produced from ingredients available in the areas of risk and without need for refrigeration. When the milk products are homogenized, the glycoproteins present aid in keeping the milk stable and prevent the oil bodies from separating. Using roasted peanuts increases the content of these protein complexes and produces a more stable final product [22]. Oxidation can occur in protein which in turn affects the stability in food applications. This has been especially studied in beverage applications [26].
The milk type product created from defatted peanut meal can be used in place of egg yolk. A mayonnaise has been produced to take advantage of the emulsifying properties of peanut protein [27]. When peanut protein was substitute for egg yolk in increasing amounts, the product held more water and was thinner than the full egg product but was an acceptable substitute. The end result is a cholesterol free product which can be considered healthier.
The functional properties of proteins are greatly influenced by their physical structure as previously determined in other types of proteins [28]. The physical structure of peanut protein can be modified using High Pressure Microfluidization (HPM) to disrupt the globular structure by interrupting the bonds between the hydrophobic amino acid side chains such as the sulfhydryldisulfide, the hydrogen bonds and the electrostatic attraction [29]. The protein unfolds in an aqueous solution and is more easily fragmented. Aggregation can then more easily occur. This aids in expanding the functionality of the peanut protein. For instance, HPM treatment of peanut protein creates small peptides with improved ACE inhibitory activity [30]. The peanut protein, arachin was found to have greater solubility, emulsion stability and increased surface hydrophobicity when high pressure was used for the extraction [31]. The peanut protein, Conarachin II was found to be more sensitive to high pressure changes in an earlier study [32]. The high-pressure treatment resulted in increased gel hardness, better water and oil holding capacity and opacity of the gels formed.
The extraction processes used to isolate the proteins from the peanut matrix influence the efficiency of the extraction and the functionality of the concentrates. Aqueous extraction was compared to solvent assisted extraction performed both before and after pressing of the peanut to remove the oil [33]. Less protein was recovered using an aqueous system. The protein recovered had less solubility and was more hydrophobic. This resulted in lower water holding capacity and weaker emulsifying capacity. Figure 1 shows the scheme for extracting protein from a emulsion of peanuts in water. The solvent extraction methods produced proteins that created better foams. In addition, when the extracts were freeze dried, the extracts had higher solubility than spray drying, which influences how they will perform in beverage applications.
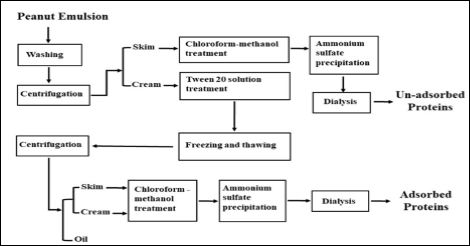
Figure 1: Extraction procedure for peanut proteins [34] (Reproduced with permission).
Using peanut protein is sometimes limited by the solubility of the proteins. Solubility has been statistically linked to protein size and cysteine content [35]. It is necessary for applications of isolated proteins in food products to have a Nitrogen Solubility Index above 80%, but for peanut, this value is usually only 65% using the normal alcohol extraction [36]. A variety of techniques have been used to increase this value. The release of peanut protein from cells to create isolates has been successful using aqueous based enzymatic extractions which eliminates the need for solvents [37-40]. Thermosonication prior to enzyme treatment has been shown to allow for better accessibility of the enzymes by unfolding of the globular proteins [41]. Subsequently, the protein is hydrolyzed for better functionality and the oil bodies present remain intact for efficient recovery. In addition, sonication can reduce particle size to make extractions of the proteins more efficient [42]. Ultrasound combined with microwave heating was reported to increase the yield and functionality of protein extracted from partially defatted peanut flour (9% fat, 55% protein) [43]. The extraction procedure used affects the functionality of the protein recovered. Meat analogs have been successfully prepared from peanut protein extracted using aqueous or solvent extraction as well as cold pressing as the gelling properties are still suitable [7]. Modification of the isolated protein by phosphorylation of certain amino acid side chains improves the physical properties [44]. From the specific protein, Arachin conarachin, the hydroxyl groups of serine, threonine and tyrosine as well as the amino group side chains of lysine and arginine are phosphorylated and become negatively charged enabling them to become hydrated which increases dissolution and solubility and emulsification creating more stable foams.
Applications of Peanut Protein
Meat analogs have continued to be a strong driver of using vegetable proteins sources and are achieving higher acceptability [45]. Soy is often used to create plant-based analogs. Peanut protein can also be used in this way but requires treatment to adequately form the fiberous structure. Use of extrusion technology with a high moisture matrix such as peanut does not produce a suitable product. The use of transglutaminases to modify the protein has been reported [46,47]. The fiberous structure characteristic of meat is the goal of creating meat analogs from vegetable proteins. The incorporation of polysaccharides such as starch, carrageenan and sodium alginate, allows for the binding of moisture to form the correct structure, tensile strength and springiness to serve as a meat analog [48]. Extrusion techniques are used to produce peanut protein products with fiberous textures. Optimization of the techniques has focused on preserving that texture. The lack of tensile strength in extruded peanut protein used to produced meat analogs have been a drawback for peanut protein [49]. The combination of peanut protein with either soy protein isolates or wheat gluten has been used to overcome this deficiency.
Protein isolated from peanut flours was supplemented with cow’s milk to produce a hybrid vegetable protein-dairy protein cheese product [50]. The heating required to produce the product resulted in destruction of the some of the essential amino acids (threonine and lysine). When compared to a product made from soy protein, the yield was less as was the firm texture, but the protein content was higher when peanut protein was used. A similar product using only peanut “milk” or water extracted peanut protein coagulated with calcium sulphate and then treated with high pressure was referred to as a peanut cheese (paneer-style) [51]. The resulting product was stable for up to 45 days and had better consumer acceptability than similar products produced from soy protein and from traditional paneer produced from cow’s milk. It is feasible to consider using peanut protein in vegan dairy substitutes.
The production of gels from peanut protein by interactions with polysaccharides is an active area of research. The conditions of pH, temperature, enzymes best suited for hydrolysis, among others have been reported [52-55]. In general, peanut proteins have poor gelation properties but these can be improved by certain enzyme treatments such trans glutaminases which allow for a better cross linking by catalyzing acyl transfers between the side chains of certain amino acids present such as glutamine and lysine [46]. The adjustment of the pH to a value of 10 and the application of heat treatment of 40°C to a solutions of peanut protein isolates resulted in increased solubility, water interactions with the protein as well as protein- protein interactions [55]. The gels produced had the best water holding capacity and gel strength. Additionally, using cold plasma to bind peanut protein to sugar molecules through bonding with the tryptophan and tyrosine present in the protein isolate creates a gel with additional strength [56]. The greater solubility of the peanut protein after cold plasma treatment assists in the water binding [57]. Gels from peanut protein isolates derive their character from the composition of the protein present. When the gels are higher in conarachins than arachins, the gels will have increased firmness and the ionic strength of the isolate determines the solubility of these proteins [58]. The cross linking between the conarachins and arachins differs due to the total number of free sulfur groups present [59]. Using a surfactant to increase the efficiency of extraction of the peanut protein was found to increase the emulsion ability and stability due to increased protein solubility [52]. The gelation strength of the protein was however found to decrease.
Breads for gluten sensitive consumers have been produced from a range of alternative grains and pulses. When pressed for oil, the remaining oilcake from peanuts can contain a significant amount of protein which can be used to make bread like products that are gluten free. A flatbread product combining peanut oil cake with a protein level of 42% combined with quinoa and broccoli was found to be acceptable to over 70% of the consumer panelists [60]. Mixtures of wheat and peanut flours to produce an acceptable bread product resulted in a range of issues with the final characteristics of the product such as color, loaf volume and weight, and crumb moisture, texture, and density [61]. The most acceptable mixture from the sensory analyses data proved to be a 10% substitution of peanut flour for wheat flour. There are cases where it is undesirable for the peanut protein to form a gel. It has been found that pH adjustments below 2 and above 12 prevent gel formation [62]. These pH values are outside those of normal foods, so this would not be a problem in food formulation.
Tofu is a protein gel type food product made using soy protein isolate that is popular in Asian cuisine. Similar to soy, a tofu product can be produced using an equivalent procedure [63]. The pressed cake from oil extraction is soaked in water to form the peanut milk. Treatment with transglutaminase followed by heating, cooling and then treatment with a coagulant, results in the curd-like material that can be formed into the tofu product. Peanut protein isolate that is used to make gels by transaminases can produce a tofu product [64]. The texture can be manipulated by changing the amount of transglutaminase used along with high temperature pressure cooking. Research using peanut protein to make tofu in China is led by the popularity of the tofu and lack of available soy protein to keep up with demand.
Hydrolysis of food proteins into small peptides results in products with Angiotensin Converting Enzyme (ACE) inhibitory activity [65]. This allows for control of blood pressure by reducing the production of the peptides responsible for vasoconstriction. Isolated peanut protein can be hydrolyzed using readily available enzymes to produce peptides with this activity [66]. These protein hydrolysates have been shown to produce foams that can serve as efficient emulsifiers in bread dough to produce gluten free bread products [67]. Rice milk fortified with hydrolyzed protein from peanut meal and calcium phosphate nanoparticles during heat processing was found to have an extended shelf life as well as added bioactivity [68]. The protein was found to have antimicrobial and antioxidant properties in the finished product. Peanut meal protein hydrolysates were also found to provide stability to selenium-based nanoparticles that was better than individual sulfur containing amino acids [69,70]. Peptide-calcium complexes are a recent type of supplement that has proven to enhance bioavailability due to the absorption mechanism of the peptides [71-73]. The stability of the enclosed bioactive compounds such as resveratrol are increased and can be released over time. Figure 2 illustrates how these nano particles are formed. High quality proteins such a peanut, soy and sunflower have been used to prepare these complexes. The better adsorption of the calcium as determined by the increase in bone density was proven in an animal model.
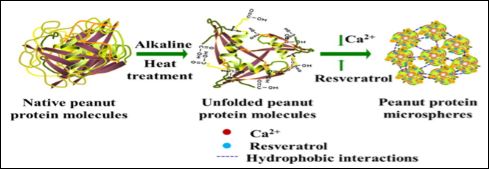
Figure 2: Formation of peanut microspheres [72] (Reproduced with permission).
Encapsulation agents have found favor in the delivery of various compounds with nutritional and pharmaceutical effects. Nanoemulsion coatings protect bioactive components during delivery. This nanoemulsions have been produced from whey, casein, soy, and wheat protein. Peanut protein isolates have also been successfully used to create nanoemulsions [74-76]. In a comparison study, peanut protein isolates were found to function better than soy or wheat protein isolates due to their ability to produce smaller, more stable droplets [77]. These complexes allow for the delivery of bioactive components with protection against deterioration. Anti-inflammatory compounds extracted from other plant products can use the peanut derived nanoparticles [76].
Isolates of peanut protein can be hydrolyzed and will form similar Maillard products upon heating with glucose. The reaction products derived from the peanut peptides have been confirmed [78,79]. Sensory analysis with human subjects also confirmed that the resulting compounds had the same or similar umami taste response as those from soy. Peanut being a legume would have potential to create products to enhance flavor similar to soy sauce. To eliminate the use of solvents such as hexane to isolate peanut oil, peanuts are extracted using enzymes to break down the matrix [34]. When oil is recovered from peanuts using enzymatic extractions in aqueous systems, the proteins can be recovered from the solutions. The emulsions formed often reduce the oil recovered. Additional proteases are used to further break the proteins into smaller peptides. Depending on the peptides produced, these compounds have distinct flavors due to the amino acids present. Sensory analysis of solutions containing the recovered peptides have shown that the basic tastes, salty and umami predominate [80]. Using Discriminate Factor Analysis (DFA), this concept was proven as seen in Figure 3. This indicates that peanut protein fractions could be used as flavor enhancing ingredients or possibly as salt substitutes.
Peanuts can also serve as a source of enzymes for industrial purposes. Amylases have been isolated and used as the immobilization agent by glycoside linkages to carbon nanotubes [81]. For food applications, amylases are used to liquidate starches which makes their uses more economical. Peanut amylases have been found to work effectively [82]. A small protease has been isolated from crude peanut oil that has applications in hydrolyzing the native proteins associated with the oil bodies, oleosin, caleosin, and steroloesin [83]. In addition, it has been proven to hydrolyze Ara h1 and one of the other arachins that are responsible for the peanut allergic response. This protease can have application in producing peanut oil that is free of peanut protein.
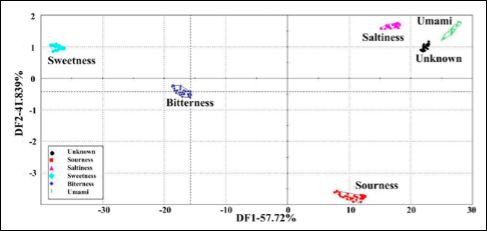
Figure 3: Analysis of the taste attributes of the five taste samples and peanut meal samples (unknown samples was peanut meal double enzymatic hydrolysis hydroly- sates at enzyme treatment times of 10 min, 1 h, 3 h, 8 h, 12 h, 24 h. Umami: 0.1%- 1.0% MSG; saltiness: 0.001%-0.2% salt; sourness: 0.02%–0.2% citric acid; bitterness: 0.025%-0.25% tannic acid; sweetness: 0.25%-2-5% sugar). Data were processed by DFA. [80] (Reproduced from free access under the terms of Creative Commons
Defatted peanut meal after carbohydrate extraction can produce an edible film material [84]. Edible films are proposed as plastic substitutes for food packaging. Use of naturally degradable materials creates a pathway for the reduction of plastic waste. Peanut meal is essentially the protein from peanut that can be solubilized and treated with glycerol as a plasticizer at a high pH to produce the film. Peanuts coated with the film are protected from oxidation during storage. Starches from other legumes, such as pea have been used to create films [85]. Pea starch at levels up to 50% were found to form films with good mechanical strength, be water resistant and have stability. Xylose, a sugar isolated from wood sources was reported to serve suitable carbohydrate modifier for powders and films prepared from peanut protein with enhanced water resistance [31,86,87]. The use of gum Arabic was found to be suitable as the polysaccharide modifier with an optimized time of 6 days to reach equilibrium [18]. The best tensile strength depended on the disulfide bond formation. Incorporation of thymol, a natural phenol compound, isolated from the herb, thyme produced an edible film that also was able to withstand microbial attack and offer antioxidant protection to the food in contact with the film [88]. Other materials, especially soy have been used to create these films along with food grade starches. One of the issues is that the films are not good moisture barriers since the ingredients are often strongly hydrophilic. One solution is to reinforce the fills using nanoparticles made from peanut protein [89]. Adding peanut nanoparticles increased the mechanical and moisture barrier strength of a soy-starch film by a proposed mechanism of creating a network that slows the movement of water molecules through the matrix.
Peanut Protein Characteristics and the Influence on Peanut Products
While vegetable proteins such as peanut are more readily available and cost less to produce than animal protein, the nutritional attributes are less. The quality of peanut protein due to the amino acid profile is considered to be very high but is not complete. Peanuts are deficient in the essential amino acids, threonine, lysine, and methionine. Most vegetable sources are also lacking in these amino acids when compared to animal sources [90]. The high protein content of peanuts of 20 to 25 g/100g compared to most grains of 7 to 15g per 100g is the advantage of peanut protein.
Peanut protein characterization reports often focus on the protein within the peanut flour matrix. Isolation of the pure proteins and protein fractions from peanut provided a more efficient way to investigate the functionality of the proteins [91]. When comparing defatted peanut flour and peanut isolate, the flour was more efficiently hydrolyzed by a protease (Flavorzyme) than the isolate [92]. This was attributed to peptide aggregation due to hydrophobic interaction and hydrogen bonding. Isolation of the proteins can be done using anion exchange chromatography [93]. The emulsifying properties of isolated peanut proteins and the stability of foams formed using them were described.
By using peanut protein isolates as emulsifiers, food products similar to margarines have been prepared from vegetable oil and water [94]. Peanut protein has been used to form a microgel using transglutaminase. The particles were manipulated at high pH (9.0) into forming monolayers that entrapped oil and water resulting in a final product with the functionality of vegetable spreads. In peanut milk products, the protein served as the agent of the emulsifier as it forms a macromolecular layer to protect the oil bodies [22]. When roasted peanuts are used, the product increases in stability due to the Maillard reaction and the enhanced solubility and conformational changes in the proteins. This was attributed to the glycoproteins formed during processing which are more protective of the oil bodies during the homogenization and thermal treatment necessary for processing. These proteins also give stability to peanut products by protecting the oil bodies [93].
Peanut Protein Isolates (PPI) are effective as emulsifiers. Heat treatment at temperatures above 80°C increased the size of the proteins in the isolates, the hydrophobicity and other physical characteristics increasing the emulsifying power of the peanut protein isolates [95]. Subjecting PPI through several cycles of freezing and thawing reduces the sulfhydryl content and surface hydrophobicity [96]. The particle size was reduced, and the microstructure became more uniform which increased the emulsification effect of the PPI. The use of ultrasound to extract protein isolates from peanuts caused changes in the peanut proteins, arachin and conarachin [97]. Less large molecular weight material and small particles resulted in droplet flocculation as well as conformational changes in the proteins. As a result, the ability of the isolates to act as emulsifiers is improved. Use of phosphates as complexing agents also improved the emulsifying properties of peanut protein by causing structural changes due to the phosphate groups binding to the hydroxyl groups on the serine, threonine and tyrosine side chains and the amino groups of the lysine side chains [44,98]. The enhanced protein hydration improved dissolution, solubility as well as the emulsification resulting in more stable foams. The changes also increased the digestibility of the protein. Carbohydrates have been used as complexing agents to increase the emulsification properties [18]. Conarachin was found to better complex with carbohydrates such as dextran and gum Arabic compared to arachin. The bonds formed were with the lysine and arginine amino side chains in the protein. In addition, pH played a role in the solubility with the dextran complex favored at low pH and gum Arabic favored above pH 7. When heat was compared to ultrasound in the complex preparation, ultrasound produced more compact structures.
Small peptides were formed from the hydrolysis of peanut protein isolates. High pressure has been used prior to enzyme catalyzed hydrolysis to increase the efficiency of the hydrolysis and increase the amounts of small molecules produce [99]. The use of the high-pressure treatment also increased the antioxidant activity of the peptides when measured using a chemical system (DPPH assay). Low pH solutions such as found in vinegar change the confirmation of the peanut protein isolates resulting in a decrease in the hydrophobicity [20]. The proteins are made more susceptible to digestion and produce bioactive peptides with higher ACE (angiotensin converting enzyme) activity increasing the health benefits.
Lectins are plant proteins that have a specific affinity for certain sugars. Although their role in plant metabolism has not been completely elucidated, they are thought to play a role in seed germination. As seeds, peanuts contain significant levels of lectin, but it is usually greatly reduced when peanuts are roasted. Their specific binding abilities have been studied as possible drug delivery systems or diagnostic techniques [100].
Nonfood Uses of Peanut Protein
Vegetable oil processing results in waste effluent that contains high levels of organic materials that needs to be removed before discharge. Defatted peanut meal that has been extracted with salt solution was found to be effective in reducing the total suspended solids, chemical oxygen demand and the turbidity of the effluent from palm oil processing facilities [101]. The activity was attributed to the extract being largely composed of peanut protein. Soy has been used to create wood adhesives. The protein levels in peanuts are higher than those in soy, so they can be considered a better source for similar applications such as wood adhesives [102]. Surfactants were used to denature proteins in defatted peanut meal and modified with ethylene glycol diglycidyl ether to increase water resistance. The material was then applied to thin wood sheets to form a plywood product.
Disadvantages of Peanut Protein
Peanuts contain a range of proteins that fill many of the roles of proteins in any organism, such as storage of amino acids needed for growth, involvement in biogenesis and stabilization of membranes, cell wall organization, intercellular signaling, have antimicrobial and insecticide properties but also induce an allergenic response in susceptible individuals [103].
The major barrier to the increased use of protein from peanuts is the allergenicity of the protein which has been increasing in areas of the Western portions of the world, especially the United States, Canada, and the United Kingdom [104]. Observations of less prevalence of peanut allergy in Asia where boiling is the preferred method of preparation over roasting indicates that peanut protein stability to the cooking process may affect the allergenic response in humans [48]. The protein in roasted peanuts is more stable than that in raw or boiled peanuts and allergenicity in those products has been seen to be less in animal models. Research has shown that allergenicity was reduced when the protein is bound to compounds with phenolic moieties from foods such as cranberries and blueberries [105-108]. The polyphenols were reported to block the binding sites of the Ara h1 protein with the IgE epitope due to conformational changes. The polyphenols isolated from peanut skins have also been found to have the same properties [109]. In addition to blocking the allergenic response, binding health promoting polyphenols such as the catechins from tea, the peanut proteins can serve as delivery systems for these compounds [110,111]. Heat treatment can denature the protein in peanut and reduce the allergenic effect but does not eliminate it [112].
The functional properties of peanut protein are largely dependent on the structural form of the protein. These properties are dependent on the conditions of storage [113]. The processing of peanut protein isolate changes the structure and can reduce functionality [114,115]. However, protection from elevated temperatures, oxidations, and humidity can be provided by vacuum packaging.
Conclusion
Peanuts have long been valued for their protein content. Isolation and use of the protein outside of the whole seed has been of interest for some time. This review shows that more recently there has been a great deal of focus on high value applications of peanut protein as much material is available after processing of peanuts for peanut oil. Applications of peanut protein isolates for food ingredients such as emulsifiers, protective carriers of bioactive ingredients, and bioactive peptides is expected to increase. Problems of solubility and degradation are being addressed by researchers, especially in China. The abatement of the allergenicity of peanut remains the major barrier to wide-spread use of peanut protein ingredients for products for human consumption.
Disclaimer
Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture or North Carolina Agricultural Research Service, nor does it imply approval to the exclusion of other products that may be suitable. USDA is an equal opportunity provider and employer.
References
- Sandefur HN, McCarty JA, Boles EC, Matlock MD (2017) Peanut products as a protein source: Production, nutrition, and environmental Chapter 13 in Nadathur SR, Wanasundara SPD, Scalin L, eds., Sustainable Protein Sources, New York, NY, Academic Press 209-221.
- Ohr LM (2020) Plant based protein market grows stronger. Food Tech 74:
- Jani BL, Devani BM (2020) Peanut protein: Rich sources as vegan HSOA J Food Sci Nutr 59.
- Wild F, Czerny M, Janssen AM, Kole APW, Zunabovic M, et al. (2014) The evolution of plant based alternatives to meat: From niche markets to widely accepted meat alternatives. Agro Food Ind High Tech 25: 45-49.
- S. Department of Agriculture, Agricultural Research Service. Food Data Central, 2019.
- USDA, Foreign Agriculture Service International Production Assessment Division (2021).
- Tu J, Wu W (2019) Critical functional properties of defatted peanut meal produced by aqueous extraction and conventional methods. J Food Sci Tech 56: 4722-4731.
- Diby NNAS, Konan KND, Ananga AO, Dodo H (2020) Improving peanut protein quality: Expression of a synthetic storage protein. Afr J Biotech 19: 265-275.
- Abdualrahm M (2013) Chemical, in-vitro protein digestibility, minerals and amino acids composition of edible seeds (Arachis hypogaea L.) Sci Int 1: 199-202.
- Lucas EW (1979) Food uses of peanut J Amer Oil Chem Soc 56: 425-430.
- Arya SS, Salve AR, Chauhan S (2016) Peanuts as functional food: A J Food Sci Technol 53: 31-41.
- Qinzhu Z, Yan-ling C, Dong-xiao S, Tian B, Yang Y, et (2018) Process optimization and anti-oxidative activity of peanut meal Maillard reaction products. LWT-Food Sci Tech 97: 573-580.
- FAS, Foreign Agricultural Service, USDA (2021) Oilseeds: World markets and trade.
- Wu H, Wang Q, Ma T, Ren J (2009) Comparative studies on the functional properties of various protein concentrate preparations of peanut protein. Food Res Int 42: 343-348.
- Labuckas DO, Lamarque AL, Maestri D (2016) Partially defatted peanut flour: A functional ingredient to improve nutritional value of bakery Rev Chil Nutr 43: 381-387.
- Seth K, Kochhar A (2017) Formulation and nutritional evaluation of baked products supplemented with partially defatted peanut flour. Nutr Food Sci 47: 808-816.
- Grace MH, Truong AN, Truong VD, Raskin I, Lila MA (2015) Novel value-added uses for sweet potato juice and flour in polyphenol-and protein-enriched functional food ingredients. Food Sci Nutr 3: 415-424.
- Li C, Xue H, Chen Z, Ding Q, Wang X (2014) Comparative studies on the physicochemical properties of peanut protein isolate-polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res Int 57: 1-7.
- De Angelis E, Bavaro SL, Monaci L, Pilolli R (2018) Effects of the varietal diversity and the thermal treatment of the protein profile of peanuts and hazelnuts. J Food Qual 2018:7635957.
- Guo Z, Chen F, Yang H, Liu K, Zhang L (2015) Kinetics of protein extraction in reverse micelle. Int J Food Prop 18: 1707-1718.
- Zhao X, Chen J, Chen X, Wang X, Wang Y (2015) Separation of peanut protein by reverse micelles: Optimization of the forward extraction. J Food Proc Pres 39: 1457-1465.
- Zaaboul F, Raza H, Cao C, Yuanfa L (2019) The impact of roasting, high pressure homogenization and sterilization on peanut milk and its oil Food Chem 280: 270-277.
- Kavitha S, Parimalavalli R (2014) Effect of processing methods on proximate composition of cereal and legume flours. J Hum Nutr Food Sci 2: 1051.
- Oduro AF, Saalia FK, Adjei MYB (2021) Sensory acceptability and proximate composition of 2-plant based dairy Foods 10: 482.
- Gama AP, Mwangwela AM, Gichohi-Wainaina WN, Adhikari K (2020) Sensory and nutritional properties of peanut-based beverages: A promising solution for undernutrition in Malawi and possibly beyond. J Sci Food Agric 100: 2460-2467.
- Ye L, Liao Y, Sun W, Zhao M (2015) Effect of protein oxidation on the stability of peanut beverage. CyTA-J Food 13: 49-55.
- Karshenas M, Goli M, Zaminder N (2019) Substitution of sesame and peanut defatted-meal milk with egg yolk and evaluation of the rheological and microstructural properties of low-cholesterol mayonnaise. Food Sci Int 25: 633-641.
- Liu CM, Zhong JZ, Liu W, Tu ZC, Wan J, et al. (2011) Relationship between functional properties and aggregation changes of whey protein induced by high pressure microfluidization. J Food Sci 76: 341-347.
- Gong K, Chen L, Xia H, Dai H, Li X, et al. (2019) Driving forces of disaggregation and reaggregation of peanut protein isolates in aqueous dispersion induced by high-pressure microfluidization. Int J Biol Macromol 130: 915-921.
- Gong K, Deng L, Shi A, Liu H, Liu L, et al. (2017) High-pressure microfluidisation pretreatment disaggregate peanut protein isolates to prepare antihypertensive peptide Int J Food Sci Tech 52: 1760- 1769.
- Lin WJ, Liu HZ, Shi AM, Liu J, Adhikari B, et (2015) Preparation and characterization of films from xylose-glycosylated peanut protein isolate powder. Int J Food Sci Tech 50: 1538-1544.
- He XH, Liu HZ, Liu L, Zhao GL, Wang Q, et al. (2014) Effects of high pressure on the physicochemical and functional properties of peanut protein isolates. Food Hydrocoll 36: 123-129.
- Liu J, Li P, Jiang Z, Yang R, Zhang W (2018) Characterization of peanut protein concentrates from industrial aqueous extraction processing prepared by spray and freeze drying methods. Int J Food Sci Tech 54: 1597-1608.
- Zhang SB, Lu QY (2015) Characterizing the structural and surface properties of proteins isolated before and after enzymatic demulsification of the aqueous extract emulsion of peanut Food Hydrocoll 47: 51- 60.
- Wang L, Liu H, Liu L, Wang Q, Li S, et al. (2017) Prediction of peanut protein solubility based on the evaluation model established by supervised principal component regression. Food Chem 218: 553-560.
- Ma T, Zhu H, Wang J, Wang Q, Yu L, et (2017) Influence of extraction and solubilizing treatments on the molecular structure and functionality properties of peanut protein 79: 197-204.
- Liu C, Hao L, Chen F, Yang C (2020) Study on extraction of peanut protein and oil bodies by aqueous enzymatic extraction and characterization of J Chem 5148967.
- Liu C, Chen FS, Niu RH, Gao YH (2020) Effects of pretreament on the yield of peanut oil and protein extracted by aqueous enzymatic extraction and the characteristics of the emulsion. J Oleo Sci 69: 1445-1453.
- Liu C, Hao LH, Chen FS, Zhu TW (2020) The mechanism of extraction of peanut protein and oil bodies by enzymatic hydrolysis of the cell J Oleo Sci 69: 1467-1479.
- Nyo MK, Nguyen LT (2019) Value-addition of defatted peanut cake by proteolysis: Effects of protease and degree of hydrolysis on functional properties and antioxidant capacity of Waste Biomass Valor 10: 1251-1259.
- Chen L, Ettelair R, Akhtar M (2019) Improved enzymatic accessibility of peanut protein isolate pre-treatment using thermosonication. Food Hydrocoll 93: 308-316.
- Nguyen TH, Le VVM (2019) Effects of technological parameter of ultrasonic treatment on the protein extraction yield from defatted peanut Int Food Res 26: 1079-1085.
- Ochoa-Rivas A, Nava-Valdez Y, Serna-Saldívar SO, Chuck-Hernández C (2017) Microwave and ultrasound to enhance protein extraction from peanut flour under alkaline conditions: Effects in yield and functional properties of protein isolates. Food Bioproc Technol 10: 543-555.
- Yu L, Yang W, Sun J, Zhang C, Bi J, et al. (2015) Preparation, characterisation and physical properties of the phosphate modified peanut protein obtained from Arachin Conarachin Food Chem 170: 169-179.
- Zhang S, Liu L, Zhang y, He N, Wang Q (2021) High-moisture extrusion process of transglutaminase modified peanut protein: Effect of tranglutaminase on the mechanics of the process forming a fibrous Food Hydrocol 112: 106346.
- Kuraishi C, Yamazaki K, Susa Y (2001) Transglutaminase: Its utilization in the food industry. Food Rev Int 17: 221-245.
- Zhang SB, Wang XH, Li X, Yan DQ (2020) Effects of Tween 20 and transglutaminase modifications on the functional properties of peanut JAOCS 97: 93-103.
- Zhang J, Liu L, Jiang Y, Faisal S, Wei L, et al. (2019) Converting peanut protein biomass waste into “Double Green” meat substitutes using a high- moisture extrusion process: A multiscale method to explore a process for forming a meat-like fibrous structure. J Agric Food Chem 67: 10713- 10725.
- Zhang J, Liu L, Zhu S, Wang Q (2018) Texturization behavior of peanut- soybean/wheat protein mixtures during high moisture extrusion Int J Food Sci Tech 53: 2535-2541.
- Salinas-Valdés A, De la Rosa-Millán J, Serna-Saldivar SO, Chuck- Hernández C (2015) Yield and textural characteristics of Panela cheese produced with dairy-vegetable protein (soy or peanut) blends supplemented with transglutaminase. J Food Sci 80: S2950-S2956.
- Chauhan OP, Kumar S, Nagraj R, Narasimhamurthy R, Faju PS (2015) Effect of high pressure processing on yield, quality and storage stability of peanut paneer. Int J Food Sci Tech 50: 1515-1521.
- Jiao B, Shi A, Liu H, Sheng X, Liu L, et (2018) Effect of electrostatically charged and neutral polysaccharides on the rheological characteristics of peanut protein isolate after high-pressure homogenization. Food Hydrocoll 77: 329-335.
- Zhu YD, Li D, Wang LJ (2019) Dynamic rheological properties of peanut protein isolate and aggregation dispersion and acid-induced gel. Powd Tech 358: 95-102.
- Jiang S, Zhang J, Li S, Zhang C (2020) Effect of enzymatic hydrolysis on the formation and structural properties of peanut protein gels. Int J Food Eng 17:167-176.
- Wang Y, Yang F, Wu M, Li J, Bai Y, et al. (2020) Synergetic effect of pH shifting and mild heating in improving heat induced gel properties of peanut protein isolate. LWT-Food Sci Tech 131: 109812.
- Yu JJ, Chen Y, Zhang YF, Zheng XC, Li SH, Chen Y (2020) Analysis of the glycosylation products of peanut protein and lactose by cold plasma treatment: Solubility and structural characteristics. Int J Biol Macromol 158: 1194-1203.
- Hui J, Dong S, Han F, Li Y, Chen G, et al. (2018) Effects of dielectric barrier discharge (DBD) cold plasma treatment on physicochemical and functional properties of peanut protein. Food Bioproc Technol 11: 344-
- Basse B, Bosc V, Salter JM, Chan-Huot M, Dupes JP, et al. (2020) Combined effects of ionic strength and enzymatic pre-treatment in thermal gelation of peanut protein extracts. Food Res Int 137: 109362.
- Qui C, Hu X, Li L, Yang X, Zhao M (2017) Effect of transglutaminase cross-linking on the confirmational and emulsifying properties of peanut arachin and conarachin fractions. Eur Food Res Tech 243: 913-920.
- Kahlon TS, Avena-Bustillos RJ, Brichta JL, Kahlon AK (2019) High- protein nutritious flatbreads and an option for gluten sensitive Foods 8: 591.
- Adeboye AS, Fayemi OE, Bamgbose A, Adewunmi A, Sobowale SS (2018) Towards the development of peanut-wheat flour composite dough: Influence of reduced-fat peanut flour on bread quality. J Food Proc Pres 42:
- Li J, Wu M, Wang Y, Li K, Du J, et al. (2020) Effect of pH-shifting treatment on structural and heat induced gel properties of peanut protein Food Chem 325: 126921.
- Chen BY, Li QZ, Hu H, Meng S, Shah F, et al. (2020) An optimized industry processing technology of peanut tofu and the novel prediction model for suitable peanut varieties. J Integ Agric 19: 2340-2351.
- Guo Y, Hu H, Wang Q, Liu H (2018) A novel process for peanut tofu gel: Its structure, microstructure and protein behavior change affected by processing conditions. LWT-Food Sci Tech 96:140-146.
- Hartman R, Meisel H (2007) Food derived peptides with biological activity: From research to food applications. Curr Op Biotech 18: 163-
- Jamdar SN, Rajalakshmi V, Pednekar MD, Juan F, Yardi V (2010) Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem 121: 178-184.
- Phongthai S, Singsaeng N, Nhoo-ied R, Suwannatrai T, Schőnlechner R, et al. (2020) Properties of peanut (KAC431) protein hydrolysates and their impact on the quality of gluten-free rice bread. Foods 9: 942.
- Abdelhamid SM, Farroh KY, Wagdy SM, Aki EM (2020) Rice milk fortification using calcium hydroxyl phosphate nanoparticles and hydrolyzed protein fractions. Egypt J Chem 63: 2301-2318.
- Ning F, Ge Z, Qiu L, Wang X, Luo L, et al. (2020) Double-induced se- enriched peanut protein nanoparticles preparation, characterization and stabilized food-grade Pickering emulsions. Food Hydrocoll 99: 105308.
- Ye MJ, Xu QL, Tang HY, Jiang WY, Su DX, et al. (2020) Development and stability of novel selenium colloidal particles complex with peanut meal peptides. LWT-Food Sci Tech 126: 109280.
- Yuan X, Bao X, Feng G, Zheng M, Ma S (2020) Effects of peptide- calcium complexes from sunflower seeds and peanuts on enhancing bone mineral density. Int J Food Sci Tech 55: 2942-2953.
- Shi A, Wang Q, Chen X, Liu H, Liu H, et al. (2017) Calcium-induced peanut protein nanoparticles for resveratrol J Contr Rel 255: e6- e7.
- Shi A, Chen X, Liu L, Hu H, Wang Q, et al. (2017) Synthesis and characterization of calcium-induced peanut protein isolate RSC Adv 7: 53247-53254.
- Liu Y, Liu C, Zhang S, Li J, Zheng H, et al. (2021) Comparison of different protein emulsifiers on physicochemical properties of β-carotene- loaded nanoemulsion: Effect on formation, stability and in vitro Nanomaterials 11: 167.
- Hua J, Liu C, Zhang S, Guo Z, Li J, et al. (2020) Comparison of protein hydrolysates against their native counterparts in terms of structural and antioxidant properties, and when used as emulsifiers for curcumin Food Func 11: 10205.
- Ning F, Wang X, Zheng H, Zhang K, Bai C, et (2019) Improving the bioaccessibility and in vitro absorption of 5-cemthylnobiletin from chenpi by se-enriched peanut protein nanoparticles-stabilized pickering emulsion. J Func Foods 55: 76-85.
- Li Y, Jin H, Sun X, Sun J, Liu C, et al. (2019) Physicochemical properties and storage stability of food protein-stabilized Nanomaterials 8: 25.
- Zhang J, Sun-Waterhouse D, Fen Y, Su G, Zha M, et al. (2019) The umami intensity enhancement of peanut protein isolate hydrolysate and its derived fractions and peptides by Maillard Food Res Int 120: 895-903.
- Zhang J, Zhao M, Su G, Lin L (2019) Identification and taste characteristics of novel umami and umami-enhancing peptides separated from peanut protein isolate hydrolysate by consecutive chromatography and UPLC- ESI-QTOF-MS/MS. Food Chem 278: 674-682.
- Wang L, Niu Q, Hui Y, Jin H, Chen S (2015) Assessment of taste attributes of peanut meal enzymatic-hydrolysates using an electronic Sensors 15: 11169-11188.
- Das R, Ranjan R, Shinha N, Kayastha AM (2018) Comparative characterization of peanut β-amylase immobilization onto graphene oxide and graphene oxide carbon nanotubes by solid state NMR. J Phys Chem C 122: 19259-19265.
- Das R, Kayastha AM (2018) An antioxidant rich novel β-amylase from peanuts (Arachis hypogaea): Its purification, biochemical characterization and potential applications. Int J Biol Macromol 111: 148-157.
- Chen L, Chen J, Yu L, Wu K, Zhao M (2018) Emulsification performance and interfacial properties of enzymically hydrolyzed peanut protein isolate pretreated by extrusion cooking. Food Hydrocoll 77: 607-616.
- Martin MP, Riveros CG, Paredes AJ, Allemandi DA, Nepote V, et al. (2019) A natural peanut edible coating enhances the chemical and sensory stability of roasted peanuts. J Food Sci 84:1529-1537.
- Sun Q, Xiong CSL (2014) Functional and pasting properties of pea starch and peanut protein isolate blends. Carbo Poly 101: 1134-1139.
- Lin WJ, Liu HZ, Shi AM, Lu L, Wang Q, et al. (2015) Effect of glycosylation with xylose on their mechanical properties and water solubility of peanut protein films. J Food Sci Tech 52: 6242-6253.
- Liu L, Lin WJ, Liu HZ, Shi AM, Hu H, et al. (2017) Effect of xylose on the structural and physicochemical properties of peanut isolated protein based films. RSC Adv 7: 52357-52365.
- Zhoug T, Liang Y, Jiang S, Yang L, Shi Y, et al. (2017) Physical antioxidant and antimicrobial properties of modified peanut protein isolate based films incorporating thymol. RSC Adv 7: 41610-41618.
- Li X, Ji N, Qui C, Xia M, Xiong L, et al. (2015) The effect of peanut protein nanoparticles on characteristics of protein- and starch-based nanocomposite A comparative study. Ind Crops Prod 77: 565-574.
- Gorissen SHM, Crombag, JJR, Senden JMG, Waterval WAH, Bierau J, et (2018) Protein content and amino acid composition of commercially available plant based protein isolates. Amino Acids 50: 1685-1695.
- Monteiro PV, Prakash V (1994) Functional properties of homogeneous protein fractions from peanut (Arachis hypogaea ). J Agic Food Chem 42: 274-278.
- Zheng L, Zhao Y, Xiao C, Sun-Waterhouse D, Zhao M, et al. (2015) Mechanism of the discrepancy in the enzymatic hydrolyses efficiency between defatted peanut flour and peanut protein isolate by Flavorzyme. Food Chem 168: 100-106.
- Boualeg I, Boutebba A (2017) Purification of water soluble proteins (2S albumins) extracted from peanut defatted flour and isolation of their isoforms by gel filtration and anion exchange chromatograpy. Sci Stud Res Chem Chem Eng Biotech Food Ind 18: 135-143.
- Jiao B, Shi A, Wang Q, Binks BP (2018) High-internal-phase Pickering emulsions stabilized solely by peanut-protein-isolate microgel particles with multiple potential applications. Angew Chemie 57: 9274-9278.
- Zhang Y, Xiong W, Lei L, Pei Y, He L, et al. (2019) Influence of heat treatment on structure, interfacial rheology and emulsifying properties of peanut protein isolate. Czech J Food Sci 37: 212-220.
- Feng H, Jin H, Gao Y, Yan S, Zhang Y, et al. (2020) Effects of freeze- thaw cycles on the structure and emulsifying properties of peanut protein Food Chem 330: 12725.
- Sun X, Zhang W, Zhang L, Tian S, Chen F (2020) Molecular and emulsifying properties of arachin and conarachin of peanut protein isolate from ultrasound-assisted extraction. LWT-Food Sci Tech 132: 109790.
- Sánchez-Reséndiz A, Rodriguez-Barrientos S, Rodriguez-Rodriguez J, Barba-Dávilia B, Serna-Saldívar SO, et al. (2018) Phosphoesterification of soybean and peanut proteins with sodium trimetaphosphate (STMP): Changes in structure to improve the functionality for food applications. Food Chem 260: 299-305.
- Dong X-h, Li j, Jiang G-x, Li H-y, Zhao M-m, et al. (2019) Effects of combined high pressure and enzymatic treatments in physiochemical and antioxidant properties of peanut proteins. Food Sci Nutr 7: 1417-1425.
- Sleiman MH, Csonka R, Arbez-Gindre C, Heropoulos GA, Calogeropoulou T, et al. (2015) Binding and stabilization effects of glycodendritic compounds with peanut agglutinin. Int J Biol Macromol 80: 692-701.
- Birima AH, Ahmed AT, Noor MJMM, Sidek LM, Muda ZC, et (2015) Application of salt extracted peanut seeds in the pretreatment of palm oil mill effluent (POME). Desalin Water Treat 55: 2196-2200.
- Li J, Li X, Li J, Gao Q (2015) Investigating the use of peanut meal: A potential new resource for wood adhesive. RSC Adv 5: 80136-80141.
- Ozias-Akins P, Breitender H (2019) The functional biology of peanut allergens and possible links to their allergenicity. Allergy 74: 888-898.
- Clarke AF, Elliot S, Pierre YS, La Vieille S, Ben-Shoshan M (2020) Temporal trends in prevalence of food allergy in Canada. J Allergy Clin Immunol Pract 8: 1428-1430e5.
- Zhang T, Shi Y, Zhao Y, Wang J, Wang M, et (2019) Different thermal processing effects on peanut allergenicity. J Sci Food Agric 99: 2321- 2328.
- He W, Zhang T, Velickovic TC, Li J, Lyu Y, et al. (2020) Covalent conjugation with (-)-epigallo-catechin 3-gallate and chlorogenic acid changes in allergenicity and functional properties of Ara h1 from Food Chem 331: 127355.
- Plundrich NJ, Kulis M, White BL, Grace MH, Guo R, et al. (2014) Novel strategy to create hypoallergenic peanut protein-polyphenol edible matrices for oral immunotheraphy. J Agric Food Chem 62: 7010-7021.
- Plundrich NJ, White BL, Dean LL, Davis JP, Foegeding EA, et al. (2015) Stability and immunogenicity of hypoallergenic peanut protein- polyphenol complexes in vitro pepsin Food Func 6: 2145-2154.
- Plundrich N, Bansode R, Williams L, Lila MA (2017) In vitro hypoallergenicity of peanut protein-blueberry polyphenol aggregate J Allerg Clin Immun 139: AB139.
- Bansode RR, Plundrich NJ, Randolph PD, Lila MA, Williams LL (2018) Peanut flour aggregation with polyphenolic extracts derived from peanut skin inhibits IgE binding capacity and attenuates RBL-2H3 cells degranulation via MAPK signaling Food Chem 263: 307-314.
- Vesic J, Stambolic I, Apostolovic D, Milcic M, Stanic-Vucinic D, et al. (2015) Complexes of green tea polyphenol, epigalocatechin-3-gallate and 2S albumins of peanut. Food Chem 185: 309-317.
- Cherry JP (1990) Peanut protein and product functionality. J Amer Oil Chem Soc 67: 293-301.
- Sun X, Jin H, Li Y, Feng H, Liu C, et (2018) The molecular properties of peanut protein: Impact of temperature, relative humidity and vacuum packaging during storage. Molecules 23: 2618.
- Gong KJ, Shi HM, Liu HZ, Liu L, Hu H, et al. (2016) Emulsifying properties and structure changes of spray and freeze dried peanut protein J Food Eng 170: 33-40.
- Singh B, Singh U (1991) Peanut as source of protein for human foods. Plant Foods Hum Nutr 41: 165-177.
Citation: Dean LL (2021) Peanut Protein-Processes and Applications. A Review. J Nutr Food Sci 4: 031.
Copyright: © 2021 Dean LL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.


