*Corresponding Author:
Javier Fernandez-Sanchez,
Department of Allergy, Miguel Hernandez University (UMH), University General Hospital of Alicante, Alicante, Spain
Tel: +34 965933018 /619663019
Email: fj.fernandez@umh.es
Abstract
Background: Carbamazepine (CBZ),often used as a first-line agent for epilepsy, is considered one of the most common causes DRESS, especially in white people carrying the HLA-A*31:01 allele. Our aim was to demonstrate how the concentration of the drug patch tests influences the results.
Case Description: A 62-year-old man, diagnosed with a left hemifa- cial spasm, developed a DRESS after three weeks of treatment with CBZ. The patient presented general malaise, fever, maculopapular exanthema, choluria, eosinophilia, altered liver function tests and a positive result for the human herpes virus-6.
Treatment and Outcome: Patch tests were applied and read at 72 hours to phenytoin at 20% and 5%; CBZ, at 5% and 1%; and pheno- barbital, at 5% and 1%. Only CBZ 5% showed positivity.
Conclusion: DRESS is a common, non-immediate hypersensitivity reaction. Patch tests are a safe diagnostic tool to identify the culprit drug, but at a very low concentration, these can be mistakenly ruled out.
Clinical Relevance: This clinical case demonstrates the importance of carrying out an adequate allergy study to identify the culprit drug for non-immediate hypersensitivity reactions, and shows the importance of testing different concentrations of the suspect drug to identify it correctly.
Keywords
Carbamazepine; DRESS syndrome; Drug hypersensi- tivity; Drug hypersensitivity syndrome
Abbreviations
DRESS: Drug Reaction with Eosinophilia and Systemic Symptoms
HHV: Human Herpesvirus
HSR: Hypersensitivity Reaction
HSV: Herpes Simplex Virus
PCR: Polymerase Chain Reaction
Introduction
Carbamazepine is a widely used anticonvulsant and first-line treatment in epilepsy and other neurological and psychiatric disorders. Hypersensitivity Reactions (HSRs) associated with carbamazepine can occur in up to 10% of patients [1]. These usually affect the skin as a maculopapular exanthema, but HSRs are also related with more severe conditions, such as Drug Reactions with Eosinophilia and Systemic Symptoms (DRESS), also known as drug-induced hypersensitivity syndrome in the absence of confirmed Eosinophilia [2]. The risk of HSRs increases when the patient presents specific HLA alleles, like the HLA-A*31:01 allele in white people [3]. About 0.89% of these patients will develop carbamazepine-induced DRESS-a potentially life-threatening reaction [2] with a mortality rate of 5% to 10% [3]. Clinical features include widespread rash, fever of more than 38.5°C, lymphadenopathy, the involvement of at least one internal organ such as the liver (in >80% of cases), and eosinophilia. However, the severity of liver damage may not be related to the causative drug [4]. The kidney is the second-most involved organ system and is associated with older age and underlying renal or cardiovascular diseases [1,4]. Other organs such as the lungs, gastrointestinal tract, spleen, heart, pancreas, thyroid, brain, muscle and/or eyes could also potentially be damaged [2]. Resolution of skin eruptions and visceral involvement generally occurs after drug removal, with a gradual recovery that lasts six to nine weeks. In about 20% of cases, the symptoms persist longer than a few months [4]. Characteristically, this syndrome can be triggered by a new infection or by reactivation of a latent human herpes virus 6 (HHV-6) [1].
Differential diagnosis with other severe drug eruptions, infections, hypereosinophilic syndrome, lymphoma, and autoimmune connec- tive tissue diseases must be done, as these conditions may mimic DRESS syndrome [2].
Case Presentation
A 62-year-old man, diagnosed with a left hemifacial spasm, began carbamazepine treatment at a dose of 100 mg every 12 hours. After three weeks, he presented general malaise, fever up to 40.5°C, ery- thematous and pruritic maculopapular exanthema, choluria, and con- junctival jaundice. On the sixth day after symptoms onset, he present- ed to the emergency department and was admitted. An initial physical examination showed: blood pressure of 157/71 mmHg, heart rate of 107 beats per minute, respiratory rate of 20 breaths per minute, tem- perature of 36.7°C, generalized maculopapular exanthema without involvement of mucous membranes, pharyngeal erythema, bilateral cervical adenopathies, and abdominal pain on the right hypochon- drium (Figure 1). Standard treatment included aspirin, rosuvastatin, gemfibrozil, and clonazepam. He was an active smoker, consuming 50 packs/year.
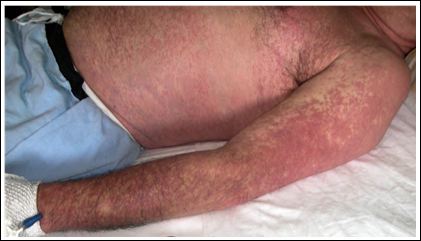
Figure 1: Maculopapular exanthema secondary to carbamazepine-induced DRESS.
During hospital admission, a chest X-ray showed no alterations, and an abdominal ultrasound showed hepatic steatosis. A comput- ed tomography soft tissue neck with intravenous administration of contrast material was also performed, revealing adenopathies in the axillary (7 mm in diameter), posterior cervical (9 mm) and hepat- ic hilar (11 mm) areas. Blood tests showed eosinophilia and elevated transaminases levels. Serological tests were undertaken for HIV, hep- atitis B and C, cytomegalovirus, Epstein-Barr virus, Herpes Simplex Virus (HSV), measles and human herpes virus-6 (HHV-6; using the Polymerase Chain Reaction (PCR) assay); the patient was positive for HHV-6 (Table 1).
Consultation with the allergy department prompted several other tests. Suspecting DRESS, carbamazepine was discontinued and intravenous methylprednisolone was initiated with improvement of symptoms. After five days, methylprednisolone was replaced with an equivalent dose of oral prednisone, and the dose was progressively tapered until resolution of the symptoms and normalization of eosinophils and liver function after 30 days. No additional medications such as antihistamines, intravenous immunoglobulin, or antiviral drugs (ganciclovir) were needed to control the disease. At the third month of follow-up, the patient had normal results on laboratory tests, and there was no recurrence of symptoms in the absence of carbamazepine.
The allergy study included the standard prick test, with 22 preva- lent inhalants, which did not show any sensitization. A biopsy of skin lesions revealed a perivascular lymphohistiocytic infiltrate in the su- perficial dermis, plus interface dermatitis with necrotic keratinocytes, suggestive of toxicoderma. The immunohistochemical study was pos- itive for CD3, CD4, and CD8, and negative for CD20 (Figure 2).
|
Variable |
Admission day |
Day 4 |
Day 7 |
Day 15 |
4 months |
|
Hematology |
|
|
|
|
|
|
White blood cell count (x103/ml) |
9.07 |
22.87 |
17.51 |
16.75 |
9.55 |
|
Eosinophils (x103/ml) |
0.87 |
4.89 |
0.79 |
0.27 |
0.29 |
|
Metabolic panel |
|
|
|
|
|
|
Glucose (mg/dl) |
98 |
96 |
94 |
89 |
70 |
|
Urea (mg/dl) |
24 |
30 |
49 |
28 |
28 |
|
Creatinine (mg/dl) |
0.68 |
0.73 |
0.81 |
0.65 |
0.86 |
|
Glomerular filtration rate (ml/min) |
139.7 |
115.7 |
102.6 |
132.3 |
100.9 |
|
Bilirubin (mg/dl) |
|
|
|
|
|
|
Direct fraction |
2.1 |
|
|
|
|
|
1.5 |
1.09 |
|
|
|
|
|
0.74 |
0.84 |
0.7 |
|
|
|
|
ALT (IU/ml) |
291 |
181 |
78 |
70 |
26 |
|
AST (IU/ml) |
119 |
54 |
51 |
34 |
20 |
|
ALP (IU/ml) |
300 |
350 |
229 |
205 |
|
|
CRP |
2.49 |
0.95 |
2.01 |
0.17 |
<0.10 |
|
Urine analysis |
|
|
|
|
|
|
Proteins (mg/dl) |
25 |
|
|
|
Neg |
|
Urobilinogen (mg/dl) |
12 |
|
|
|
Neg |
|
Bilirubin (mg/dl) |
3 |
|
|
|
Neg |
|
White cells (x HPF) |
0-2 |
|
|
|
0-2 |
|
Red cells (x HPF) |
0-2 |
|
|
|
0 |
|
Eosinophils (x HPF) |
0 |
|
|
|
0 |
|
Immunology |
|
|
|
|
|
|
ANA |
|
|
Neg |
|
|
|
Hepatitis B serology |
|
Neg |
|
|
|
|
Hepatitis C virus serology |
|
Neg |
|
|
|
|
HIV 1 and 2 |
|
Neg |
|
|
|
|
CMV (IgG/IgM) |
|
Pos/Neg |
|
|
|
|
Variable |
Admission day |
Day 4 |
Day 7 |
Day 15 |
4 months |
|
EBV (IgG/IgM) |
|
Pos/Neg |
|
|
|
|
HSV (IgG/IgM) |
|
Neg |
|
|
|
|
Measles (IgG/IgM) |
|
|
Pos/ Neg |
|
|
|
HHV-6 PCR assay |
|
|
Pos |
|
|
|
Microbiology |
|
|
|
|
|
|
Blood cultures |
|
Neg |
|
|
|
|
Urine culture |
|
Neg |
|
|
|
Furthermore, patch tests to phenytoin at 20% and 5%; carbamaze- pine at 5% and 1%; and phenobarbital at 5% and 1% were placed, and the reading was made 72 hours later. There were positive results only for carbamazepine 5% (Figure 3). Suspecting DRESS, we requested the HLA genotype, identifying the allele A*24:03/A*31:01.
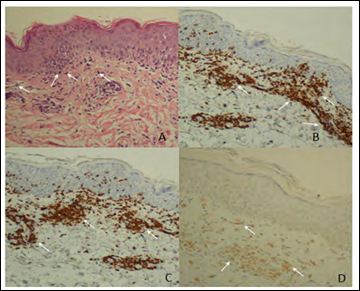
Figure 2: Pathological findings. A) Eosin hematoxylin stain shows the epidermis with discrete focal spongiosis/par- akeratosis. Some necrotic keratinocytes (pointedwith white arrows) are apparent in the epidermal basal layer, and in the superficial dermis, there is a dense perivascular lymphohistiocytic infiltrate, with exocytosis of lymphocytes in the epidermis and with extravasated red cells; B) Immunohistochemical analysis markers CD3 (dark brown cells); C) Immunohistochemical analysis markers CD8 (dark brown cells); D) Immu- nohistochemical analysis markers CD4 (light brown cells).

Figure 3: Patch test results.
Discussion
DRESS is a severe, drug-induced HSR with an elevated mortality rate. Although the drug provocation test is the gold standard for confirming the culprit, this procedure is contraindicated in DRESS due to the severe nature of the reaction. Instead, patch testing is often considered the first choice in the diagnosis, as it is a safe and valid procedure [5]. Therefore, the diagnostic sensitivity is based on positive test results in patients with a suggestive clinical history [6].
Aromatic anticonvulsants have been described as causing up to 35% of all DRESS cases [3], and carbamazepine is one of the most fre- quent culprits, especially in white people carrying the HLA-A*31:01 allele. Our patient was a carrier of this allele, which increases the risk from 5.0% to 26.0%, while its absence reduces the risk by 5.0% to 3.8%, especially in people of European descent [3].
About10% of individuals treated with carbamazepine and other anticonvulsant drugs develop transient adverse effects, including cutaneous reactions, which can occur from day 8 to day 16 after treatment initiation and spontaneously abate within a similar time- frame when medication is discontinued [1]. However, sometimes the reactions are not transient and affect other organs besides the skin, requiring treatment to control them. This is the case of HSRs including DRESS, with an incidence rate of1.0 to 4.1 per 10,000 patients [1,2]. In the case we presented, the simultaneous involvement of several organs in addition to skin lesions was suggestive of an HSR and not just a transient effect of the drug.
The pathogenesis of DRESS syndrome is partially understood. Different mechanisms have been implicated, including slow acetylation, reactivation of human herpes and detoxification defects, which can lead to the formation and accumulation of reactive metabolites that directly cause cell death or act as prohaptens that bind to T cells and evoke immune response [1,2].
Diagnosis of DRESS syndrome requires vigilance, careful clinical observation, and a detailed laboratory examination. The most frequently used diagnostic criteria are shown in table 2. The Bocquet criteria are based on three clinical characteristics that must be present to establish the diagnosis [7]. On the other hand, the European Registry of Severe Cutaneous Adverse Reactions to Drugs and Collection of Biological Samples (RegiSCAR) criteria [2] include seven characteristics, scored from - 1 to2 points each, with DRESS diagnosis based on the total score: < 2 points, no case; 2-3 points, possible case; 4-5 points, probable case; >5 points, definite case. In our case, the patient had a total score of 6 points (Table 1), meriting his classification as a definite case.
|
Bocquet criteria |
RegiSCAR criteria |
J-SCAR criteria |
|
1. Cutaneous drug eruption. 2. Internal organ involvement: - Ly mp h aden o p - athies (>2 cm in diameter), hepati- tis (transaminases value >2 times upper limit of nor- mal), interstitial nephritis, and in- terstitial pneumo- nia or carditis 3. Hematologic ab- normalities: Eo- sinophil count >1.5×103/μL or presence of atypi- cal lymphocytes |
1. Hospitalization. 2. Reaction suspected to be drug-related. 3. Acute rash. 4. Fever above 38°C. 5. Enlarged lymph nodes involving at least two sites. 6. Involvement of at least 1 internal organ. 7. Blood count abnormali- ties: - Lymphocytes above or below normal laboratory limit. - Eosinophil count above laboratory limit. - Platelets below laborato- ry limit. |
1. Maculopapular rash devel- oping >3 weeks after start- ing a limited number of drugs. 2. Persistent clinical symptoms 2 weeks after discontinua- tion of causative drug. 3. Fever above 38°C. 4. Liver transaminases level (ALT) >100 U/L or other organ involvement. 5. Leukocyte abnormalities [at least 1): leukocytosis (>11×109/L), atypical lym- phocytosis (>5%), eosino- philia (1.5×109/L). 6. Lymphadenopathy. 7. HHV-6: Human herpes vi- rus-6 reactivation. |
|
The 3 criteria are nec- essary to establish the diagnosis. |
The diagnoses is based on the total score: <2 points: no case; 2-3 points: Possible case; 4-5 points: probable case; >5 points: definite case. |
Typical DRESS: All 7 criteria are necessary Atypical DRESS: All criteria are necessary except lymphadenopa- thy and HHV-6 reactivation. |
The third diagnostic criterion for DRESS was proposed by the Japanese group of Severe Cutaneous Adverse Reactions to Drugs (SCAR-J). These criteria comprise seven items, which are quite similar to those in the RegiSCAR criteria. The most important difference is the inclusion of the HHV-6 reactivation into the diagnostic criteria for the disease. Following these criteria, the confirmation of the HHV-6 in our patient allowed us to diagnose typical DRESS case [7].
Dong-Hyun K et al. [8] compared the three criteria mentioned above, showing that RegiSCAR could be the most accurate for the diagnosis of DRESS syndrome. However, operator experience is necessary to adequately calculate the score in each patient, and unlike the SCAR-J, the critical role for other herpes viruses such as Epstein Barr virus, HHV-7 and cytomegalovirus is recognized in the pathogenesis of the disease. Additionally, other possible triggers such as spider bites or parasitosis such as to xocariasis have recently been described without being considered diagnostic criteria [9,10].
The mechanisms by which viruses induce DRESS are unclear, although it seems to be secondary to an activation and expansion of drug-specific CD4+ and CD8+ T lymphocytes, favored by the release of proinflammatory cytokines including TNF-a due to an increase in the replication of HHV-6 [7]. The pathology findings in the skin biopsy, and HHV-6 infection, could suggest that this case of DRESS may have been triggered by a viral infection in a genetically susceptible patient.
The literature supports the usefulness of patch tests for studying delayed HSRs to anticonvulsants, identifying the culprit drug in up to 95% of the cases and also elucidating the cross-reactivity between drugs [11]. The position of the European Network on Drug Allergy and Drug Allergy Interest Group of the European Academy of Allergy and Clinical Immunology recommend patch tests at concentrations of 10%, however in case of severe reactions the recommended concentration is 1% [5]. In our case, we tested carbamazepine at 1% and 5%, eluding higher concentrations to avoid a flare-up. This strategy enabled identification of the culprit drug at a concentration of 5%, but not at 1%. We also ruled out cross-reactivity with phenytoin and phenobarbital (Figure 3).
In case of negative results, in vitro tests have shown promising results in the diagnosis. Positivity is particularly high when the lymphocyte transformation test is combined with cytokines like IL-5 and cytotoxic markers measurement, but this technique has not been validated, and its use in clinical practice is still limited [6,12].
Conclusion
This clinical case illustrates the value of maintaining a high index of suspicion for DRESS in patients with maculopapular exanthema and eosinophilia. Given the mortality rate, it is essential to start treatment quickly. RegiSCAR criteria could be the most accurate for the diagnosis of the disease. Rapid recognition and removal of the offending agent are also crucial for managing the disease. When anticonvulsants are involved, patch tests could be a safe diagnostic tool, bearing in mind that if the concentration at which the drug is tested is too low, it could be ruled out by mistake. In vitro tests combining a lymphocyte transformation test with cytokines and cytotoxic markers seem to show promising results; however, further studies are needed.
Acknowledgements
To Meggan Harris for the English translation. This study was supported with research grants from ISABIAL and Carlos III Institute: RD16/06/32 (ARADYAL) and the Ministry of Science and Innovation, Spain.
References
- 1.Fricke-Galindo I, LLerena A, Jung-Cook H, López-López M (2018) Carba- mazepine adverse drug Expert Rev Clin Pharmacol 11: 705-718.
- 2.Behera SK, Das S, Xavier AS, Selvarajan S (2018) DRESS syndrome: A de- tailed Hosp Pract 46: 152-162.
- 3.McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperavičiūtė D, et al. (2011) HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in N Engl J Med 364: 1134-1143.
- 4.Wang L, Mei X-L (2017) Drug reaction with eosinophilia and systemic symp- toms: Retrospective analysis of 104 cases over one decade. Chin Med J (Engl) 130: 943-949.
- 5.Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, et (2013) Skin test concentrations for systemically administered drugs -- an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 68: 702- 712.
- 6.Bergmann MM, Caubet J-C (2019) Role of in vivo and in vitro tests in the diagnosis of Severe Cutaneous Adverse Reactions (SCAR) to drug. Curr Pharm Des 25: 3872-3880.
- 7.Watanabe H (2018) Recent advances in drug-induced hypersensitivity syn- drome/drug reaction with eosinophilia and systemic symptoms. J Immunol Res 2018: 1-10.
- 8.Dong-Hyun K, Young-Il K (2014) Comparison of diagnostic criteria and deter- mination of prognostic factors for drug reaction with eosinophilia and systemic symptoms Allergy, Asthma Immunol Res 6: 216-221.
- 9.Wutte N, Palfner M, Auer H, Ruckenbauer G, Valentin T, et al. (2014) Toxo- carosis and putative DRESS syndrome in an oncological patient: A case re- Wien Klin Wochenschr 126: 51-55.
- 10.Eyraud A, Boursault L, Darrigade AS, Taieb A, Milpied B (2017) First case of DRESS syndrome attributed to a spider bite. J Allergy Clin Immunol Pract 5:1135-1136.
- 11.Ben Mahmoud L, Bahloul N, Ghozzi H, Kammoun B, Hakim A, et al. (2017) Epicutaneous patch testing in delayed drug hypersensitivity reactions in- duced by antiepileptic Therapie 72: 539-545.
- 12.Martin M, Wurpts G, Ott H, Baron JM, Erdmann S, et al. (2010) In vitro detec- tion and characterization of drug hypersensitivity using flow cytometry. Allergy 65: 32-39.
Citation:Jimenez-Rodriguez TW, Gonzalez-Delgado MP, Ruano-Zaragoza M, Soriano- Gomis V, Greco-Bermudez L, et al. (2020) Patch Test Diagnosis of Carbamazepine- Induced Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Case Report. J Case Repo Imag 4: 024.
Copyright: © 2020 Jimenez-Rodriguez TW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


