*Corresponding Author:
Prashant D Sawant,
Intraceuticals Pty Ltd, Melbourne, Australia
Tel: +61434304306
E-mail: pdsawant@yahoo.com
Abstract
Theranostics is a new promising medical paradigm that synergistically utilizes therapeutic and diagnostic capabilities of agents for diagnosis, drug delivery or therapy and therapy response recording. This field is further evolving fast into the nano-theranostic which is immensely benefitted by multifunctional nanomaterials that can enhance not only the imaging quality but also the specific targeting of disease sites, particularly cancer sites. Nano-theranostic therapies can be assisted by nano-theranostic agents will help to reduce toxicity and reduce the time of both diagnosis and therapy and help to develop personalized therapies for cancer management.
Most of the published reviews focused on one particular type of nanotheranostic agents or methodologies for the cancer treatment. The present review article compiles recent advances and future growth of the nano-theranostic agents for cancer management. Additionally, examples of light-triggered or ultrasound triggered nanotheranostic methodologies are also provided in the present review. Moreover, key limiting factors such as nanoparticle induced toxicity, particularly, autophagy are discussed.
Introduction
John Funkhouser (PharmaNetics) was first introducing “Theranostic” in 2002 while describing PharmaNetics’s diagnostic tests that are synergistically connected to the application of specific therapies [1,2]. This new medical paradigm [1-3] uses single multifunctional agent for therapy and diagnostics and can be used for personalized medical treatments that are more specific to individuals needs such as improved prognosis and reduce dose-related toxicity or side-effects.
One of the first theranostic agent, radioiodine has used in the 1940s to image and treat thyroid cancers [4] and the first molecular imaging using radioiodine was performed by Dr. Benedict Cassen in 1950 at UCLA [5,6]. Dr. Cassen has used the rectilinear scanner to image the gland and revealed biologic characteristics of the thyroid tissues using radioiodine. An example of radioiodine theranostic is provided in Figure 1 which depicts images of two patients A and B who were diagnosed with advanced ovarian cancer. The pretreatment F-18 FDG PET imaging (Figure 1) has successfully visualized multiple cancerous lesions in the neck and abdominal cavity of both patients. The images revealed that Patient A has achieved complete remission after chemotherapy whereas Patient B has progressed to disease status after the chemotherapy. Nanoparticles (NPs) offer enhanced physico-bio-chem and optoelectronic properties due to high specific surface area, shape and (quantum) size effects and may help to deliver the concentrated dose of radiation locally to the disease site without affecting healthy cells [7-12]. Therefore, NPs can be used to achieve fast and improved continuous imaging or diagnosis (during the treatment) and site-selective drug delivery individually. Also, NPs can easily overcome cellular, anatomical, and physiological barriers including the blood-brain barrier and detect changes at cellular and molecular levels. The modern therapeutics such as proteins or peptides [13], antibodies [14,15], and water-insoluble drugs [16] need specialized delivery systems to maximize therapeutic efficacy by achieving precision in drug targeting, improving drug bioavailability and reducing cytotoxicity. The same nanotheranostic agents (NTAs) should be able to load the drug and co-deliver the drug to the specific site for the treatment and perform continuous imaging of the site during the treatment task (and not just before and after the treatment) and treat the disease area. However, both diagnostic and therapy need sufficient accumulation of NTAs at the specific disease site. Also NTAs should exhibit long half-lives in blood circulation, broad surface chemical functionality to adsorb and desorb drug molecules, maximum loading of the drug per particle, non-toxicity, efficacious biodistribution and clearance, and avoid innate immunosystem [17]. NPs used as NTAs are summarized in Table 1 [1]. NPs are better than microparticles for intravenous delivery [18,19]. The high surface to volume ratio of nanoparticles is helpful to reduce the dose and frequency of administration and increase the patient compliance.
For the cancer treatment, NTAs should exhibit permeability-and-retention (EPR) effect in tumor targeting. NTAs need a suitable biomarker that can selectively expressed on the cancer cell surface and its cognate binding vector for maximum loading on the particle and efficient imaging and/or therapeutic efficacy and have maximum EPR with long circulation half-lives without recognizing the innate immunosystem. A typical functionalized nanoparticle theranostic agent is depicted in Figure 2. As seen from Figure 2, the drugs such as RNA and DNA are generally attached on the internal or external surfaces of a suitable nanocarrier (e.g. Au nanoparticles) through physical adsorption and/or chemical bonding or biofunctionalization [20].
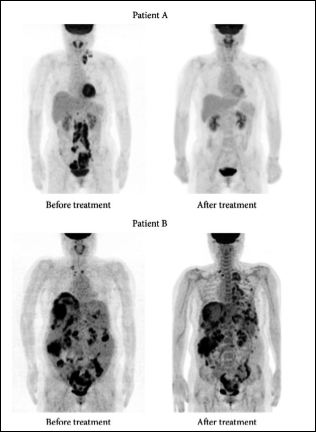
Figure 1: Patients A and B were diagnosed with advanced ovarian cancer. Pretreatment F-18 FDG PET imaging successfully visualized multiple cancerous lesions in the neck and abdominal cavity. However, the imaging was not able to predict the therapeutic response to the subsequent chemotherapy. Patient A achieved complete remission after chemotherapy; however, patient B progressed to disease status after chemotherapy. Source: Biomed. Res. Int. 2016; 2016: 1680464. Copyright © 2016 Byeong-Cheol Ahn.

Table 1: Nanotheranostic agents for cancer management.
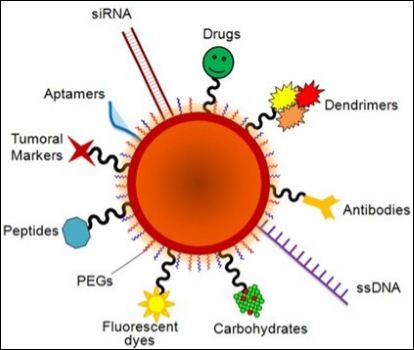
Figure 2: Schematic representation of a multifunctional nanocarrier. These innovative NPs comprise of nucleic acids such as RNA and DNA used for gene silencing approaches and in colorimetric assays, respectively. Aptamers and anticancer drug molecules are also used for delivery to the target tissue. Carbohydrates may be useful as sensitive colorimetric probes. PEG is used to improve solubility and decrease immunogenicity. Responsive nanocarriers can also trigger reaction upon external stimuli through the functionality of valuable tumor markers, peptides, carbohydrates, polymers and antibodies that can be used to improve nanocarrier circulation, effectiveness, and selectivity. Multifunctional systems can also carry fluorescent dyes that are used as reporter molecules tethered to the particle surface and employed as tracking and/or contrast agents.(Source: Conde J, et al. [20] Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front Chem. 2:48. Copyright: Frontier in Chemistry.).
The targeted anticancer therapy constructs are formed by connecting the ligands to the carriers of chemotherapeutic agent. The resultant construct facilitates the bioconjugation with suitable receptors of cancer cells which supports the over expression of cancer cell receptors and the affinity of ligand to receptor [21-23]. The chemotherapeutic agent is then delivered to the most resistant cancer cells with internal longtime circulation and achieves the high drug concentration in situ the tumor without releasing back to the blood circulation. The ligand immunogenicity is important from the safety and efficacy point of view [24]. Some examples of the nano-theranostic agents are discussed as follows.
Iron Oxide Nanotheranostic Agents
Crystalline magnetite or hematite based Iron Oxide Nanoparticles (IONPs) exhibit saturation magnetization values at room temperature. Moreover, IONPs smaller than 20 nm provide super paramagnetic properties. The thermal energy is enough to overcome the anisotropy energy of each magnetic nanoparticle that can lead to random fluctuation of the magnetizations and result in zero net coercivity. The superior magnetic properties, inherent biocompatibility and inexpensiveness, can make IONPs as contrast agents for Magnetic Resonance Imaging (MRI). High magnetic moments of IONPs can result in the reduction of T2 relaxation time and attenuation a signal on a T2 or T2* weighted map. This signal alteration can be used to target specificity to report abnormal biological activity. During synthesis IONPS additives or ligands such as hydrophilic polymers and dendrimers are used to modify the nanocrystal surface to avoid particle aggregation. Sawant et al. [10] has demonstrated the use of nanosize Tc+99 Fe2O3 for the lung imaging by lung photoscientigraphy. Figure 3 depicts a typical lung image of a healthy volunteer obtained using nanosize Tc+99 Fe2O3. The nanosize Tc+99 Fe2O3 can be used to image and treat lung tumors after appropriate modifications. Kohler et al. [25,26] have coupled an anti-cancer drug methotrexate (MTX) onto an aminated IONP surface to form MTX-IONPs which are internalized into cells and accumulated in lysosomes where the MTX are released due to the low pH and the presence of proteases. Thus, MTXIONPs can be used as nano-theranostic agents for MRI and drug delivery to treat cancers. Hwu et al. [27] have coupled paclitaxel (PTX) to IONP surfaces using a phosphodiester at the (C-2’)-OH position and achieved to couple around 83 PTX molecules per nanoparticle. The effective release of PTX from PTX-IONPs is achieved upon exposure to phosphodiesterase. Huh et al. [28] and Lee et al. [29] have developed meso-2,3-dimercaptosuccinic acid (DMSA) derivative of IONPs, and coupled herceptin antibody molecules onto the surface of resultant DMSA-IONP surface through the covalent bond using succinimidyl-4-[N-maleimidomethyl]cyclohexane-1-carboxylate (SMCC), a heterobifunctional protein crosslinker. Herceptin was used as nanotheranostic agent (NTA) for both specific targeting for imaging using the magnetic resonance method and treating cancer. Instead of covalent coupling, both drug molecules and IONPs can be co-capsulated into polymeric matrices. Jain et al. have encapsulated doxorubicin (DOX) and PTX, along with oleic acid coated IONPs in pluronic-stabilized nanoparticles [30]. Yu et al. also loaded DOX into which are IONPs coated an anti-biofouling polymer [31]. These DOX loaded IONP nanoconjugates have exhibited better pharmacokinetics and therapeutic effects than DOX alone in a Lewis lung carcinoma xenograft model due to the anti-biofouling feature of the particles. To differentiate the antiproliferative effect of drugs (PTX, DOX and their combination) in solution and loaded in magnetic NPs (MNPs), MCF-7 cells are treated with the drug solution and drug-MNPs and cell viability is measured using an MTS assay on fifth day. Small active molecules or drugs (low molecular weight) can also be loaded into porous nanostructures through physical absorption. Hollow IONPs are created by first forming spindle-shaped β-FeOOH NPs us- ing the hydrolysis of FeCl3 followed by three step “wrap-bake-peel” treatment. DOX is then loaded into such hollow nanoparticles via simple physical absorption and released from the hollow MNPs in a sustained manner under physiologic conditions [32-34]. Cheng et al. [35] have developed porous IONPs with a nano-size cavity (~2-4 nm) using controlled oxidation and acid etching of Fe particles and encapsulated cisplatin through diffusion into IONPs’s nanocavities and subsequently coupled herceptin onto the particle surfaces to confer targeting specificity. The resulting nanoconjugates have exhibited selective affinity to ErbB2/Neu-positive breast cancer cells with IC(50) reaching 2.9 muM which is much lower than 6.8 muM needed for free cisplatin. A sustained cytotoxicity towards cancer cells can be attributed to the controlled release of cisplatin from the porous IONPs. Thus, the low pH-responsive Fe3O4NTAs can be used as a potential cisplatin delivery vehicle for target-specific therapeutic applications. There are several other examples of nano-theranistics used to treat cancer [36]. Seven patients with metastatic breast cancer were infused with epirubicin-loaded IONPs (100 nm in diameter, at 0.5% of the estimated blood volume), and after that a magnetic field was established around the tumor. The magnetic field proved successful in directing the ferrofluid to the tumor to induce tumor regression.
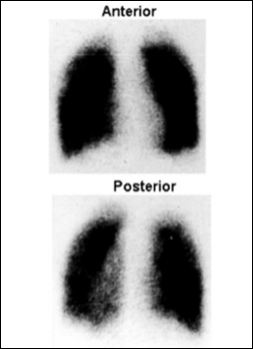
Figure 3: Photo scintigraphic images of anterior and posterior of human lung.
Namiki et al. [37] have screened cationic lipid coated IONPs, and developed LipoMag using lipids and found to be better than commercially available PolyMag in both transfection and gene knockdown. These authors [37] have also loaded modified siRNA with IONPs and evaluated its therapeutic potency in two gastric cancer models and found a 50% reduction in tumor volume after a 28-day treatment, in addition to the inhibition of angiogenesis and the induction of apoptosis. The gene knockdown was found to be significant only in the presence of magnetic fields at the tumor sites. Also, IONPs were used as a nanotheranostic agent in hyperthermia because IONPs can act as antennae in an external alternating magnetic field (AMF) to convert electromagnetic energy into heat. When phospholipid coated IONPs were injected into a subcutaneous tumor model in F344 rats, and were exposed to an AMF, these IONPs have found to raise the temperature of tumor above 43°C to cause tumor regression. However, tumor regression was not found in the control group treated without IONPs. Additionally, Fab fragment of anti-human MN antigen-specific antibody was chemically anchored onto IONP surfaces and resultant IONPs were administrated systemically into tumor-bearing mice. Resultant IONPs exhibited high tumor uptake presumably due to an antibody-antigen interaction, and induced efficient tumor hyperthermia upon AMF exposure [7].
Quantum Dot Nanotheranostic Agents
Quantum dots (QDs) are semiconductor light-emitting nanocrystals which exhibit unique size-dependent properties such as photo-stability and chemical stability and have a narrow emission spectrum [9]. QDs of CdSe, CdTe and PbS, can emit light in visible spectrum depending on their sizes but the QD based therapy is not efficient in in vivo applications due to the limited tissue penetration of visible light. Therefore, QDs with near-infrared emission have been developed using CdTe/CdSe, Cd3P2, in As/ZnSe and In As/InP/ZnSe. Also inorganic coatings using ZnS on the QDs surface as found to be useful in enhancing the photoluminscent quantum efficiencies of the resultant QD particles. Also sulphur of the ZnS layer is use as a species-mounting site. Moreover, ZnS coatings have also helped to improve the biofate of QDs. Also cysteine modified QDs are water soluble and exhibit hydrodynamic diameter less than 5.5 nm which can rapidly excreted via renal clearance without trapping in reticuloendothelial system (RES) organs such as the liver and spleen. Moreover, amphiphilic compounds, such as phospholipids, amphiphilic saccharides, acrylic acid polymers and others can be used to improve the efficiency of theranostic QDs. Smith et al. [38] have modify QDs and conjugated onto them a prostate-specific membrane antigen (PSMA) targeting antibody using a triblock copolymer consist of chains of polybutylacrylate, polyethylacrylate and polymethacrylic acid and a hydrophobic hydrocarbon side chain for prostate cancer studies. When these QD-nanoconjugates were administrated into prostate cancer bearing mice, these nano-conjugates were found to be accumulated in the tumor area only due to both the EPR effect and specific antibody-antigen interactions. Similarly, Nurunnabi et al. [39] have synthesized water soluble QDs-herceptin-PEG-10,12-pentacosadiynoic acid (PEG-PCDA), and stabilized by cross linking the coating shell UV irradiation and demonstrated an efficient tumor targeting rate and therapeutic effects on an MDA-MB-231 tumor model. On the other hand, Bagalkot et al. [40] have developed a QD-aptamer-DOX conjugate (QD-Apt-Dox) to achieve simultaneous cancer imaging, therapy and therapy monitoring. The fluorescence activities from QD and DOX were found to be attenuated by their interaction with DOX and RNA, respectively, and are both QD and Dox were found to be in the quenched state in the QD-Apt-Dox nanosystem. When these nano-theranostic QDs were delivered into targeted tumor cells, DOX was gradually released from the nanosystem, and triggered both the therapeutic functions and QD fluorescence. Yuan et al. [41] have used reversible physical adsorption to load MTX onto QD surfaces and induced photoluminescence quenching of QDs. The physical adsorption of MTX was reversed when exposed to higher affinity species such as DNA and an exchange of the MTX coating by DNA led to a restoration of the QD photoluminescence. This facile reversible ligand exchange process can be used to monitor the delivery of drug molecules to cancer site.
QDs can be used as either photosensitizers or carriers in photodynamic therapy (PDT) to treat cancer because as a photosensitizer, QDs can be activated by light and transfer the triplet state energy to nearby oxygen molecules to cause cell damage through the generation of reactive oxygen intermediates (ROIs) [42,43]. Photo activated ROIs impart damage to purine and pyrimidine bases. However, the quantum yield of QD-generated singlet oxygen is less than 5%, which is much lower than that of classic photosensitizers (40-60%).
Gold Nanotheranostic Agents
Gold nanoparticles (Au NPs) exhibit unique size and morphology dependent properties such as strong surface plasmon absorption, stability, biosafety and ease of modification and therefore are used to build functional agents for both imaging and therapy applications. Spherical Au NPs of 10 nm size exhibit characteristic surface plasmonic absorption at ~520 nm which can be red-shifted to maximum of 575 nm by increasing the Au particle size. Additionally, the absorption can be shifted to the near infrared (NIR) region (650-900 nm) by changing morphology of NPs from spherical to rod shape. Because of the size and morphology dependent optoelectronic properties, Au NPs have been used for PDT and imaging purposes in computed tomography (CT), photoacoustics and surface-enhanced Raman spectroscopy (SERS).
In the PDT based cancer treatment, Au NPs are first directed towards the tumor sites and accumulated, followed by the exposure of laser irradiation to convert light into heat to kill cancerous cells specifically without damaging normal tissue damage. Thus, Au NPs based PDT treatment is safer than the conventional laser irradiation treatment. However, the characteristic absorption at 500-600 nm of spherical Au NPs is not appropriate for the PDT therefore; the morphology of Au NPs needs to be changed to a nanorod, nanocage or nanoshell from spherical shape to shift the absorption to the NIR region.
Cheng et al. [44] have adsorbed a PDT agent, Pc4, onto PEGylated Au NPs and used it as a drug carrierin addition to PDT function. Authors [44] have demonstrated the fast release of Pc4 delivery (less than 2 hours) as compared to that of free drug (2 days). Similarly, Prabaharan et al. [45] have developed an amphiphilic block-copolymer coated Au NPs based system for tumor targeting and drug delivery. This copolymer-Au system consisted of an Au NP core, a hydrophobic poly-aspartic acid (PAsp) inner shell and a hydrophilic, folate-conjugated PEG outer shell (PEG-OH/FA) which can load up to17 wt% DOX through covalent conjugation onto the hydrophobic inner shell. This nanosystem possesses both a tumor targeting mechanism (folate on the outer layer) and an intracellular drug release mechanism through hydrazone linkage of DOX on the inner layer. Moreover, PEG coated Au nanocages [46,47] were accumulated in a U87MG xenograft model which helped to increase the tumor surface temperature to 54°C within 2 min upon exposure to NIR light (light triggered nanotheranostics). Lu et al. [48] have coupled used α-melanocyte-stimulating hormone (MSH) analog, [Nle4, D-Phe7] α-MSH (NDP-MSH) onto Au nanoshells and the resultant nanoconjugates are administered to a B16/F10 melanoma model. Authors [48] have found that the resultant nanoconjugates have accumulated in tumor in large quantities due to NDP-MSH. B16/F10 melanoma found in the tumor was efficiently ablated upon exposure to laser illumination only and the contralateral tumor was not ablated if it was not expose to the laser illumination. This PDT success was validated by histological and PET studies. Additionally, [18F] fluorodeoxyglucose (18F-FDG) PET has found a remarkable decrease in the tumor up take indicating a drop in a metabolic activity. Lu et al. [49] have also demonstrated the use of Au nanoshells as light-controllable siRNA carriers for chemotherapy. The specific tumor targeting specificity was achieved by coupling Au nanoshells with folic acid and siRNA with a sequence that targets NF-κB P65 (a protein complex that controls transcription of DNA, cytokine production and cell survival) to the nanoparticle surface via thiol-Au interaction. A stable thiol-Au covalent bond helps to carry the siRNA payload even after cell uptake which was destroyed upon the exposure of NIR light irradiation. The NIR light irradiation damages the endolysosomal membrane and releases siRNA from the Au nanoshell surface into cytoplasms which was confirmed by the observation of light-inducible siRNA release and subsequent NF-κB P65 down regulation both in vitro and in vivo experiments. Additionally, the down regulation of NF-κB P65 was resulted in an increased sensitivity to the chemotherapy which is evidenced by an improved therapeutic index when PDT was combined with irinotecan treatment (particularly for treating colon cancer). This is also good example of light triggered nanotheranostics.
Carbon Nanotube Nanotheranostics
Carbon nanotubes (CNTs) exhibit a graphite-like structure which is inert and inhibitive to most of conjugation chemistry but have potential applications in Raman and photoacoustic imaging and drug delivery [50,51]. The strong optical absorbance of single-walled carbon nanotubes (SWNTs) is an intrinsic property of SWNTs which can be used for optical stimulation of nanotubes inside living cells to afford multifunctional nanotube biological transporters [52,53]. CNTs have found to be internalized by cells and effectively cross biological barriers therefore CNTs can be used as nano-vectors for therapeutic agent delivery. The internalization of CNTs by cells can occur via different routes such as endocytosis and passive diffusion depending upon surface coatings. The solubility and bio-functions of CNTs can be improved by modifying CNT’s surface with organic molecules and use in the delivery of drugs, antigens and genes [54]. Additionally, a high degree of CNT functionalization (f-CNTs) can reduce toxic effects [55]. Water-soluble f-CNTs and CNTs can be further coated with proteins [52], polymers [56] or single-stranded DNA [57] to improve their interactions with mammalian cells, leading to their cytoplasmic translocation [58,59] whereas ammonium-functionalized cationic nanotubes can condense and deliver plasmid DNA (pDNA) intracellularly, leading to enhanced marker gene expression [59,60]. The strong optical absorbance of CNTs in the NIR region makes CNTs as a potential nano-theranostic agent in cancer PDT. Upon exposure to NIR light, internalized CNTs in cells are capable of triggering endosomal rupture and cell death [61]. Samori et al. [62] have coupled MTX onto 1,3-dipolar cycloaddition functionalized CNTs and internalized into human cells including breast cancer cells. Authors [62] have found that the cytotoxic activity of the resultant CNT-MTX construct was strongly dependent on the presence and type of linker [63]. Additionally, phospholipid-CNT nanoconjugates have been used for both imaging and therapy. siRNA was coupled to phospholipid-CNT nanoconjugates using a disulfide bond and the resultant CNT transporter exhibited high transfection efficiency while outperforming lipofectamine in inducing RNAi. Selective cancer cell destruction was achieved without harming receptor-free normal cells by folate functionalized SWNT followed by the selective internalization of SWNTs inside cells labelled with folate receptor tumor markers, and subsequent exposure to NIR. PEGylated CNTs can improve the pharmacokinetics and therapeutic effects because PEGylation brings an extra stability to CNTs. The PEGylated CNTs was coupled with either Pt (IV) prodrug or PTX onto PTX through a cleavable ester bond and the resultant construct was tested in a murine 4T1 breast cancer model. The PTX-Pegylated-CNT nanoconjugates have exhibited a 10-fold increase in tumor homing than PTX alone and prolonged the circulation half-life of the PTX-Pegylated-CNT nanoconjugates. The PTX-Pegylated-CNT nanoconjugates have demonstrated better tumor suppression outcome than clinically used taxol [64]. Additionally, Moon et al. have demonstrated the use of PEGylated SWNT and NIR irradiation to eradicate tumors in a human epidermoid mouth carcinoma model with no further tumor recurrence over six months [61]. Also, Ghosh et al. have encapsulated CNT using DNA to improve the heat emission efficacy and induce complete tumor eradication after the internalization of nano-conjugates in a PC3 xenograft model intratumorally and subsequent NIR irradiation [65].
Silica nanoparticles as nanotheranostic agents
Silica is generally considered as a safe and is used in surgical implants. Silica nanoparticles themselves do not have characteristic optoelectronic properties for imaging but can help facile loading of a broad range of imaging and therapeutic functions. It is easy to create multi-functional surface of silica nanoparticles and encapsulate small molecules, IONPs and QDs. Alternatively, these nanoparticles can also be easily incorporated into silica matrices to combine both magnetic and optical properties [66,67]. Roy et al. [68] have used the micellization method to trap water-insoluble photosensitizing anticancer drug 2-devinyl-2-(1-hexyloxyethyl) pyropheophorbide on ~30nm organically modified silica NPs. The resulting drug-doped NPs are spherical, highly monodispersed, and stable in aqueous system. The entrapped drug is found to be more fluorescent in an aqueous medium than the free drug, permitting use of fluorescence bioimaging studies. Upon irradiation, the photosensitizing drug entrapped in nanoparticles has efficiently generated singlet oxygen. In vitro studies have demonstrated the uptake of drug-doped silica NPs into the cytosol of tumor cells and significant damage to impregnated tumor cells after the light irradiation at 650 nm. Thus silica NTAs can be used for imaging and PDT functions [68].
Mesoporous silica nanostructures (MSNs) consist of hundreds of empty nano/micro channels or molecular-sieve structures and exhibit a large surface area (>900 m2/g), MSNs can be used to encapsulate small molecules via simple physical interactions and control the drug delivery properties. The mesopores can be capped or sealed using Au NPs after the loading of anticancer drugs (e.g. PTX) to inhibit the premature drug release. Au NP capping is photolabile and can be uncapped upon photo-irradiation to release guest drug molecules. Similar seals or caps based on QDs, IONPs, coumarin and diethylenetriamine can be also used. Park et al. [69] have developed biodegradable luminescent porous silicon nanoparticles (LPS iNPs) and loaded with DOX. These silica nanotheranostic particles can self-destruct in vivo and be renally cleared within a relatively short time which helps to reduce the toxicity risk to normal organs. Further authors [69] used these nanotheranostic nanoparticles for tumor imaging and slow release of DOX upon degradation of nanoparticles.
Liposome Nanotheranostics
Liposomes are spherical vesicles composed of a lipid bilayer of either synthetic or natural phospholipids with diameters ~100 nm and can encapsulate both therapeutic and diagnostic agents, protect the encapsulated agents from external environments, prolong systemic circulation lifetime of the encapsulated agents. Liposomes are wide- ly used as drug delivery vehicles for cancer therapy [70,71] and can be functionalized with various targeting ligands to achieve cell- or tissue-specific delivery [72]. Additionally, liposomes are used to en- capsulate a variety of contrast agents such as superparamagnetic iron oxide NPs (SPIOs) [73,74], gadolinium-based, and manganese-based compounds [73,75,76] for MRI applications to enhance the contrast in T2-weighted MRI for better in vivo visualization. Iron oxide par- ticles are encapsulated into a cholesterol/DOPE/DSPC liposome to form multifunctional nanotheranostic agents [77]. Soundararajan et al. have encapsulating 186Re and DOX into liposome interior to de- velop radiolabeled liposomes for cancer chemoradionuclide therapy [78] and tested in male nude rats bearing xenografts of head and neck squamous cell carcinoma. These radiolabeled liposomes have demon- strated a prolonged circulation time with minimum liver accumula- tion. Moreover, these radiolabeled liposomes are combined with che- motherapeutic drugs to demonstrate real-time imaging and increases efficacy conferred by chemoradionuclide therapy [78].
Polymer-Based Nanotheranostics
Polymeric NPs have been used for drug delivery applications [79] with their core were loaded with a variety of therapeutic or imaging agents. Polymer NPs have helped the sustained and controlled release of these agents through various mechanisms such as surface or bulk erosion, diffusion through the polymer matrix, swelling followed by diffusion or stimulation by the local environment results [79]. Additionally, polymeric NPs have been used as effective carriers for MRI contrast agents such as SPIOs and Gd-based compounds [72,80,81]. The mixture of SPIOs and DOX is encapsulated by using an amphiphilic block co-polymer, maleimide-PEG-poly(lactic acid) (PLA), which self-assembles to form polymeric NPs and used as NTAs for both drug delivery and MRI [80,81]. Surface-presenting maleimide groups help the conjugation of either cRGD molecules to target αvβ3 integrins [80] or short peptides containing 10 amino acids to target αvβ6 integrins [81]. The resultant NTAs are used for the evaluation of pharmacokinetics by real-time MRI in tumor-bearing mice. Additionally, targeted NTAs have demonstrated a higher tumor accumulation due to the integrin-mediated endocytosis leading to enhanced tumor retardation as compared to the non-targeted NPs. The strategy of encapsulating SPIO-DOX mixture to develop NTAs is applicable to many existing polymeric NP systems [82]. Radionuclide compounds such as 11C, 18F, 64Cu, 76Br, 99mTc, 111In and 90Y, have been used for radionuclide imaging are combined with a wide range of copolymers such as N-(2-hydroxypropyl)methacrylamide (HPMA) to develop robust nano-sized delivery systems also [83]. Such NTAs can be used as image-guided drug delivery systems to monitor drug pharmacokinetics, intratumoral drug distribution, and drug tumor accumulation in real-time [84]. Peng et al. [85] have developed multifunctional polymeric NTAs of PEG-polycaprolactone (PCL) di-block co-polymer with a NIR florescent dye (IR-780) for both NIR imaging and PDT followed by labelling with 188Re and used for micro SPECT-guided tumor imaging. A preferential tumor accumulation of these NTAs is observed in BALB/c athymic nude mice bearing HCT-116 colorectal carcinoma followed by enhanced tumor inhibition by NIR irradiation as compared with control groups without NTAs (control groups are treated with PBS, NIR irradiation only, or micelles only). Recently, Zhang et al. [86] have described the use of a biopolymer (hyaluronic acid) NPs for optical/photoacoustic image-guided photothermal therapy which can potentially be used as NTAs. Cheng et al. have developed multifunctional PEGylated Prussian Blue nanocubes (PB NCs) for simultaneous cancer imaging and photothermal therapy [87]. These PB-NCs are clinically approved NTAs and exhibit strong NIR absorbance and intrinsic paramagnetic properties that are useful for in vivo bimodal imaging-guided photothermal therapy. Polyethylene glycol (PEG) coated PB-NCs (PB-PEG-NCs) are highly stable in various physiological solutions. In vivo T1-weighted magnetic resonance and photoacoustic tomography bimodal imaging showed that PB-PEG-NCs have high uptake in the tumor after the intravenous administration in a mouse tumor model. After the uptake of PB-PEG NCs into tumor, in vivo cancer treatment is then conducted using NIR laser irradiation of the tumors to achieve excellent therapeutic efficacy without any apparent toxicity. Recently, Kang et al. [88] have used the ability of hydrogen peroxide (H2O2) to overproduce reactive oxygen species (ROS) and induce oxidative stress, inflammation and cellular damages during ischemia/reperfusion (I/R) injury. This phenomena is used to develop H2O2 triggered CO2 generating antioxidant poly (vanillin oxalate) (PVO) NTAs for simultaneous ultrasound imaging and therapeutic effects for the hepatic I/R treatment. PVO NPs generate CO2 through H2O2 triggered oxidation of peroxalate esters and release vanillin, which exerts antioxidant and anti-inflammato- ry activities. Intravenously administrated PVO NPs have found to enhance the ultrasound signal in the site of hepatic I/R injury and effectively suppressed the liver damages by inhibiting inflammation and apoptosis [88]. Tumor-homing echogenic glycol chitosan based nanoparticles (Echo-CNPs) are constructed to concurrently execute cancer-targeted ultrasound imaging and ultrasound triggered drug delivery [89]. Echo-CNPs produced by simultaneously encapsulating an anticancer drug and bio-inert perfluoropentane (PFP), an ultrasound gas precursor into glycol chitosan NPs using the oil in water (O/W) emulsion method [89]. The resulting nanosize (432 nm) Echo-CNPs are composed of hydrophobic anticancer drug/PFP inner cores and a hydrophilic glycol chitosan polymer outer shell. The resultant Echo-CNPs have showed the prolonged echogenicity via the sustained microbubble formation process of liquid-phase PFP at the body temperature and exhibited an ultrasound triggered drug release profile. Echo-CNPs have further exhibited a significant increase of tumor-homing ability with lower non-specific uptake by other tissues in tumor-bearing mice through the nanoparticle’s EPR effect [89].
Future Challenges and Opportunities
Despite technological development of NTAs, there are many unsolved issues that need to be address in the future. Some of the challenges are provided as follows.
Toxicity and Safety: The toxicity of QDs, CNTs and metal particles will be a prime concern for both regulatory agencies and patients. Autophagy plays a key role in cancer and neurodegeneration and nanoparticles may induce it [90,91]. Professor Yoshinori Ohsumi discovered and elucidated mechanisms underlying autophagy, a fundamental process for degrading and recycling cellular components and has won the 2016 Nobel Prize in Physiology or Medicine [92]. Recently Mao et al. have showed that interference of Ag NPs with ubiquitination may account for Ag NPs induced defective autophagy and cytotoxic effects [93]. TiO2 NPs can accumulate in the brain and induce brain dysfunction and may cause neurotoxicity through oxidative stress, apoptosis, inflammatory response, genotoxicity, disrupted signaling pathways, dysregulated neurotransmitters, synaptic plasticity and direct impairment of cell components [94]. Similarly, Huang et al. [95] have demonstrated that the cytotoxicity of aggregated gold nanoparticles is significantly higher than well-dispersed ones and attributed the dispersity-dependent cytotoxicity to the increase of cellular endocytosis and reactive oxygen species. Autophagy may be used to kill cancer selectively but more research is needed to understand autophagy mechanisms using NTAs. Further studies are also needed to address this important issue before commercializing NTAs.
Biodegradability and Biofate: Non-biodegradable nature of CNTs and silica nanoparticles may pose some serious issues to use as NTAs. More studies are needed to prove non-toxicity or safety of that these NTAs in addition to their efficacy.
Site Specific Drug Delivery: Researchers are always inspired to develop site specific and controlled release drug delivery systems. The initial burst release followed by the sustain release of drugs will be a desired outcome to reduce site-specific toxicity and treatment cost, and improve patient compliance.
Clinical standards: To get the regulatory approvals, gold standards or controls are required to validate the functional integration of imaging and therapeutic synergy in NTAs at nanoscale.
Cost of Development and Treatment: The production cost of NTAs is generally high. However, economies of scale, new production methods and more consumer demand along with government support may help to solve this issue.
Conclusions
Nano-theranostics is a new promising medical paradigm that synergistically utilizes therapeutic and diagnostic capabilities of multifunctional nanoparticles for diagnosis, site specific drug delivery or therapy and recording of therapy response. Additionally, nano-theranostic agents will help to reduce toxicity, improve patient compliance and reduce time of both diagnosis and therapy and can be used to develop personalized therapies.
The present review focused on the use of different types of NTAs for the cancer treatment. Despite excellent progress so far, there are many key challenges such as nanoparticle induce toxicity and autophagy remained to be solved. In future, it is safe to assume that we will be efficacious, cost-effective and safer nano-theranostic agents for the treatment of cancers.
References
- Revia RA, Zhang M (2016) Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Materialstoday 19: 157-168.
- Jeelani S, Reddy RC, Maheswaran T, Asokan GS, Dany A et (2014) Theranostics: A treasured tailor for tomorrow. J Pharm Bioallied Sci 6: 6-8.
- Moghimi SM, Farhangrazi ZS (2015) “Theranostics,” in Encyclopedia of Cancer, Springer, Berlin, Germany Pg no: 1-3.
- Silberstein EB (2012) Radioiodine: the classic theranostic Semin Nucl Med 42:164-170.
- Ahn BC (2016) Personalized medicine based on theranostic radioiodine molecular imaging for differentiated thyroid cancer. Biomed Res Int 2016:1680464.
- Lee DY, Li KC (2011) Molecular theranostics: a primer for the imaging profes AJR Am J Roentgenol 197: 318-324.
- Xie J, Lee S, Chen X (2010) Nanoparticle-based theranostic agents. Adv Drug Deliv Rev 62: 1064-1079.
- Sawant PD Niranjane A (2006) Formation of nanoparticles of sparingly soluble salts (caso4 and caso4:dy) using liquid-liquid separation method and application for detection of α-radiations. IET Micro & Nano Letters 1: 108-111.
- Sawant PD, Ramaniah LM, Manohar C (2006) Capacity of nano-reactors of AOT micro-emulsions to form and sustain ultra small semiconductor quantum dots. J Nanosci Nanotech 6: 241-247.
- Sawant PD, Sawant SP (2005) Tc99m doped Nano-Hematite for Lung Pho J Biomed Nanotech 1: 406-409.
- Tzhayik O, Sawant P, Efrima S, Kovalev E, Klug JT (2002) Xanthate capping of silver, copper, and gold colloids. Langmuir 18: 3364-3369.
- Sawant PD, Kovalev E, Klug JT, Efrima S (2001) Alkyl xanthates: New capping agents for metal Capping of platinum nanoparticles. Langmuir 17: 2913-2917.
- Kuang Y, Jiang X, Zhang Y, Lu Y, Ma H, et al. (2016) Dual functional peptide-driven nanoparticles for highly efficient glioma-targeting and drug Mol Pharm 13: 1599-1607.
- Chakroun N, Hilton D, Ahmad SS, Platt GW, Dalby PA (2016) Mapping the aggregation kinetics of a therapeutic antibody Mol Pharm 13: 307-319.
- England CG, Hernandez R, Eddine SB, Cai W (2016) Molecular imaging of pancreatic cancer with antibodies. Mol Pharm 13: 8-24.
- Liu J, Ma X, Jin S, Xue X, Zhang C, et al. (2016) Zinc oxide nanoparticles as adjuvant to facilitate doxorubicin intracellular accumulation and visualize pH-responsive release for overcoming drug Mol. Pharm 13:1723- 1730.
- Alexis F, Pridgen F, Molnar LK, Farokhzad OC (2008) Factors affecting the clearance and biodistribution of polymeric Mol Pharm 5: 505- 515.
- Zhang M, Yudasaka M (2014) Potential application of nanocarbons as a drug delivery system. Carbon 69: 642.
- Hughes GA (2005) Nanostructure-mediated drug Nanomedicine 1:22-30.
- Conde J, Dias JT, Grazú V, Moros M, Baptista PV, et (2014) Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front Chem 2: 48.
- Liu Y, Miyoshi H, Nakamura M (2007) Nanomedicine for drug delivery and imaging: a promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles. Int J Cancer 120: 2527-2537.
- Torchilin VP (2006) Multifunctional Adv Drug Del Rev 64: 302-315.
- Parveen S, Misra R Sahoo SK (2012) Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and Nanomedicine Nanotechnology Biology and Medicine 8: 147-166.
- Mehra NK, Mishra V, Jain NK (2014) A review of ligand tethered surface engineered carbon nanotubes. Biomaterials 35: 1267-1283.
- Kohler N, Sun C, Fichtenholtz A, Gunn J, Fang C, et al. (2006) Methotrexate-immobilized poly(ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small 2: 785-792.
- Kohler N, Sun C, Wang J, Zhang M (2005) Methotrexate-modified superparamagnetic nanoparticles and their intracellular uptake into human cancer Langmuir 21: 8858-8864.
- Hwu JR, Lin YS, Josephrajan T, Hsu MH, Cheng FY et al. (2009) Targeted paclitaxel by conjugation to iron oxide and gold nanoparticles. J Am Chem Soc 131: 66-68.
- Huh YM, Jun YW, Song HT, Kim S, Choi JS et (2005) In vivo magnetic resonance detection of cancer by using multifunctional magnetic nanocrystals. J Am Chem Soc 127: 12387-12391.
- Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, et al. (2007) Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med 13: 95-99.
- Jain TK, Richey J, Strand M, Leslie-Pelecky DL, Flask CA et (2008) Magnetic nanoparticles with dual functional properties: drug delivery and magnetic resonance imaging. Biomaterials 29: 4012-4021.
- Yu MK, Jeong YY, Park J, Park S, Kim JW, et al. (2008) Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo. Angew Chem Int Ed Engl 47: 5362-5365.
- Park J, Lee E, Hwang NM, Kang M, Kim SC et (2005) One-nanometer-scale size-controlled synthesis of monodisperse magnetic iron oxide nanoparticles. Angew Chem Int Ed 44: 2872-2877.
- Piao Y, Kim J, Bin Na H, Kim D, Baek JS et al. (2008) Wrap-bake-peel process for nanostructural transformation from bold β-FeOOH nanorods to biocompatible iron oxide nanocapsules. Nature Materials 7: 242-247.
- Kim J, Lee JE, Lee J, Yu JH, Kim BC et al. (2006) Magnetic fluorescent delivery vehicle using uniform mesoporous silica spheres embedded with monodisperse magnetic and semiconductor nanocrystals. J Am Chem Soc 128: 688-689.
- Cheng K, Peng S, Xu C, Sun S (2009) Porous hollow Fe3O4 nanoparticles for targeted delivery and controlled release of J Am Chem Soc 131: 10637-10644.
- Medarova Z, Pham W, Farrar C, Petkova V, Moore A (2007) In vivo imaging of siRNA delivery and silencing in tumors. Nat Med 13: 372-377.
- Namiki Y, Namiki T, Yoshida H, Ishii Y, Tsubota A et (2009) A novel mag- netic crystal-lipid nanostructure for magnetically guided in vivo gene deliv- ery. Nat Nanotechnol 4: 598-606.
- Smith AM, Duan H, Mohs AM, Nie S (2008) Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev 60: 1226-1240.\
- Nurunnabi M, Cho KJ, Choi JS, Huh KM, Lee YK (2010) Targeted near-IR QDs-loaded micelles for cancer therapy and Biomaterials 31: 5436- 5444.
- Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW et al. (2007) Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy Nano Lett 7: 3065-3070.
- Yuan J, Guo W, Yang X, Wang E (2009) Anticancerdrug-DNA interactions measured using aphoto induced electron-transfer mechanism based on lu- minescent quantum dots. Anal Chem 81: 362-368.
- Tsay JM, Trzoss M, Shi L, Kong X, Selke M et al. (2007) Singlet oxygen production by peptide-coated quantum dot-photosensitizer J Am Chem Soc 129: 6865-6871.
- Samia AC, Dayal S, Burda C (2006) Quantum dot-based energy transfer: perspectives and potential for applications in photodynamic Photo- chem Photobiol 82: 617-625.
- Cheng Y, C Samia A, Meyers JD, Panagopoulos I, Fei B, et (2008) Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J Am Chem Soc 130: 10643-10647.
- Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S (2009) Gold nanopar- ticles with a monolayer of doxorubicin-conjugated amphiphilic block copoly- mer for tumor-targeted drug delivery. Biomaterials 30: 6065-6075.
- Chen J, Saeki F, Wiley BJ, Cang H, Cobb MJ, et (2005) Gold nanocages: bio conjugation and their potential use as optical imaging contrast agents. Nano Let 5: 473-477.
- Chen J, Glaus C, Laforest R, Zhang Q, Yang M, et al. (2010) Gold nano- cages as photothermal transducers for cancer treatment. Small 6: 811-817.
- Lu W, Xiong C, Zhang G, Huang Q, Zhang R, et al. (2009) Targeted photo- thermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold Clin Cancer Res 15: 876-886.
- Lu W, Zhang G, Zhang R, Flores LG, Huang Q, et (2010) Tumor site-spe- cific silencing of NF-κB p65 by targeted hollow gold nanospheres-mediated photothermal transfection. Cancer Res 70: 3177-3188.
- Welsher K, Liu Z, Daranciang D, Dai H (2008) Selective probing and imag- ing of cells with single walled carbon nanotubes as near-infrared fluorescent Nano Lett 8: 586-590.
- Liu Z, Li X, Tabakman SM, Jiang K, Fan S, Dai H (2008) Multiplexed multicol- or Raman imaging of live cells with isotopically modified single walled carbon J Am Chem Soc 130: 13540-13541.
- Kam NW, Dai H (2005) Carbon nanotubes as intracellular protein transport- ers: Generality and biological J Am Chem Soc 127: 6021-6026.
- Shi Kam NW, Jessop TC, Wender PA, Dai H (2004) Nanotube molecular transporters: internalization of carbon nanotube-protein conjugates into Mammalian cells. J Am Chem Soc 126: 6850-6851.
- Bianco A, Kostarelos K, Partidos CD, Prato M (2005) Biomedical applica- tions of functionalised carbon nanotubes. Chem. Comm. 5: 571-577.
- Sayes CM, Liang F, Hudson JL, Mendez J, Guo W, et (2006) Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett 161: 135-142.
- Cherukuri P, Bachilo SM, Litovsky SH, Weisman RB (2004) Near-infrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic J Am Chem Soc 126: 15638-15639.
- Heller DA, Baik S, Eurell TE, Strano MS, et al. (2005) Single-walled carbon nanotube spectroscopy in live cells: Towards long-term labels and optical Adv Mater 17: 2793-2799.
- Pantarotto D, Briand JP, Prato M, Bianco A (2004) Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun 7: 16-17.
- Pantarotto D, Singh R, McCarthy D, Erhardt M, Briand JP, et (2004) Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew Chem Int Ed Engl 43: 5242-5246.
- Cai D, Mataraza JM, Qin ZH, Huang Z, Huang J, et (2005) Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat Methods 2: 449-454.
- Moon HK, Lee SH, Choi HC (2009). In vivo near-infrared mediated tumor destruction by photothermal effect of carbon ACS Nano 3: 3707- 3713.
- Samorì C, Ali-Boucetta H, Sainz R, Guo C, Toma FM et (2010) Enhanced anticancer activity of multi-walled carbon nanotube-methotrexate conjugates using cleavable linkers. Chem.Comm. 46: 1494-1496.
- Kostarelos K, Lacerda L, Pastorin G, Wu W, Wieckowski S et (2007) Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat Nanotech 2: 108-113.
- Kam NW, O’Connell M, Wisdom JA, Dai H (2005) Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA 102: 11600-11605.
- Ghosh S, Dutta S, Gomes E, Carroll D, D’Agostino R, et (2009) Increased heating efficiency and selective thermal ablation of malignant tissue with DNA-encased multiwalled carbon nanotubes. ACS Nano 3: 2667-2673.
- Sathe TR, Agrawal A, Nie S (2006) Mesoporous silica beads embedded with semiconductor quantum dots and iron oxide nanocrystals: dual-function microcarriers for optical encoding and magnetic separation. Anal. Chem. 78: 5627-5632.
- Koole R, van Schooneveld MM, Hilhorst J, Castermans K, Cormode DP et (2008) Paramagnetic lipid-coated silica nanoparticles with a fluorescent quantum dot core: a new contrast agent platform for multimodality imaging. Bioconjug Chem 19: 2471-2479.
- Roy I, Ohulchanskyy TY, Pudavar HE, Bergey EJ, Oseroff AR et al. (2003) Ceramic-based nanoparticles entrapping water-insoluble photosensitizing anticancer drugs: A novel drug-carrier system for photodynamic therapy. J Am Chem Soc 125: 7860-7865.
- Park JH, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN et al. (2009) Biodegradable luminescent porous silicon nanoparticles for in vivo applica Nat Mater 8: 331-336.
- Malam Y, Loizidou M, Seifalian AM (2009) Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci 30: 592-599.
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, et (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotech 2: 751-760.
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, et al. (2008) Nanoparticles in medicine: therapeutic applications and Clin Pharmacol Ther 83: 761-769.
- Martina MS, Fortin JP, Ménager C, Clément O, Barratt G, et al. (2005) Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J Am Chem Soc 127: 10676-10685.
- Wang YX, Hussain SM, Krestin GP (2001) Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR im Eur Radiol 11: 2319-2331.
- Kabalka GW, Davis MA, Moss TH, Buonocore E, Hubner K, et al. (1991) Gadolinium-labeled liposomes containing various amphiphilic Gd-DTPA derivatives: targeted MRI contrast enhancement agents for the liver. Magn Reson Med 19: 406-415.
- Viglianti BL, Abraham SA, Michelich CR, Yarmolenko PS, MacFall JR, et (2004) In vivo monitoring of tissue pharmacokinetics of liposome/drug using MRI: Illustration of targeted delivery. Magn Reson Med 51: 1153-1162.
- Erten A, Wrasidlo W, Scadeng M, Esener S, Hoffman RM, et (2010) Magnetic resonance and fluorescence imaging of doxorubicin-loaded nanoparticles using a novel in vivo model. Nanomedicine Nanotechnology Biology and Medicine 6: 797-807.
- Soundararajan A, Bao A, Phillips WT, Perez R, Goins BA (2009) [(186)Re] Liposomal doxorubicin (Doxil): in vitro stability, pharmacokinetics, imaging and biodistribution in a head and neck squamous cell carcinoma xenograft Nucl Med Biol 36: 515-524.
- Fang RH, Zhang L (2011) Dispersion-based methods for the engineering and manufacture of polymeric nanoparticles for drug delivery J Nanoeng Nanomanuf 1: 106-112.
- Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, et al. (2006) Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery sys Nano Let 6:2427-2430.
- Guthi JS, Yang SG, Huang G, Li S, Khemtong C, et al. (2010) MRI-visible micellar nanomedicine for targeted drug delivery to lung cancer cells. Mol Pharm 7: 32-40.
- Lai JR, Chang YW, Yen HC, Yuan NY, Liao MY et al. (2010) Multifunctional doxorubicin/superparamagnetic iron oxide-encapsulated Pluronic F127 micelles used for chemotherapy/magnetic resonance imaging. J Appl Phys 107:09B318.
- Lu ZR (2010) Molecular imaging of HPMA copolymers: Visualizing drug delivery in cell, mouse and man. Adv Drug Deliv Rev 62: 246-257.
- Kobayashi H, Ogawa M, Alford R, Choyke PL, Urano Y (2010) New strategies for fluorescent probe design in medical diagnostic imaging. Chem Rev 110: 2620-2640.
- Peng CL, Shih YH, Lee PC, Hsieh TM, Luo TY, et al. (2011) Multimodal image-guided photothermal therapy mediated by 188Re-labeled micelles containing a cyanine-type photosensitizer. ACS Nano 5: 5594-5607.
- Zhang L, Gao S, Zhang F, Yang K, Ma Q, et al. (2014) Activatable hyaluronic acid nanoparticle as a theranostic agent for optical/photoacoustic image-guided photothermal therapy. ACS Nano 8: 12250-12258.
- Cheng L, Gong H, Zhu W, Liu J, Wang X, et al. (2014) PEGylated Prussian blue nanocubes as a theranostic agent for simultaneous cancer imaging and photothermal therapy. Biomaterials 35: 9844-9852.
- Kang C, Cho W, Park M, Kim J, Park S, et al. (2016) H2O2-triggered bubble generating antioxidant polymeric nanoparticles as ischemia/reperfusion targeted nanotheranostics. Biomaterials 85: 195-203.
- Min HS, You DG, Son S, Jeon S, Park JH, et al. (2015) Echogenic glycol chitosan nanoparticles for ultrasound-triggered cancer theranostics. Theranostics 5: 1402-1418.
- Huang D, Zhou H, Gao J (2015) Nanoparticles modulate autophagic effect in a dispersity-dependent manner. Sci Rep 5: 14361.
- Peynshaert K, Manshian BB, Joris F, Braeckmans K, De Smedt SC et (2014) Exploiting intrinsic nanoparticle toxicity: The pros and cons of nanoparticle-induced autophagy in biomedical research. Chem Rev 114: 7581-7609.
- https://www.nobelprize.org/nobel_prizes/medicine/laureates/2016/press. html
- Mao BH, Tsai JC, Chen CW, Yan SJ, Wang YJ (2016) Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy Nanotoxicology 10: 1021-1040.
- Song B, Zhang Y, Liu J, Feng X, Zhou T, et al. (2016) Unraveling the neurotoxicity of titanium dioxide nanoparticles: focusing on molecular mecha Beilstein J Nanotech 7: 645-654.
- Huang D, Zhou H, Liu H, Gao J (2015) The cytotoxicity of gold nanoparticles is dispersity-dependent. Dalton Trans 44: 17911-17915.
Citation: Sawant PD (2016) Nano-Theranostics for Cancer Management. J Nanosci Nanomed Nanobio 1: 001.
Copyright: © 2016 Sawant PD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.


