*Corresponding Author:
Sha Zhang,
Division of Research and Development, Mellitas Health Foods, LLC, Ithaca 14850, New York, USA
Tel: +1 8008618916
E-mail: shaz@mellitasfoods.com
Abstract
This study investigates the ability of a proprietary carbohydrate blocker (Max Bloc bean protein) extracted from Phaseolus Vulgaris to inhibit pancreatic α-amylase. Results show that Max Bloc bean protein can inhibit >96% of the pancreatic α-amylase activity (1.0 μL/ mL). The proteinaceous inhibitor gradually binds with amylase and decreases its starch digestion activity. It has also been found that Max Bloc bean protein has 10-16 times higher α-amylase inhibitory activity compared to three other brands on market. Max Bloc bean protein contains no residues of toxic hemagglutinins (0 HAU/g), while significant amount of hemagglutinins (256,000-640,000 HAU/g) can be found in the other three brands. In addition, Max Bloc bean protein can survive the gastric digestion without losing any α-amylase inhibitory activity. The active binding sites of Max Bloc bean protein are not affected in presence of various food grade polysaccharides. The bean protein also shows excellent stability when stored at room temperature. More importantly, the in vitro study shows that Max Bloc bean protein can significantly reduce the digestion of starchy food by 69.2% - 93.3%, including white bread, rice and pasta. These results suggested that the highly bioactive Max Bloc bean protein can be used as a functional or medicinal food for glucose control and weight management.
Keywords
Amylase inhibition; Carb blocker; Glucose control; Max Bloc bean protein; Ultrahigh activity
Introduction
In 1975, Marshall and Lauda discovered and reported a protein from white kidney beans (Phaseolus Vulgaris) that acted as an α-amylase inhibitor, naming it “Phaseolamin” [1]. This inhibitor has been shown to non-competitively inhibit the activity of mammalian salivary and pancreatic α-amylase, preventing them from acting on the α-1,4-glycosidic bonds of starches and other polysaccharides [2-6]. This activity has led to the marketing of supplements containing the inhibitor as a mean to reduce the caloric absorption from and post- prandial glycemic response to the consumption of starchy foods, and are often marketed as “carb blockers”, “carb controllers”, or “starch blockers” [7-10].
In humans and other mammals, the digestion of starch is a complex process, starting in the mouth and continuing in the small intestines [11-13]. The majority of starch digestion happens in the small intestines, where pancreatic α-amylase acts on the starch chain, randomly breaking it into smaller molecules, each containing two or more glucose units. The oligosaccharides are further digested by other enzymes, primarily α-glucosidase until the end result is single glucose units, which are absorbed through the intestinal wall and into the bloodstream [14]. Inhibition of α-amylase by compounds such as phaseolamin causes the enzyme to lose its catalytic activity, preventing the hydrolysis of the starch molecule [3,15]. This, in turn, results in the starch being undigested and passed into the large intestines without releasing glucose (Figure 1), which greatly decreases the number of calories provided by starchy foods [10,16]. The undigested starch also acts like soluble dietary fibers and/or prebiotics in large intestines, either bulking the stool or encouraging the growth and activity of beneficial gut microbiota [17-19]. These phenomena highlight the potential for the amylase-inhibiting bioactive white kidney bean protein to be used as an aid for human health, promoting weight loss, improving glucose control, and assisting with the management of type 2 diabetes. During the supplement craze of the 1980s, commercial white kidney bean extracts were first introduced to the market, and over the past 30 years, additional researches have been performed attempting to better understand the amylase inhibition of these natural products [10,19,16]. Despite promising potential, the early commercially available products have shown little to no effect on human health, primarily due to the low purity and resulting low α-amylase inhibitory activity of the products [20].
In 1985, a group of researchers at Mayo Clinic reported a partial purification procedure for white beans that was able to enrich amylase inhibitory protein content 6-8 fold [16]. This partially purified inhibitor was later shown to inactivate more than 90% of intra-duo-denal, intra-ileal, and salivary amylase in vitro [19]. Subsequent human studies were able to confirm that consumption of 2.9 to 5.0 g of white bean amylase inhibitor significantly reduced the postprandial glucose peak and improved carbohydrate tolerance in both normal and diabetic subjects [10,18,17]. Unfortunately, unpleasant side effects from the studies, such as nausea and vomiting, were also seen, most likely due to the presence of high level of toxic hemagglutinins in the enriched extract [18,17]. Unless denatured, hemagglutinins, also called phytohemagglutinins or bean lectins, can bind with the villi of the small intestine, causing serious damage to the intestinal walls, and this phenomenon has been linked to multiple incidents of foodborne illness associated with undercooked kidney beans that have been reported in Canada, Australia, and in the United Kingdom [21]. While it is trivially simple to deactivate the hemagglutinins in kidney beans by denaturation, requiring exposure to boiling temperature for an hour or less, such a treatment would also completely deactivate the amylase inhibitor in the beans [22]. Thus, while the early clinical trials of partially purified white bean inhibitors showed great promise as a natural medicine to control blood glucose and block starch digestion, the products’ high level of hemagglutinins greatly restricted their use and thus prevented any commercialization of the technology.
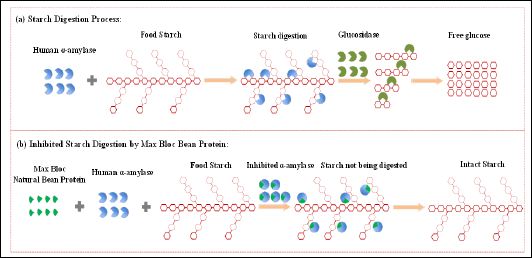
Figure 1: Scheme of starch digestion: (a), the normal process of starch digestion; (b), the process of starch digestion when α-amylase was inhibited by Max Bloc bean protein.
In 2013, the authors began researching and investigating various techniques to selectively deactivate the hemagglutinins in kidney beans while keeping the α-amylase inhibitory activity of the bean protein intact. A proprietary process was developed that allows the production of a purified protein with ultra-high α-amylase inhibitory activity and fully denatured hemagglutinin, and the product is currently available on the market under the trade name “Max Bloc bean protein”. This study aims to characterize the inhibitory properties of Max Bloc bean protein against pancreatic α-amylase and compare its efficiency with three other commercial α-amylase inhibitor products made from white kidney beans, currently available in the United States and/or China, and to demonstrate the efficacy of Max Bloc bean protein at reducing the digestibility of common starchy foods, including rice, pasta, and white bread.
Materials and Methods
Materials and reagents
Sodium hydroxide, sodium carbonate, sodium phosphate dibasic, sodium phosphate monobasic, and hydrochloric acid were purchased from a scientific supply house (Innovating Science, Aldon Corp, Avon NY). Porcine pancreatic α-amylase (A6255), pepsin (P7000), and 2-chloro-4-nitrophenyl-α-D-maltotrioside (CNPG3) were purchased from Sigma-Aldrich (St Louis, MO, USA) and used without further purification. Food-grade guar gum, xanthan gum, carrageenan, and acacia gum were from TIC gum Inc. (White Marsh, MD, USA). Distilled water, rice, pasta, and bread were purchased from a local super-market.
Production of Max Bloc bean protein with ultra-high inhibitory activity
USDA Grade No. 1 dry beans (Phaseolus vulgaris) were soaked in purified water for 8 hours (Figure 2). The resultant softened beans were then milled into a slurry using a high shear disintegrator (Bredo Likwifier, Ensight Solutions, Strafford, MO 65757). Crude protein extraction was performed at room temperature by continuously stirring the bean slurry for 1 hour to allow soluble proteins to hydrate and enter the aqueous phase of the slurry. The slurry was then sent to a centrifugal decanter to remove insoluble matter, and the clear stream from decanter, i.e. primary protein solution, was subjected to a proprietary secondary protein extraction process designed to increase the bioactivity of protein. The enhanced protein solution was then purified and treated with a second proprietary process in order to fully deactivate the hemagglutinins at low temperatures. The resultant inhibitory protein solution was then pasteurized using the high-temperature short time (162 ºF/67 ºC, for 18 s) and spray dried into protein powders
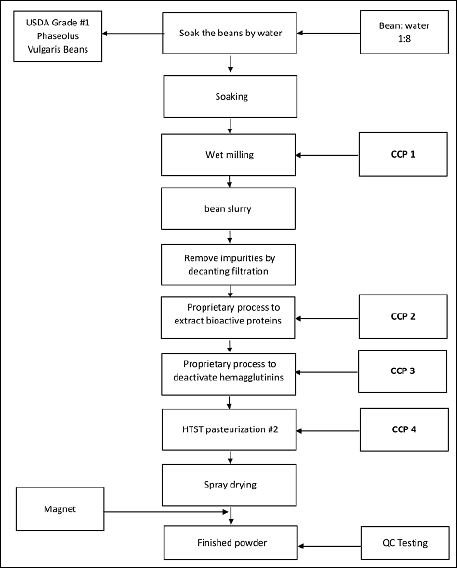
Figure 2: The process to isolate highly active amylase inhibitor protein from dried beans (Phaseolus vulgaris).
Measurement of the α-amylase inhibitory activity of bean protein
Dry Max Bloc bean protein was hydrated in 100 mM of phosphate buffer (pH 6.8) at concentrations from 0.2% to 1.0% (m/v). Aliquots (0.5 mL) of each protein solution were mixed with an equal volume of α-amylase solutions (0.1 – 1.0 μL/mL). The resulting mixtures were incubated at 37 ºC for 10 mins to allow for binding between the bean protein and α-amylase. Then 0.5 mL of a model substrate solution, comprised of 20 mM of CNPG3 in phosphate buffer, was added to each mixture and incubated at 37 ºC for an additional 5 min. The reaction was then halted by the addition of 2.0 mL of a 10% sodium carbonate solution. A UV-visible spectrophotometer (Laxco Alpha-1000) was then used to measure the absorption at 410 nm in order to determine the degree of hydrolyzation of the CNPG3, which releases a yellow moiety upon hydrolysis [23]. Control samples consisting of phosphate buffer (without bean protein) were also prepared and used to determine the original α-amylase activity. The inhibitory activity of bean protein was calculated according to previous reports [24,25] and expressed as the α-amylase inhibitory activity per gram of bean protein (AIU/g).
Measurement of preincubation time on the α-amylase inhibitory activity
To measure the effect of preincubation time on an α-amylase inhibitory activity, solutions of Max Bloc bean protein in phosphate buffer at 0.2% concentration and α-amylase solution at 0.5 μL/mL were used. As before, equal volumes of each were mixed and incubated at 37 ºC, this time for 0, 1.0, 5.0, 7.5, 10, 20, 30, and 60 min to allow the binding of inhibitor protein to α-amylase. These preincubated solutions were then mixed with the 0.5 mL of CNPG3 and assayed as stated above to determine what effect increasing or decreasing the preincubation time would have on the inhibitory activity of protein.
Comparison of α-amylase inhibitory activity between Max Bloc bean protein and other commercial α-amylase inhibitor products
Three different commercially available white kidney bean extract powders were obtained directly from their respective companies, during various tradeshows. To compare activity, each of the powdered samples, including Max Bloc bean protein, was dissolved in phosphate buffer at a concentration of 0.2% (m/v). Then the activities of the solutions were measured as above, using α-amylase solutions with a concentration of 0.5 μL/mL, and incubating for 10 min prior to adding CNPG3. As before, the reaction of amylase and GNPG3 was stopped after 5 min by adding 10% of sodium carbonate solution, and amylase inhibitory activity is expressed as AIU/g.
Effect of model gastric digestion time on the α-amylase inhibitory activity of Max Bloc bean protein in powder form and in gelatin capsules
An in vitro digestion system model was used to determine what effect exposure to gastric juice may have on the activity of the α-amylase inhibitor. Gastric juice was simulated using a solution of hydrochloric acid and pepsin (3.2 mg/g) with pH adjusted to 1.2. Max Bloc bean protein powder was added to beakers containing the model gastric juice (pepsin to protein weight ratio of 1:2) under continuous stirring and held at 37 oC from 0 to 60 min, with an intermittent sampling of the fluid. Immediately upon sampling, the pH of each sample aliquot was adjusted to 7.0 using sodium carbonate in order to inactivate pepsin and the samples were then diluted with phosphate buffer to ensure the Max Bloc bean protein concentration was 0.2% and the α-amylase inhibitor activity was determined using the method described in section 2.3. To better simulate the use of Max Bloc bean protein in nutritional supplements, the powdered sample was also filled into size #00 gelatin capsules (PurecapsUSA) at 500 mg per capsule. These capsules were then placed into the model digestive system and samples were collected as for the powdered protein suspension.
Interaction of Max Bloc bean protein with complex polysaccharides and the storage stability of Max Bloc bean protein powder
Guar gum, xanthan gum, carrageenan, and acacia gum, were thoroughly dissolved in distilled water at a concentration of 10 g gum/ kg water. Max Bloc bean protein was also dissolved in distilled water at a concentration of 20 g protein/ kg water, and then aliquots of each polysaccharide solution were added to aliquots of the protein solution to obtain a polysaccharide to protein ratio of 1:10. After 30 min incubation at room temperature with stirring to allow for interaction between the protein and polysaccharides, the mixture was diluted to produce a protein concentration of 0.2%, and aliquots of α-amylase (0.5 μL/mL) were used to test inhibitory activity according to the procedure above. In addition, samples of dried Max Bloc bean protein powder were stored at ambient temperature and tested for inhibitory activity every month for 6 months to determine the storage stability of the dried bean protein product.
In vitro starch digestion study
Rice, pasta, and bread, three widely consumed starch-rich foods, were selected for the in vitro starch digestion study. Rice was cooked using an automatic rice cooker (Arc 150, AROMA), using rice to water ratio of 1:2 by volume. Cook time was controlled by the appliance but was approximately 30 min. The pasta was cooked in an excess of boiling water for 10 mins before draining. White bread (D’Italinao Italian bread) was purchased from a local market the day prior to sampling. Samples (50 g) of each starch food (rice, pasta, and bread) were individually mixed with 150 g phosphate buffer (pH 6.8) and blended into a paste using a high-shear juice blender (Oster Classic Series Blender), before placing the samples into 37 ºC continuously stirred water bath. In the control samples, α-amylase solution (1.0 μL/mL) was added to the pastes in order to initiate starch digestion, while in the experimental samples, the α-amylase solution was preincubated with Max Bloc bean protein (1.0%) for 15 min prior to addition to the pasted starchy food. Samples (2.0 mL) of each mixture were taken at 10 min intervals for one hour, and each was immediately centrifuged at 4000 rpm for 5 min (Heraeus Multifuge 3S+) to separate the starch and other insoluble materials from the clear liquid phase which contained the soluble sugars resulting from starch digestion. These solid contents of the clear liquids were then measured by a refractometer (Clinical Refractometer Veterinary RHC-200ATC) and used to indicate the speed of starch digestion.
Results and Discussion
Rationale of the extraction process
It is a relatively straightforward process to extract and enrich proteinaceous compounds, including inhibitors from dried kidney beans. However, toxic bean hemagglutinins are also proteinaceous, and will also likely be co-extracted and enriched, resulting in a potentially dangerous product. These toxic hemagglutinins must be deactivated for safe human consumption, and while the traditional process is quite capable of doing so, it relies upon severe heat treatment to partially deactivate hemagglutinins, which also denatures amylase inhibitor proteins, creating an extract with very low amylase inhibitory activity. The proprietary process used to produce Max Bloc bean protein is able to completely and selectively deactivate the toxic hemagglutinins from kidney beans while keeping the amylase inhibitory activity.
Effect of preincubation time on amylase inhibitory activity of Max Bloc bean protein
In order to utilize Max Bloc bean protein as a means of glucose control and weight management, it is essential to understand how quickly the inhibitors in the product can interact with α-amylase and inhibit the activity of the enzyme. Prior to this work, the binding speed for bean amylase inhibitors had not been thoroughly investigated in previous publications. The results of the binding study are presented in Figure. 3, which clearly shows that the binding of pancreatic α-amylase with Max Bloc bean protein does not occur instantaneously, but rather is a gradual process. The inhibition of enzymatic activity increases logarithmically as the preincubation time increases. At 5 min, Max Bloc bean protein can inhibit ~38% of the α-amylase activity. Max Bloc bean protein can inhibit ~63% and ~82% of the amylase activity if preincubated with the amylase for 10 min and 20 min. Further extending the incubation time to 30-60 min enhances the inhibitory activities to >90%. This suggests that if Max Bloc is being used as a dietary supplement for glucose control or weight management, the product would need to be taken 10-20 mins prior to a meal in order to maximize its efficiency, lest the proteinaceous inhibitor not have enough time to bind and inhibit significant amounts of α-amylase.
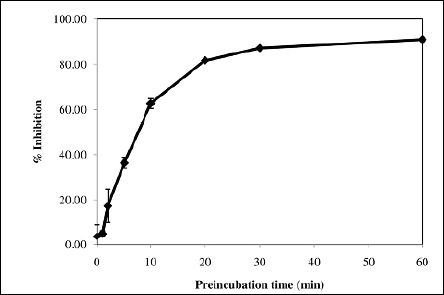
Figure 3: Effect of the preincubation time of Max Bloc bean protein and α-amylase on the inhibitory efficiency.
The effect of different concentrations of Max Bloc bean protein on inhibition is shown in Figure. 4. At a concentration of 0.2%, enzymatic activity can be reduced by 76% when the concentration of α-amylase is 0.5 μL/mL, but if the concentration of α-amylase is increased to 1.0 μL/mL, the inhibition drops to 30%. However, at a concentration of 1.0%, Max Bloc bean protein can inhibit >96% of amylase activity for all of the amylase concentrations tested in this study (0.1 - 1.0 μL/ mL), which is a promising finding, give that blocking more than 90% of amylase activity is thought to be necessary to effectively suppress starch digestion in small intestines [10, 16,19].
Comparison of amylase inhibitory activity of Max Bloc bean protein with other commercially available products
Current commercial amylase inhibitor products from white kidney beans are usually marketed as white kidney bean extract due to their low purities. Individual suppliers have their own internal testing procedures to determine the amylase inhibitory activity, which makes it extremely difficult to directly compare inhibitory activity between products from different suppliers based on the specifications they provide. In this study, the three commercial white kidney bean extracts from the United States and China and the Max Bloc product were all compared using the same testing procedure, with results shown in figure 5. Of particular note is that the inhibitory activity of Max Bloc bean protein is 10-16 times higher than other brands, indicating that gram for gram, Max Bloc bean protein has 10 -16 times more starch-blocking power than the other three brands. And because the majority of white kidney bean products found in the U.S. market rely on extracts produced in these countries, it is likely that similar results would be seen in virtually all commercially available products [26]. Moreover, as shown in table 1, the levels of toxic hemagglutinins in the other brands’ hemagglutinins (256,000-640,000 HAU/g) are much higher compared with Max Bloc, which contains 0 HAU/g of toxic hemagglutinins.
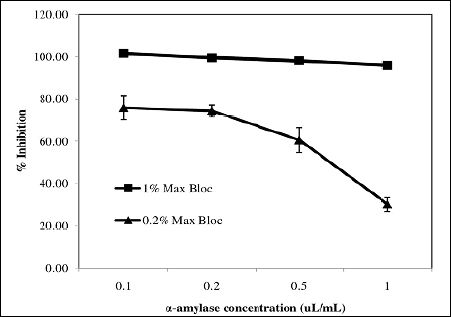
Figure 4: Effect of concentrations of α-amylase and Max Bloc bean protein on the inhibitory efficiency.
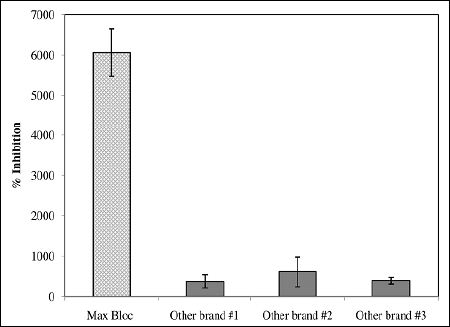
Figure 5: Comparison of α-amylase inhibitory activity of Max Bloc bean protein with other commercial products.
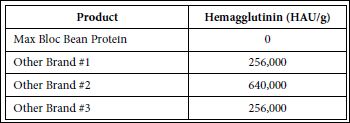
Table 1: Comparison of hemagglutinin in Max Bloc bean protein with other brands.
Effect of gastric digestion on the inhibitory activity of Max Bloc bean protein
Being the first major digestive organ, the stomach can be an extremely damaging environment for many healthful food/ drug components. Gastric juice contains proteolytic pepsin and a large amount of hydrochloric acid in order to begin breaking down food for digestion [27]. Determining whether a potential supplement or treatment can survive gastric digestion is critical because if it cannot, protective measures such as encapsulation will need to be used, potentially increasing the overall cost of the product. As seen in figure 6, the inhibitory activity of Max Bloc bean protein fares well, even after being subjected to gastric digestion at 37 ºC for as long as 60 min. This strongly suggests that Max Bloc bean protein can survive the environment of the stomach during digestion and reach small intestines with the necessary activity to inhibit pancreatic α-amylase. The data also show that the use of gelatin capsules likely causes a gradual release of the inhibitor due to the time required to dissolve the gelatin capsules. This delays the amylase inhibitory activity early on in the model digestion, with activity following a logarithmic progression, plateauing at 30 min. This suggests that if Max Bloc is to be sold in capsule form, dosage instructions would need to take in to account both the binding time necessary for inhibition, plus the amount of time needed for sufficient amounts of the protein to be released from the gelatin capsule.
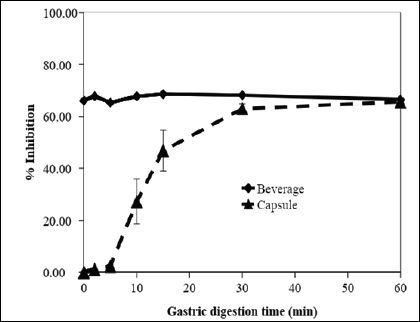
Figure 6: Effect of model gastric digestion time on α-amylase inhibitor activity of Max Bloc bean protein as a powdered beverage mix or as a dietary supplement in gelatin capsules.
Interaction of Max Bloc bean protein with polysaccharides and the storage stability of Max Bloc bean protein powder
It is well known that proteins can form complexes with polysaccharides, such as guar gum and xanthan gum [28-30]. These complexes have the potential to block or limit critical binding sites and/ or prevent conformational changes necessary for optimal activity. As polysaccharides are a common food ingredient, it was important to determine whether an interaction might occur between them and the inhibitory proteins in Max Bloc. As shown in Figure. 7a, among the four polysaccharides, guar gum, xanthan gum, carrageenan, and acacia gum, no influence on amylase inhibitory activity of Max Bloc bean protein was observed. Not only does this suggest there is no need to avoid certain foods when using the product, but it also could allow the formulation of different formulations of the product such as gummies or gels using polysaccharides without sacrificing potential activity.
Finally, it is important to determine the practical shelf-life of health supplements and treatments, and as shown in figure 7b, the inhibitory activity of Max Bloc follows a flat linear trend, showing no indication of activity loss up to 6 months of room temperature storage. This lack of downward trend suggests the actual shelf life is likely much longer, and thus with proper packaging, it is not unrealistic to assume that Max Bloc bean protein could be stored at room temperature for years.
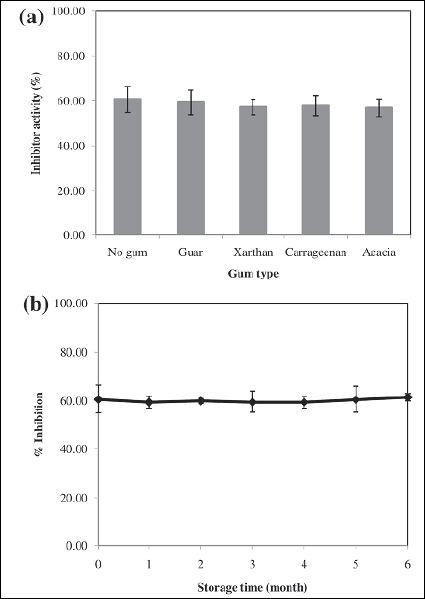
Figure 7: Effect of addition of different types of polysaccharides (a) and storage time (b) on the α-amylase inhibitory activity of Max Bloc bean protein.
Inhibition of starch digestion in vitro
Starch-rich foods, rice, pasta, and bread, were selected and used for the in vitro digestion study and figure 8 shows the digestion curve of the cooked samples. In figure 8a, it is clear that sugar content is liberated in the control sample, which contained only rice and α-amylase, the soluble sugar content gradually increased from 3.0% to 6.9% during the digestion period (0-60 min). Surprisingly the sample which had been treated with one of the commercial inhibitor products also saw an increase of 3.0% to 6.9% during the same digestion period, suggesting the commercial inhibitor had no inhibitory activity in the model system. In contrast, the Max Bloc sample showed a much lower increase (3.0% to 4.2%). Similar or better trends were seen in the cooked pasta (Figure 8b) and white bread samples (Figure 8c), with Max Bloc bean protein blocking 75.9% and 93.3% of starch digestion in cooked pasta and white bread, respectively. These data strongly suggest that were this to be in a living human, roughly 69-93 % of the carbohydrates from theses starchy foods would not be absorbed by the small intestines, resulting in a significant calorie reduction and a profound decrease in the postprandial glucose spike.
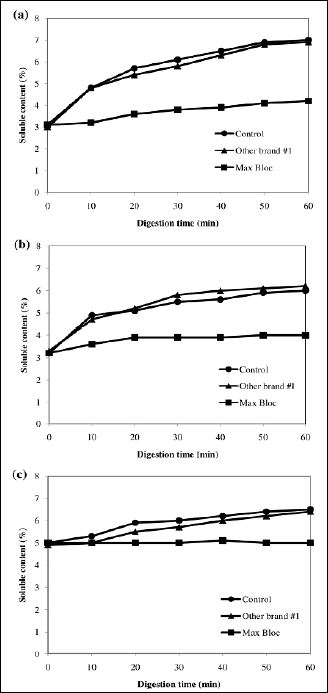
Figure 8: In vitro digestion of various food starch: (a) cooked rice; (b) cooked pasta; (c) white bread by α-amylase with and without Max Bloc bean protein.
Conclusions
Max Bloc bean protein is a proprietary bean extract with zero hemagglutinins and 10-16 times the amylase inhibitory activity compared to the current commercial products. In vitro modeling indicates that the product can readily survive a gastric environment without protective encapsulation or use of capsules, and reduce the amount of starch digested by 69.2% to 93.3% in common starchy foods (rice, pasta, and white bread). This strongly suggests that the highly bioactive protein has significant potential as either a dietary supplement or a medicinal food for glucose control and weight management. Max Bloc also helps boost the fiber intake by transforming the undigested starch into dietary fiber. Further validation of the effects in vivo will, of course, need to be done, and are forthcoming.
References
- Marshall JJ, Lauda CM (1975) Purification and properties of phaseolamin, an inhibitor of alpha-amylase, from the kidney bean, Phaseolus vulgaris. J Biol Chem 250: 8030-8037.
- Bo-Linn GW, Ana CAS, Morawski SG, Fordtran JS (1982) Starch blockers-their effect on calorie absorption from a high-starch meal. N Engl J Med 307: 1413-1416.
- Sales PM, Souza PM, Simeoni LA, Magalhães PO, Silveira D (2012) α-Amylase inhibitors: a review of raw material and isolated compounds from plant source. J Pharm Pharm Sci 15: 141-183.
- Frels JM, Rupnow JH (1985) Characterization of two α-amylase inhibitors from black bean (Phaseolus vulgaris). Journal of Food Science 50: 72-77.
- Lebenthal E (1987) Role of salivary amylase in gastric and intestinal digestion of starch. Digestive diseases and sciences 32: 1155-1157.
- Gibbs BF, Alli I (1998) Characterization of a purified α-amylase inhibitor from white kidney beans (Phaseolus vulgaris). Food Research International 31: 217-225.
- McCarty MF (2005) Nutraceutical resources for diabetes prevention-an up- Med Hypotheses 64: 151-158.
- Wolever TM, Jenkins D, Vuksan V, Jenkins A, Buckley G, et al. (1992) Beneficial effect of a low glycaemic index diet in type 2 diabetes. Diabet Med 9: 451-458.
- Preuss HG (2009) Bean amylase inhibitor and other carbohydrate absorption blockers: effects on diabesity and general J Am Coll Nutr 28: 266-276.
- Layer P, Rizza Ra, Zinsmeister Ar, Carlson GL, Dimagno EP (1986) Effect of a purified amylase inhibitor on carbohydrate tolerance in normal subjects and patients with diabetes mellitus. Mayo Clin Proc 442-447.
- Butterworth PJ, Warren FJ, Ellis PR (2011) Human α-amylase and starch digestion: An interesting marriage. Starch-Stärke 63: 395-405.
- Björck I, Granfeldt Y, Liljeberg H, Tovar J, Asp NG (1994) Food properties affecting the digestion and absorption of carbohydrates. Am J Clin Nut 59: 699-705.
- Levin RJ (1994) Digestion and absorption of carbohydrates-from molecules and membranes to The American journal of clinical nutrition 59: 690- 698.
- Dhital S, Lin AH-M, Hamaker BR, Gidley MJ, Muniandy A (2013) Mammalian mucosal α-glucosidases coordinate with α-amylase in the initial starch hydrolysis stage to have a role in starch digestion beyond glucogenesis. PLoS One 8: 62546.
- Lajolo FM, Genovese MI (2002) Nutritional significance of lectins and enzyme inhibitors from legumes. J Agric Food Chem 50: 6592-6598.
- Layer P, Carlson GL, Dimagno EP (1985) Partially purified white bean amylase inhibitor reduces starch digestion in vitro and inactivates intraduodenal amylase in humans. Gastroenterology 88: 1895-1902.
- Boivin M, Flourie B, Rizza RA, Go VLW, DiMagno EP (1988) Gastrointestinal and metabolic effects of amylase inhibition in Gastroenterology 94: 387-394.
- Boivin M, Zinsmeister Ar, Go Vl, Dimagno EP (1987) Effect of a purified amylase inhibitor on carbohydrate metabolism after a mixed meal in healthy hu In: Mayo Clinic Proceedings 4: 249-255.
- Layer P, Zinsmeister AR, DiMagno EP (1986) Effects of decreasing intraluminal amylase activity on starch digestion and postprandial gastrointestinal function in humans. Gastroenterology 91: 41-48.
- Carlson GL, Li B, Bass P, Olsen WA (1983) A bean alpha-amylase inhibitor formulation (starch blocker) is ineffective in man. Science 219: 393-395.
- Nciri N, Cho N (2018) New research highlights: Impact of chronic ingestion of white kidney beans (Phaseolus vulgaris L. var. Beldia) on small-intestinal disaccharidase activity in Wistar rats. Toxicology reports 5: 46-55.
- Thompson LU, Rea RL, Jenkins DJ (1983) Effect of heat processing on hemagglutinin activity in red kidney Journal of Food Science 48: 235-236.
- Tysoe C, Williams LK, Keyzers R, Nguyen NT, Tarling C, et al. (2016) Potent human α-amylase inhibition by the β-defensin-like protein ACS central science 2: 154-161.
- Shi L, Mu K, Arntfield SD, Nickerson MT (2017) Changes in levels of enzyme inhibitors during soaking and cooking for pulses available in Journal of food science and technology 54: 1014-1022.
- Iguti AM, Lajolo FM (1991) Occurrence and purification of alpha-amylase iso inhibitors in bean (Phaseolus vulgaris L.) Journal of agricultural and food chemistry 39: 2131-2136.
- https://daxueconsulting.com/china-vds-market-vitamin-and-dietary-supplements/. Accessed Oct. 2019
- Hur SJ, Lim BO, Decker EA, McClements DJ (2011) In vitro human digestion models for food applications. Food Chemistry 125: 1-12.
- Laneuville S, Paquin P, Turgeon S (2000) Effect of preparation conditions on the characteristics of whey protein-xanthan gum Food Hydrocolloids 14: 305-314.
- Zhang S, Hsieh F-H, Vardhanabhuti B (2014) Acid-induced gelation properties of heated whey protein-pectin soluble complex (Part I): Effect of initial Food Hydrocolloids 36: 76-84.
- Zhang S, Vardhanabhuti B (2014) Intragastric gelation of whey protein–pectin alters the digestibility of whey protein during in vitro pepsin Food & Function 5: 102-110.
Citation:Zhang S, Cavender GA, Allen CJ (2020) Max Bloc® Carb Blocker from Phaseolus Vulgaris with Ultra-high α-Amylase Inhibitory Activity for Glycemic Control and Weight Management. J Nutr Food Sci 3: 011.
Copyright: © 2020 Zhang S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


