*Corresponding Author:
Thana’ Y Aljaraedah,
Department of Nutrition and Food Technology, Human and Dietetics, Faculty of Agriculture, The University of Jordan, Jordan
E-mail: thanaana67@gmail.com
Abstract
Pregnancy is one of the most critical periods for mother and child. It involves a tremendous flow of physiological changes and metabolic adaptations week by week, and even small deviations from the norm might have detrimental consequences at different pregnancy stages. Third trimester human pregnancy is characterized by a 2-3-fold increase in plasma triglyceride and lesser increases in cholesterol and phospholipid. Maternal hypertriglyceridemia at late gestation results from the juxtaposition of several factors. Enhanced adipose tissue lipolysis facilitating the availability to the liver substrates for triglyceride synthesis and contributing to augmented flux of Very Low Density Lipoproteins (VLDL) into the circulation. There is a commonality among mammals in the adaptive responses during lactation and in their general regulation. Acylated fatty acids can be metabolized by the enzymes of the β-oxidation pathway or incorporated into TAG for secretion into milk. In the mammary gland the mRNA for most enzymes of the β-oxidation pathway falls two- to threefold at secretory activation directing fatty acids to TAG synthesis. Lipogenesis stops when lactation starts. Then, mammary adipocytes Trans-differentiate into secretory epithelial cells to promote lipid transfer during milk production. Lipids are major constituents of the milk of most mammals and a singularly important source of the calories required for neonatal growth and signaling molecules that promote postnatal development.
Keywords
Breast feeding; Fatty acids; Lactation; Lipids; Metabolism; Pregnancy
Abbrevations
AFPs Alpha-Fetoproteins
ARA Arachidonic
BMI Body Mass Index
DHA Docosahexaenoic Acid
FA Fatty Acid
FATPs Fatty Acid Transport Proteins
FFA Free Fatty Acids
GWG Gestational Weight Gain
IR Insulin Resistance
IL-6 Interleukin-6
LCPUFA Long Chain Polyunsaturated Fatty Acids
MVM Microvillous Plasma Membrane
NAFLD Nonalcoholic Fatty Liver Disease
PL Phospholipid
PM Plasma Membrane
SFAs Saturated Fatty Acids
subQ Subcutaneous
TAG Triacylglycerides
uMSC Umbilical Mesenchymal Stem Cell
VLDL Very Low Density Lipoproteins
Introduction
Pregnancy is one of the most critical periods for mother and child. It involves a tremendous flow of physiological changes and metabolic adaptations week by week, and even small deviations from the norm might have detrimental consequences at different pregnancy stages [1]. Third trimester human pregnancy is characterized by a 2-3-fold increase in plasma triglyceride and lesser increases in cholesterol and phospholipid [2]. Similar but less dramatic changes occur with administration of estrogens alone or in combination with a progestin as an oral contraceptive [3]. These changes have a pathological extreme in massive hypertriglyceridemia and pancreatitis in both pregnancy and oral contraceptive therapy [1].
Breast is consists of epithelial ducts associated with adipose lobules and the adipose tissue is the major contributor to the volume of the breast, and it plays a crucial role in the morphogenesis of mammary glands. Within the breast, special female-specific adipocytes were described and called “pink adipocytes.” Throughout pregnancy, the size of mammary adipocytes increases to store lipids [4]. The processes responsible for secreting lipids are particularly unique to mammary secretory cells and represent original adaptations of apocrine secretion. All eukaryotic cells rest on on Phospholipid (PL) and Fatty Acid (FA) transport to sustain membrane structure and organization, and to fuel and regulate cellular functions. The abundant milk secretion, including large quantities of lipid in some species, requires adaptation and integration of PL and FA synthesis and transport processes to encounter secretion demands [5]. Lipids are main constituents of the milk of most mammals and a exceptionally important source of the calories required for neonatal growth and signaling molecules that promote postnatal development The principal lipid constituents of milk are neutral lipids, which include fatty acid esters of glycerol (diacyland triacylglycerol esters) and cholesterol (cholesteryl esters), and polar phospholipids that contain glycerophospholipids and sphingolipids [5]. Triglycerides, cholesterol, and phospholipids comprise 98–99%, 0.3–1.3%, and 0.6–1.3%, respectively, of total milk lipids, Lactating women, who produce milk with an average fat content of 4.2%, secrete around 0.5 g of fat/day/kg body weight [5].
These changes have a pathological extreme in massive hypertriglyceridemia and pancreatitis in individually pregnancy and oral contraceptive therapy. For the duration of pregnancy, the distribution of fatty acids and lipids between the three compartments (maternal, placental, and fetal) is dependent on gestational stage [6]. The third trimester of pregnancy is characterized by improved lipolysis. This catabolic state is supported by the maternal insulin- resistant state which reduces the suppression of lipolysis. The higher concentration of lipids in the maternal circulation is also the consequence of the hormone estrogen that suppresses the activity of LPL in the maternal liver [3]. Maternal fat gathered in the adipose tissue during early pregnancy thus becomes available for placental transfer during the later stages of pregnancy, satisfying the increased fetal demand for fatty acids [3].
Consequently, the rate of maternal β-oxidation also decreases as the pregnancy progresses. The maternal plasma carnitine levels have also been found to reduce up to 40% at delivery as compared to twelve weeks of gestation which could be in response to lower fatty acid oxidation at delivery. The dietary balance of omega-6 and omega-3 fatty acids is important for sustaining good health and successful pregnancy [6]. In the course of time the ratio of omega-6 to omega-3 fatty acids in the diet has changed from 1:1 to 20:1. The constituents of maternal lipoproteins are taken up by the placenta via the activity of lipoprotein receptors, lipases, Fatty Acid-Binding Proteins (FABPpm), and other mechanisms [6].
Lipogenesis ends when lactation starts. At that time, mammary adipocytes Trans-differentiate into secretory epithelial cells to promote lipid transfer during milk production. This process participates actively to breast remodeling as breast adipocytes display an extraordinary plasticity [7]. Breast adipose tissue is a secretory organ like the other depots. It produces prolactin and steroid hormones like estrogens thanks to an aromatase. In addition, it participates in epithelial cell growth, angiogenesis, intercellular communication, and milk production, and it secretes many growth factors and enzymes involved in beast reshaping during development [7]. Dissimilar endocrine and also in the buttocks is sensitive to estrogens, in contrast to WAT in the upper back which is more sensitive to glucocorticoids [8]. This unique association between adipose tissue and the mammary epithelium outlines functional differences with other body fat depots [9].
Maternal obesity and excessive Gestational Weight Gain (GWG) are recurrently associated with alterations in carbohydrate and lipid metabolism, abnormal levels of pregnancy hormones, and a pro- inflammatory state [10]. These disturbances increase the risk of maternal and fetal complications such as gestational diabetes mellitus, hypertensive disorders of pregnancy, cesarean delivery, lung disease, miscarriage, still birth, fetal chromosomic anomalies, preterm birth, and fetal macrosomia ) [11]. Furthermore, adiposity excess alters the placental nutrient transfer and modifies the composition of breast milk, affecting the development and genetic programing of multiple fetal organs (liver, adipose tissue, skeletal muscle, and brain, among others) [11]. Maternal pre-pregnancy obesity is independently associated with fetal overgrowth and with total body adiposity, abdominal fat accumulation, and lower fat free-mass in neonates. These features are aggravated by an excessive GWG during mid and late pregnancy at the time of higher fat accretion in the fetus [10]. Moreover, high adiposity in neonates is related to insulin resistance, hyperinsulinemia, and a pro-inflammatory status characterized by high circulating levels of leptin and InterLeukin-6 (IL-6) [12]. Furthermore to these features, the intrahepatic fat content of neonates during the first week after birth is correlated with maternal Body Mass Index (BMI) in obese women with gestational diabetes [11].
Lipids Homeostasis in Pregnancy
Maternal hypertriglyceridemia at late gestation outcomes from the juxtaposition of several factors [3]: Enhanced adipose tissue lipolysis facilitating the availability to the liver substrates for triglyceride synthesis and contributing to augmented flux of Very Low Density Lipoproteins (VLDL) into the circulation. Maternal hyperphagia and unmodified gut lipid absorption increasing chylomicron formation from dietary lipid [3]. Reduced LiPoprotein Lipase (LPL) activity in extrahepatic tissues (especially adipose tissue) which does not allow a triglyceride removal proportional to their enhanced production [3]. The dynamics of changes in the fat content of fetal tissue is different from found in the mother. First, there is no catabolic period. Second, the anabolic period starts much later than it starts for the mother, i.e., around weeks 20–22 of pregnancy and continues gradually completed the following 10–12 weeks. There is a sharp rise in fetal fat around approximately 32 weeks which continues till the time of birth [13]. Throughout the first trimester human fetal FA synthesis is lower than fetal FA requirements and the majority of fat is obtained via placental transport [13]. After the fatty acids cross the placenta and reach the fetal circulation, they bind to Alpha-FetoProteins (AFPs) and enter the fetal liver for the synthesis of TG. The majority of fatty acids transferred across the placenta are effiuxed to serum albumin [13].
AFP has a binding preference for Long Chain PolyUnsaturated Fatty Acids (LCPUFA) particularly DocosaHexAenoic acid (DHA). From the physiological point of view, LCPUFAs are considered the most important in maternal–fetal metabolism. Fetal brain, retina, and CNS development requires LCPUFA in abundant amounts and the only source for the fetus is through the maternal circulation [2]. The percentage of DHA in the fetal blood increases exponentially after 20 weeks of gestation. From postmortem studies of fetuses and infants, it was assessed that the fetus accretes 50–60mg omega-3 fatty acid during the last trimester of pregnancy, most of which is DHA (7–10mg) and is probably transferred from the placenta. Fetal body fat is 13%–15% of body weight at birth, with 45%–50% of it being palmitic acid, much of which is derived from endogenous synthesis in the fetus [2]. For the period of last trimester of pregnancy, there is a high fetal demand for Fatty Acids (FA), as they are critical for normal neural and vascular development [14]. The fetus is able to synthesize saturated and monounsaturated FAs from glucose and ketone bodies. Nevertheless depends entirely on placental transport for its supply of the Essential Fatty Acids (EFA) linoleic and a linolenic acid. The EFAs and their derivatives the (LCPUFAs) arachidonic (ARA) and (DHA) are essential constituents of membranes, act as precursors of cellular signaling molecules, and are particularly important for the developing brain and retina [14].
Triglycerides are transported in the bloodstream as lipoproteins. TGs must be hydrolyzed into FFA before they can be transferred across the syncytiotrophoblast of the human placenta. This transporting epithelium is a multinucleated syncytium, polarized with the apical or MicroVillous plasma Membrane (MVM), bathed in maternal blood, and the basal Plasma Membrane (PM), oriented toward the fetal capillary [15]. At least two types of TG hydrolases have been recognized in MVM, a placenta-specific hydrolase and LPL. LPL has been suggested to be the most important hydrolase in the initial step for transfer of TG-derived FA, as the placenta-specific TG hydrolase is inhibited by serum [14].
In the late 2nd and 3rd trimesters, when adipogenesis accelerates [13]. Coordinated by placental hormones, normal pregnancy metabolism is characterized by a marked increase in Insulin Resistance(IR), increased postprandial glucose, a2-to3-fold increased insulin production, and increased plasma FFAs, TGs, total cholesterol, and phospholipids similar to a metabolic syndrome as defined outside of pregnancy [14]. Phospholipids, and FFAs occur with advancing gestation and are even higher in mothers with obesity and GDM. Placenta secretes large quantities of estrogen that stimulate hepatic Very Low Density Lipoprotein (VLDL)-TG production, further promoting the doubling of maternal TGs [14].
Fetal Fat Development and Storage
Maternal triglycerides TGs and FFAs may be potential substrates for fetal fat development and storage [6]. Figure 1 shows how women enter pregnancy with preexisting overweight/ obesity, features of the metabolic syndrome, and poor nutritional patterns. When the Insulin Resistance (IR) of pregnancy is superimposed, the placenta is exposed to additional nutrients that are sensed, broken down by placental lipases (TGs), metabolized or transported (glucose, amino acids [AAs], FFAs) to the fetus. High maternal glucose and AAs promote fetal hyperinsulinemia, which drives fetal subcutaneous (subQ) fat accretion. High FFA exposure may promote increased fetal intrahepatic lipid storage, serving as one of multiple “first hits” that drive childhood Nonalcoholic Fatty Liver Disease (NAFLD) and propagate increased newborn subQ fat mass. Maternal obesity further alters fetal umbilical Mesenchymal Stem Cell (uMSC) differentiation potential, increasing their propensity to differentiate into adipocytes versus skeletal muscle cells, also contributing to higher adiposity at birth. Maternal over nutrition as a function of pre-pregnancy and pregnancy IR, combined with poor lifestyle, fetal placental exposure to excess nutrients, and fetal hyperinsulinemia with altered uMSC differentiation and intrahepatic fat, fuel increased adiposity at birth, which underscores a heightened risk for later childhood obesity, NAFLD, and metabolic disease [6].

Figure 1: Making fat from fat, CM=chylomicron; EL=endothelial lipase; G=glucose; pLPL=placental lipoprotein lipase; VLDL=very-low-density lipoprotein (Barbour and Hernandez, 2018) [6].
Fatty acid and lipid metabolism during pregnancy
For maternal metabolism, pregnancy ends not with delivery, but with weaning. Variation in metabolism, mitochondrial function, and oxidative stress between females that breastfed and those that did not breastfeed after giving birth have not been previously documented in detail. Liver, adipose tissue and, during lactation, the mammary gland, are the major sites of fatty acid metabolism. During gestation, visceral fat accumulates, and insulin resistance and lipid and TG levels increase [13]. These changes appear to reverse more quickly, and more completely, with lactation. Figure 2 shows the Fatty acid and lipid metabolism during pregnancy, After digestion of maternal dietary fat by lipases in the intestinal lumen, the fatty acids associate with bile salts and phospholipids to form micelles. LipoProtein Lipases (LPL) hydrolyze these micelles releasing triglycerides that are taken up by the enterocytes of the intestines where they combine with Apolipoproteins (Apo) and cholesterol to form nascent chylomicrons. After the addition of additional Apo mature chylomicrons are formed which are released into the lymphatic and blood circulation. Chylomicrons release 90% of triglycerides (TGs) with the action of LPL and form remnant chylomicrons and High-Density Lipoproteins (HDLs) [13].
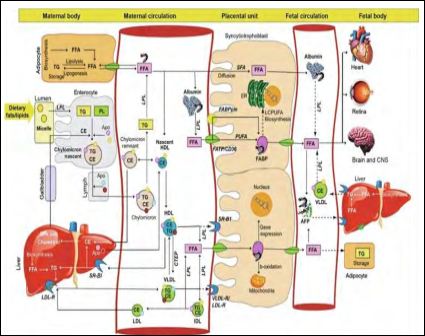
Figure 2: Fatty acid and lipid metabolism during pregnancy (Chavan-Gautam et al., 2018) [13].
Remnant chylomicrons are taken up by the liver and HDL collects the FFA (free fatty acid) from the peripheral tissues including adipocytes to deliver it to liver. The released FFAs from the chylomicrons are taken up by the adipocytes and reesterified to form TGs for storage as lipid droplets [13]. The adipocytes release de novo synthesized and stored FFA into the circulation in the third trimester when the fatty acids are required by the fetus. VLDL carries lipids from liver to the placenta and releases FFA by LPL activity. Scavenger Receptor class B member 1 (SR-BI) is the receptor for HDL and LDL-R and VLDL-R are the receptors for LDL and VLDL, respectively. Most of the FFAs bind to albumin in the circulation and placenta takes up the FFA released from all these lipoproteins by releasing LPL. Short-chain and Saturated Fatty Acids (SFAs) can cross the placenta by diffusion, while PolyUnsaturated Fatty Acids (PUFAs) require Fatty Acid Transport Proteins (FATPs), Fatty Acid Translocases (FATs/CD36), and plasma membrane Fatty Acid-Binding Proteins (FABPpm). The FABP binds to FFA and helps in cytoplasmic movement of fatty acids. The FFAs transferred to the fetal circulation bind to Albumin or α-FetoProtein (AFP) to be carried to the liver or other peripheral tissues of the fetus.
Pathways that are not clearly established are indicated using dashed lines [13].
Stress during pregnancy
The major changes occurring in the mother during the second half of gestation which are responsible of her triglyceridemia are summarized in Figure 3. Enhanced adipose tissue lipolysis facilitates the availability to the liver of substrates for TG formation which, together with endogenous changes, promote enhanced flux of TG into the circulation in the form of VLDL [16]. This pool of TG is enriched by chylomicron TG from dietary lipids, the production of which is also augmented as a result of the maternal hyperphagia and unmodified gut lipid absorption. Hypertriglyceridemia remains stable until late gestation because reduced LPL activity in the extrahepatic tissue, does not permit removal of TG proportional to its enhanced production. These variations, together with maternal energy stores (mainly in the form of fat deposits), contribute to fulfill maternal and fetal metabolic needs [16].
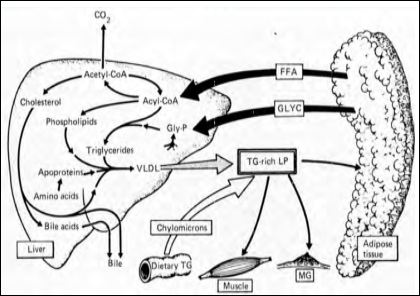
Figure 3: Major changes occurring in the mother during the second half of gestation (Herrera et al., 1987) [16].
In conditions of maternal food deprivation, the use of glycerol released from adipose tissue as a preferential gluconeogenetic substrate together with the enhanced maternal ketogensis demonstrate the important contribution of maternal fat to ensure the availability of fuels for the fetus. These conditions are modified shortly prior to parturition because an increase in mammary gland LPL activity (probably mediated by the augmented prolactin release which occurs before parturition facilities the uptake of circulating TG to the gland, causing a reduction in circulating TG and preparing the mother for lactation [16].
Lipids metabolism in Lactation
For maternal metabolism, pregnancy ends not with delivery, but with weaning. Variation in metabolism, mitochondrial function, and oxidative stress between females that breastfed and those that did not breastfeed after giving birth have not been previously documented in detail. Liver, adipose tissue and, during lactation, the mammary gland, are the major sites of fatty acid metabolism [1]. During gestation, visceral fat accumulates, and insulin resistance and lipid and triglyceride levels increase. These changes appear to reverse more quickly, and more completely, with lactation. Fat mobilization appears to increase after the first 3 months postpartum, perhaps reflecting changes in the endocrine effects of lactation on maternal appetite as frequency of infant feeds decreases [1]. In the first 2 to 3 months postpartum, Stuebe et al [17] have found that formula-feeding mothers consumed 600 to 800 fewer calories than breast-feeding mothers and lost substantially more weight. From 3 to 6 months post- partum, however, weight loss among breast-feeding women increased substantially. Key enzymes of synthesis de novo are acetyl CoA carboxylase and fatty acid synthase, while the initial step of fatty acid esterification is catalyzed by Glycerol-3-Phosphate Acyl Transferase (GPAT) [17].
As well as synthesis de novo, adipose and mammary tissues secrete LipoProtein Lipase (LPL). This allows these tissues to obtain fatty acids from TAG of chylomicrons and VLDL (secreted by intestinal and liver cells respectively) in the blood. In adipocytes, the TAG droplet is bounded by a network of proteins such as perilipin, which restrict hydrolysis and so keep the cellular concentration of fatty acids themselves low. Furthermore, adipocytes have high levels of fatty acid binding protein, which reduce the amount of free fatty acid in the cell. Throughout periods of negative energy balance and during stress, adipocytes release NEFA into the blood; these NEFA are produced by hydrolysis of TAG by the action of hormone-sensitive lipase [1]. In the course of such periods, adipose tissue secretes vasoactive factors (e.g. adenosine, prostacyclin, prostaglandin E), which help remove fatty acids from the tissue. In well-fed women, the energy balance usually remains positive [18]. Nevertheless, in situations of low-energy supply, the stored lipid can be critical to establishment of lactation and maintenance of maternal health. There is a commonality among mammals in the adaptive responses during lactation and in their general regulation. Acylated fatty acids can be metabolized by the enzymes of the β-oxidation pathway or incorporated into TAG for secretion into milk. In the mammary gland the mRNA for most enzymes of the β-oxidation pathway falls two- to threefold at secretory activation directing fatty acids to TAG synthesis. Glycerol- 3-P synthesis is increased by the upregulation of the mammary- and brain-specific aldolase C as well as glycerol kinase. Increased cholesterol production through the upregulation of the mRNA for enzymes of cholesterol synthesis occurs at secretory activation. Conversely, at midlactation expression of the relevant genes was much higher in the liver, suggesting that much of the cholesterol exported into milk is synthesized in the liver and transported through the blood stream [1].
Human milk lipids afford a major portion of the energy supply to breastfed infants as well as essential vitamins, polyunsaturated fatty acids, complex lipids, and bioactive components. Human milk lipids provide the infant with energy and essential vitamins, polyunsaturated fatty acids, and bioactive components. Adding complex lipids and milk fat globule membranes to vegetable oil-based infant formula has the potential to enhance infant development and reduce infections [19].
Conclusion
Pregnancy is one of the most critical periods for mother and child. It involves a tremendous flow of physiological changes and metabolic adaptations week by week, and even small deviations from the norm might have detrimental consequences at different pregnancy stages. Third trimester human pregnancy is characterized by a 2-3-fold increase in plasma triglyceride and lesser increases in cholesterol and phospholipid. Maternal hypertriglyceridemia at late gestation results from the juxtaposition of several factors. Enhanced adipose tissue lipolysis facilitating the availability to the liver substrates for triglyceride synthesis and contributing to augmented flux of very VLDL into the circulation. Maternal hyperphagia and unmodified gut lipid absorption increasing chylomicron formation from dietary lipid. Reduced LPL activity in extrahepatic tissues (especially adipose tissue) which does not allow a triglyceride removal proportional to their enhanced production. Variation in metabolism, mitochondrial function, and oxidative stress between females that breastfed and those that did not breastfeed after giving birth have not been previously documented in detail. Liver, adipose tissue and, during lactation, the mammary gland, are the major sites of fatty acid metabolism [17].
References
- Bastianelli C, Farris M, Bruni V, Brosens I, Benagiano G (2020) Pharmacodynamics of combined estrogen–progestin oral contra- ceptives: Effects on uterine and cervical epithelia. Expert Review of Clinical Pharmacology 13: 163-182.
- Berberoglu Z (2017) Inflammation, adipocyte mediators, and lipid metabolism in gestational diabetes mellitus. Gestational Diabetes: Risk Factors, Management and Outcomes 45-116.
- Farris M, Bastianelli C, Rosato E, Brosens I, Benagiano G (2017) Pharmacodynamics of combined estrogen-progestin oral contra- ceptives: Effects on hemostasis. Expert Review of Clinical Phar- macology 10: 1129-1144.
- Ladoux A, Peraldi P, Chignon-Sicard B, Dani C (2021) Distinct Shades of Adipocytes Control the Metabolic Roles of Adipose Tis- sues: From Their Origins to Their Relevance for Medical Applica- Biomedicines 9: 40.
- McManaman J (2020) Lipid Transport Across the Mammary In Ion Transport Across Epithelial Tissues and Disease.
- Barbour L, Hernandez T (2018) Maternal lipids and fetal over- growth: Making fat from fat. Clinical Therapeutics 40: 1638-1647.
- Kothari C, Diorio C, Durocher F (2020) The importance of breast adipose tissue in breast cancer. International Journal of Molecular Sciences 21: 5760.
- Cinti S (2018) Pink adipocytes. Trends in Endocrinology & Metab- olism 29: 651-666.
- Wang Q, Song A, Chen W, Schwalie P, Zhang F, et al. (2018) Re- versible de-differentiation of mature white adipocytes into preadipo- cyte-like precursors during lactation. Cell metabolism 28: 282-288.
- Lindsay K, Brennan L, Rath A, Maguire O, SmithT, et (2018) Ges- tational weight gain in obese pregnancy:Impact on maternal and foetal metabolic parameters and birthweight. Journal of Obstetrics and Gynaecology 38: 60-65.
- Álvarez D, Muñoz Y, Ortiz M, Maliqueo M, Chouinard-Watkins R, et (2021) Impact of Maternal Obesity on the Metabolism and Bio- availability of Polyunsaturated Fatty Acids during Pregnancy and Breastfeeding. Nutrients 13: 19.
- Ruchat S, Allard C, Doyon M, Lacroix M, Guillemette L, et (2016) Timing of excessive weight gain during pregnancy modulates new- born anthropometry. Journal of Obstetrics and Gynaecology Cana- da 38: 108-117.
- Chavan-Gautam P, Rani A, Freeman D (2018) Distribution of fatty acids and lipids during In Advances in clinical chemistry 84: 209-239.
- Jayalekshmi V, Ramachandran S (2020) Maternal cholesterol levels during gestation: Boon or bane for the Molecular and Cel- lular Biochemistry. Pg no: 1-16.
- Segura Moreno M (2019) Effect of maternal obesity and gestational diabetes on placental fatty acid uptake, metabolism and transfer to the fetus.
- Herrera E, Gomez-Coronado D, Lasunción M (1987) Lipid metabo- lism in pregnancy. Neonatology 51: 70-77.
- Stuebe A, Rich-Edwards J. (2009) The reset hypothesis:Lactation and maternal American Journal of Perinatology 26: 81- 88.
- Rudolph M, McManaman J, Phang T, Russell T, Kominsky D, ea (2007) Metabolic regulation in the lactating mammary gland: A lipid synthesizing machine. Physiological genomics 28: 323-336.
- Brasaemle D, Rubin B, Harten I, Gruia-Gray J, Kimmel A, et al. (2000) Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. Journal of Biological Chemistry 275: 49.
Citation: Aljaraedah TY (2021) Lipids Metabolism in Pregnancy and Lactation in Human: A General Review. J Obes Bod Weig 2: 002.
Copyright: © 2021 Aljaraedah TY. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


