*Corresponding Author:
Cheemalapati Venkata Narasimhaji,
Central Ayurveda Re- search Institute for Drug Development, Kolkata, India
Email: vnarsimhaji@yahoo. com; vnjicheemalapati@gmail.com
Abstract
The present study was aimed to evaluate the purity of the ingredients through the assay of marker compound to ensure the quality of the prepared poly herbal formulation in the course of standardization. It was carried out by isolation of piperine from poly herbal formulation Trikakuchurna besides one of its ingredient namely Piper nigrum; quantitative evaluation of Piperine through densitometry, using HPTLC for poly herbal formulation of in-house prepared and market purchased samples besides ingredients. The isolated compound was identified by UV, H1 NMR, and LC-MS. Thus the presence of marker not only identified in the formulations but also physically found with the isolated marker compound to ensure the quality of the finished product.
Introduction
Globally, the scenery for Traditional and Complementary Medicine (T&CM) has been improving consistently. In line with the WHO Traditional Medicine strategy 2002-2005 and the WHO TM strategy 2014-2023, and relevant World Health Assembly resolutions, member states took steps between 2005 and 2018 to promote the safety, quality and effectiveness of T&CM. They also took steps for the appropriate integration of T&CM into health systems (particularly health services) by developing national policies, regulatory frameworks and strategic plans for T&CM products, practices and practitioners. Based on current information, 88% member states have acknowledged their use of T&CM which corresponds to 170 member states [1]. These are the countries that have formally developed policies, laws and regulations for T&CM, and the actual number of countries using T&CM is likely to be even higher. Herbal medicine and/or Traditional medicines (TMs) demand and popularity are increasing regularly, as80% of people in developing country relays on traditional medicines [2]. An account of increasing popularity for TMs and botanical products of poly herbal formulations, their global market value expected more; accordingly its standardization protocols to be developed for the high purity and quality for the global acceptance. The same has emphasized to ensure the quality of medicinal plant products by using modern controlled technique and applying suitable standards [3,4] by World Health Organization (WHO). For standardization of natural product drugs such as poly herbal formulations and its extracts, single chemical entities, “marker compounds,” may be used as potency standards in modern techniques such as high performance thin layer chromatography (HPTLC), HPLC [5]and hyphenated techniques.
Trikatuchurna [6] is a unique ploy herbal formulation, is a combination of equal parts of Piper nigrum L., (Fruit), Piper longum L. (Fruit), and Zingiber officinale Roscoe. (Rhizome). In preparation of churna the ingredients are collected, dried, powdered individually and passed through sieve number 44 to prepare a fine powder, as per procedure given in Ayurvedic Formulary of India, Part I, 7:14 [6,7]. Trikatuchurna when used along with other herbs, it enhances the bio-availability and is used one of the ingredients in many Ayurvedic medicines [7,8]. Trikatuchurna acts as immunomodulatory and anti-inflamatory [9] etc.
Piperine is main active compound in Piper nigrum (Black pepper) one of the raw botanical ingredient in Trikatuchurna. Piperine is the main compound leading to bioactivity of black pepper. Its pungency has been estimated as 100000-200000 Scoville Unit [10,11]. Piperine is an alkaloid and it is the carboxamide of Piperic acid and Piperidine [12]. In recent decades, Piperine came into the spotlight of pharmaceutical research. It has antibacterial [13], antioxidant [14], anti-inflammatory [15], antiarthritic [15] and other effects. The most interesting point is that Piperine increases the bioavailability of a number of therapeutic drugs as well as phytochemicals [16].
Isolation of markers from the raw botanical ingredients ensures their purity and isolation of the markers as well as from the formulation ensures the quality of the formulation in terms of strength for its efficacy. For standardization of natural product drugs, single chemical entities that are “marker compounds,” would be used as standards for potency check and its quality assurance by quantitative evaluation through a basic, economic, and well versed technique, High Performance Thin Layer Chromatography (HPTLC) analysis [5] and hyphenated techniques such as HPLC and LC-MS etc.,
HPTLC fingerprint profiles [17] for the analysis for marker compounds with qualitative and quantitative evaluation of ingredients along with finished formulation and market samples may provide added value in the quality evaluation of formulation to ensure the identity, purity and strength. The present study was carried out for isolation of piperine from Trikakuchurna as a wholeand piper nigrum one of the raw ingredients of formulation, TC; quantitative evaluation of piperine for ingredients, in-house prepared formulations and market samples.
Materials and Method
Collection of plant materials
The raw plant materials namely, fruit of Piper nigrum L., fruit of Piper longum L. and rhizome Zingiber officinale Roscoe. Were procured from the crude drug market from Chennai. The single drugs were identified and authenticated by the botanist of CSMRADDI, institute of CCRAS, Ministry of AYUSH, Government of India, Chennai [18].
Preparation of trikatuchurna
The plant materials of each ingredient were ground to get fine powder and sieved through sieve number 44 to prepare a fine powder each sample. The fine powders of each ingredient in equal quantities were thoroughly mixed to get the finished formulation as per procedure given in Ayurvedic Formulary of India, Part I, 7:14. In the same way the three batches of TC finished formulation were prepared, stored in airtight container and three standard commercial market samples were procured for further comparative evaluation.
Instrumentation used for the evaluation
- a. A CAMAG HPTLC system: A CAMAG HPTLC system (Muttenz, Switzerland) equipped with a sample applicator TLC autosampler 4, twin trough plate development chamber, TLC Scanner 3, win CATS software version 4.4. and Hamilton (Reno, Nevada, USA) Syringe: HPTLC studies such as fingerprint profiles development and quantitative analysis of the piperine were carried out following the method by [19-21].
- b. LCMS-Agilent 6310 Ion Trap: Mass spectrum for isolated piperine was carried out to confirm the marker compound from the mass
- c. Shimadzu UV 1601-UV Visible spectrometer: UV spectrum was
- d. Bruker 400 MHz NMR spectrometer: NMR spectrum was recorded for the isolated marker compound from the institute of CSIRIICT,
Extraction & Isolation of Piperine from Piper nigrum and Trikatuchurna
Extraction of samples: In general, about 50 g of both Piper nigrum and Trikatuchurna were weighed accurately and soaked in 500 ml of alcohol separately to extract through cold maceration method.
Method A: Isolation of Piperine from Piper nigrum and Trikatuchurna
The above residues obtained in both the cases of Piper nigrum and Trikatuchurna were taken in 10 ml of alcohol to this 10 % alcoholic KOH was added and kept for crystallization. But it was observed the formation of an oily layer. To the above solution, 5 to 10 ml of water was added drop wise until turbid formation and kept aside for the formation of solid crystals. Again precipitation has not occurred, but oil sedimentation was observed in both the case. It was then partition between water and n-hexane, hexane layer was kept aside overnight and pale yellow needle shaped crystals were observed. The crystals were filtered separately, washed with cold ether to purify and weighed to got 0.16 g and 0.19 g respectively as of pure solids. These were further confirmed as piperine by various analytical parameters with measurement of preliminary melting point [22].
Method B: Isolation of Piperine from Trikatuchurna
About 50 g of Trikatuchurna was weighed and soaked for two days in alcohol, filtered and concentrated to a thick residue. The above residue was taken and to this 10% alcoholic KOH was added and water drops were added until the turbid formation was observed and kept aside for crystallization. It was observed that the formation of crystals after one week, kept for two more days aside and filtered and washed with cold ether to got 0.21 g of the pure solid compound. The solid compound was further confirmed as Piperine with melting point, UV absorption data through UV-visible spectroscopy (λmax340.5 nm), by H1 NMR spectroscopy in CDCl3 (Figure1) and LCMS (Figure 2). The M.Wt of the isolated solid compound was observed 286 (M+H)+in ESI +Ve mode (Figure 2 and 3). The data was confirmed with the reported data for piperine [23-24].
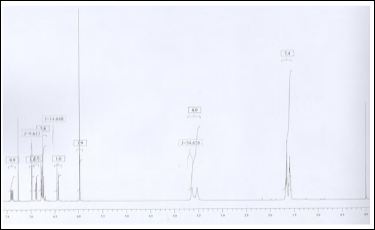
Figure 1: NMR Spectrum of Piperine in CDCl3.
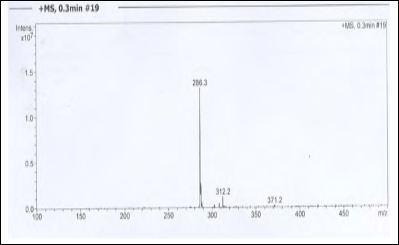
Figure 2: ESI Mass Spectrum of Piperine.
Quantitative Evaluation through HPTLC
Test solution
Extracted about 5 g accurately weighed of each powdered drug sample with methanol in a Soxhlet apparatus for 8 h each. Filtered and concentrated the combined extract under vacuum to get residue. Dissolved the whole residue obtained in ethanol in a 100 ml volumetric flask and made up the volume.
Standard solution
Dissolved 2 mg of piperine in 10 ml of methanol in a volumetric flask and made up to the volume.
HPTLC method
Silica gel 60 F254 pre-coated plates (20 x 10 cm) were used with Toluene: Ethyl acetate (7: 3) as solvent system. The bandwidth applied on plate was 6 mm and ascending mode was used for development of thin layer chromatography. Saturation time was 20 minutes along with humidity level45% ± 5% RH and room temperature 25°C ± 2°C. TLC plates were developed up to 8 cm. The TLC plate was scanned at 254 nm for quantification purpose. TLC Photographs were taken and documented at 254 nm, 366 nm and after dipping in vanillin sulphuric acid for identification and documentation (Figure 4).
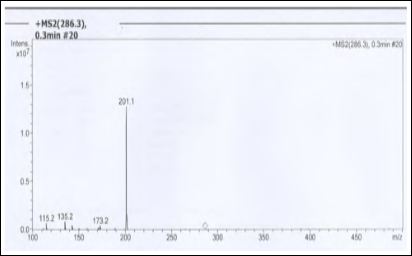
Figure 3: ESI Mass Spectrum Fragment of Piperine.
Calibration curve
1, 2 and 4 ml of standard solutions corresponding to 0.2, 0.4 and
0.8 mg of piperineon the TLC plate were applied. The plate was developed in a mobile phaseof Toluene: Ethyl acetate(7: 3), in a twin trough chamber previously saturated for 30 min, to a distance of 8 cm. The developed plate was air dried and carried out scanning in TLC Scanner 3 at 254 nm through densitometry. Record the respective peak areas and prepare a calibration curve (Figure5) by plotting peak area vs concentration of the piperine applied.
Estimation of Piperine in the drug
Apply 1 to 2 ml of the test solution and 1 to 4 ml of the standard solution on the TLC plate. Develop the plate in the solvent system to obtain the chromatogram (Figure 5-7) and determine the area of the peak for the test solution corresponding to that of piperine as described above for the calibration curve. Calculate the amount of piperine present in the sample from the calibration curve.
Linearity
The linearity of the method was checked with working standards piperine with the calibration curve in the concentration range of 20 to 80 ng/spot based on a 2 to 8 μl sample volume.
Results and Discussion
In the course of standardization, the identification of raw botanical ingredients qualitatively and quantitatively is essential to verify for the presence of ingredients in desirable purity and strength. Carrying out physicochemical parameters and preliminary phyto-chemical evaluations along with various fingerprinting techniques used for the verification of ingredients in the formulation may employed for the course of standardization [8,18] and quality assurance. In the present study, it was carried out for the isolation of piperine in two methods described in the methods&metrials from one of its ingredient Piper nigrum besides finished poly herbal formulation TC, to indentify and is to verify the presence of the marker compound physically in both ingredient and finished products to ensure the purity. Quantitative evaluation of piperine in in-house prepared finished formulations of Trikatuchurna [TC (B-I to B-III)]; two of its ingredients namely piper nigrum and piper longumbesides comparing three numbers of market samples (MS-I to MS-III).
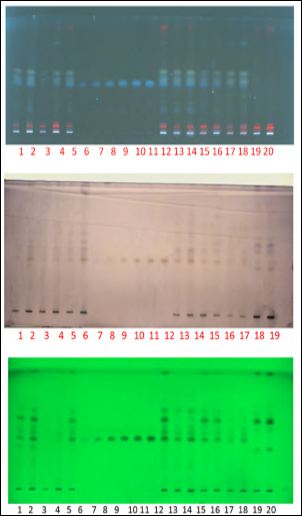
Figure 4: TLC Photo documentation of Trikatuchurna and its ingredients [UV 366nm, derivatized plate and UV 254 nm]
Note: Track 1, 2: TC[B-I]-1µl, 2µl; Track 3, 4:TC[B-II]-1µl, 2µl; Track 5, 12: TC[BIII]-1µl, 2µl; Track 6, 7, 8, 9, 10 & 11:Piperine-1µl, 2µl, 4µl, 6µl, 8µl & 10µl; Track 13, 14:MSI-1µl, 2µl; Track 15: MSII-1 µl; Track 16:MSIII-1µl; Track 17, 18: Piper nigrum-1µl, 2µl; Track 19 and 20: Piper longum-1µl and 2µl.
TC is a good source of piperine, the isolation was carried out in Method-A and B as described in methods & materials. The isolated solid compound was crystalline; pale yellow in color, the weight obtained 0.19 g to 0.21 g (% yield 0.38 to 0.42) observed m.p. was 129 oC. λmax was observed 340.5 nm (342 nm reported). 1H NMR (400 MHz, CDCl3) (δ, ppm) 6.0 (2H, s,) 7.4 (1H, ddd, H-3), δ 6.63 (1H, d J=15Hz, H-2), δ 4.0 (4H, m, H-c), δ 1.91.7 (5H, m, H-a,b). M. Wt of the solid compound was observed 286 (M+H)+in ESI +ve modeand even further confirmed with fragment of ion at m/z 201(Fig-3) observed in ESI-Ms spectrum. With this observed data of melting point, UV absorbance, 1H NMR and mass spectroscopy of isolated compound was found comparable with the reported data [22-24] and confirmed as a Piperine.
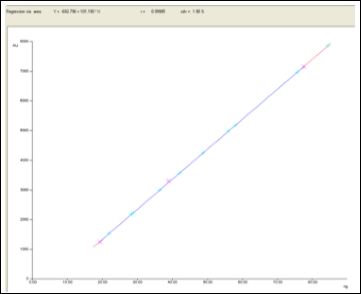
Figure 5: Calibration curve
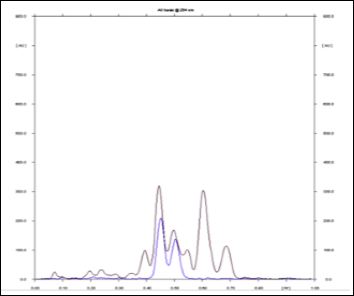
Figure 6: HPTLC densitometric scan at UV 254 nm; A. Test sample, B.Piperine standard.
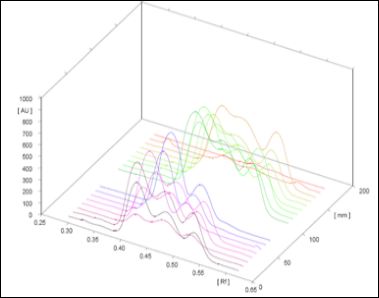
Figure 7: Chromatogram of all the Tracks.
Piperine quantitative evaluation was carried out through densitometry by A CAMAG HPTLC system scan at UV 254 nm, with the using above isolated marker compound piperine as standard. The chromatograms of standard and test solutions are given in figure 5-6. The regression equations (Y = 692.796 +101.190 × X) and correlation coefficient were obtained with 5 replicate analysis for each concentration. Correlation coefficients were obtained in the range of 0.9998 indicated excellent linearity of the procedure for working standard piperine analyzed. Calibration curve of working standard piperine is shown infigure5. Its comparative evaluation in three batches of inhouse prepared and three market samples of TC along with its ingredients namely Piper nigrum and Piper longum. The all chromatograms observed in figure7. The result was given in Table 1. The in house prepared strength in terms of assay of piperine ranges from 1.44 to 4.23%, with mean value of 3.10% and market samples assay ranges 2.40 to 3.80% with mean value of 2.89% is completely considerable in comparison. Freshly prepared in-house sample [batch-III] with high of piperine content is about 4.23%, even indicates the importance of usage of freshly prepared drug in general when compared to market samples.
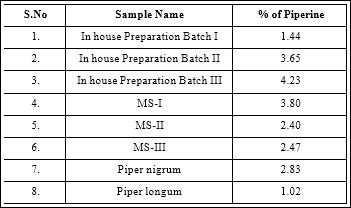
Table 1: Results.
Conclusion
Here in the study we achieved the isolation of the marker compound from formulation and its quantitative evaluation in the inhouse prepared samples besides comparing with market samples and ingredients in course of standardization of ploy herbal formulation TC and its quality assurance. In conclusion assay of certain phyto-chemical compound piperine for the ingredients as well as in the formulation will ensure the quality of a raw drug and formulation. Isolation of phyto-chemical marker piperine from ingredient will help in ensuring the purity of the raw drug, whereas isolation the of the same marker compound from formulation further to evaluate the quality in terms of its purity, identity of the ingredient in formulation in terms of physical verification and assay of the bio-active markers in terms of strength for the quality assurance.
References
- World Health Organization global report on traditional and complementary medicine 2019. Pg no: 1-226.
- Joshi NB, Shankar MB (2013) Recent Trends in the Usage of Herbo Mineral Formulations in Healthcare System. International Journal of Pharmaceutical Innovations 3: 116-129.
- World Health Organization Quality control methods for medicinal plant materials Geneva Pg no: 1-15.
- Jain V, Saraf S, Saraf S (2007) Standardization of triphalachurna: Spectrophotometric Asian J Chem19: 1406-1410.
- Shukla SS, Saraf S, Saraf S (2009) Approaches Towards Standardization And Quality Assessment Of Herbals. J Res Educ Indian Med 15: 25-32.
- Anonymous, TheAyurvedic Formulary of Part-I, Second Revised English Edition, Ministry of Health & Family Welfare, Govt. of India, Dept. of Indian Systems of Medicine & Homoeopathy. Pg no:1-182; 2003.
- Atal CK, Zutshi U, Rao PG 1981 Scientific evidence on the role of Ayurvedic herbals on bioavailability of drugs. J. Ethnopharmacol. 4: 229-232.
- Narasimhaji CV, Maheshwari B, Meena AK, Singh R, IlavarasanR, et (2018) JDRAS 3: 77-84.
- Vachana M, Rasool MK (2014) Cellular Immunology 287: 62-68.
- Tausig F, SuzukiJI, MorseRE (1956) Food Technology 10; 151-154.
- Raghuveer KG, Ananthakrishna SM (1980) Journal of Food Science and Technology 17: 268-272.
- Margraf M, Borstel R, Risch S, Marten S (2013) Knauer, VFD0117N, Germany,
- Karsha PV, Lakshmi OB (2010) Antibacterial activity of black pepper (Piper nigrum Linn.) with special reference to its mode of action on bacteria. Indian Journal of Natural Products and Resources 1: 213-215.
- Vijayakumar RS, Surya D, Nalini N (2004) Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative Redox Rep 9: 105-110.
- Bang JS, Oh DH, Choi HM, Sur BJ, Lim SJ, et al. (2009) Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis Arthritis Res Ther 11:49.
- Srinivasan K (2007) Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food SciNutr 47: 735-748.
- Lazarowych NJ, Pekos P (1998) Drug Information Journal 32: 497-512.
- Nartunai G, Arunachalam C, Maheswari B, Venkata NC, Kusuma G, et (2016) Pharmacognostical and Phytochemical evaluation of a polyherbal Ayurvedic formulation Trikatu Churna. J Ayu Med Sci 1: 34-40.
- Sethi PD (1996) High Performance Thin Layer Chromatography (1st Edn). CBS Publishers and Distributors, New Delhi,
- Stahl I (1969) Thin Layer Chromatography. A Laboratory Hand Book (Student Edn). Springer-Verlag, Berlin, Germany.
- Wagner H, Baldt S, Zgainski EM (1996) Plant drug analysis: A Thin Layer Chromatography Atlas (2nd Springer-Verlag, Berlin, Germany.
- Singh A, Sharma H, Venkata NCH, Singh R, Srikanth N (2019) HPTLC Detection of Carica papaya Dried Seed (Adulterant) in Trikatu Based on Journal of Biologically Active Products from Nature 9: 141-161.
- Ravindran PN (2003) Black pepper: Piper Boca Raton,Fla. CRC Press, UK.
- Peter KV (2006) Handbook of Herbs and spices, Sawston, Wood-head Publishing Cambridge, UK.
Citation: Narasimhaji CV, Murugammal S, Kusuma G, Meena AK, Sing R, et al. (2019) Isolation of Piperine from a Poly Herbal Formulation and its Quantitative Evaluation. J AcupunTradit Med 3: 005.
Copyright: © 2019 Narasimhaji CV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


