*Corresponding Author:
Surjit Singh,
Department of Pharmacology, All India Institute of Medical Sciences, 342005, Jodhpur
Tel: +91 8003996895
Email: sehmby_ss@yahoo.com, ss.sehmby@gmail.com
Statins and Diabetes Risk
There has been change in the safety information by United States Food and Drug Administration (US FDA) with regard to the statin use. Recently, there has been concern for increase in fasting plasma glucose levels with the use of statins. Labeling information of the statins like Rosuvastatin has been modified as ”Increases in HbA1c and fasting serum glucose levels have been reported with HMGCoA reductase inhibitors, including Rosuvastatin” [1].
Review of the literature has highlighted the concern. The study done by, Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) review group has reported a 27% increase in Diabetes Mellitus (DM) with the use of rosuvastatin as compared to placebo [2]. A Women’s Health Initiative (WHI) study data analysis, reported that statin therapy (Pravastatin, Fluvastatin, Simvastatin, Lovastatin and Atorvastatin) was associated with 48% increased risk of newonset diabetes in postmenopausal women after adjustment for age and ethnicity and concluded that it is a class effect [3]. Study done by Liew et al., 4 of 1060 subjects revealed 29% (adjusted OR = 1.290, p = 0.044, 95% CI 1.006, 1.654) increased risk of higher HbA1c levels with the use of statin as compared to no statin use.
PubMed and Google Scholar search identified about 14 clinical trials which has evaluated the incidence of diabetes events in the subjects receiving statin therapy. A metaanalysis by Sattar et al., of 13 statin trials with 91,140 participants, reported that statin therapy was associated with a 9% increased risk for incident diabetes (Odds Ratio [OR] 1.09; 95% Confidence Interval [CI] 1.021.17), with little heterogeneity (I2=11%) between trials [5]. Similar metaanalysis by Rajpathak et al., of 6 statin trials with 57,593 participants, reported a small increase in diabetes risk (Relative Risk [RR] 1.13; 95% CI 1.031.23), with no evidence of heterogeneity across trials [6]. Metaanalysis by Preiss et al., of 5 statin trials with 32,752 participants, revealed a 16% decrease in incident cardiovascular events but a 12% (Odds Ratio [OR] 1.12; 95% Confidence Interval [CI] 1.041.22) increase in risk of incident diabetes with the intensive statin therapy as compared to moderate statin therapy [7]. Metaanalysis of 14 clinical trials as done by us, revealed a 30% increased risk of incident diabetes with the use of statins (Figure 1) [2,3,819]. Subgroup analysis revealed that Pravastatin, Atorvastatin, Simvastatin, Rosuvastatin, Lovastatin and Fluvastatin was associated with 22%, 30%, 30%, 17%, 44% and 81% increased risk of incident diabetes respectively. As the heterogeneity is large (I2 = 80), we used random effect model for metaanalysis of the studies. Deleting the data of WHI study from the metaanalysis decreases the heterogeneity to 11% but has resulted in decrease in power of metaanalysis. So we did analysis including the WHI study.
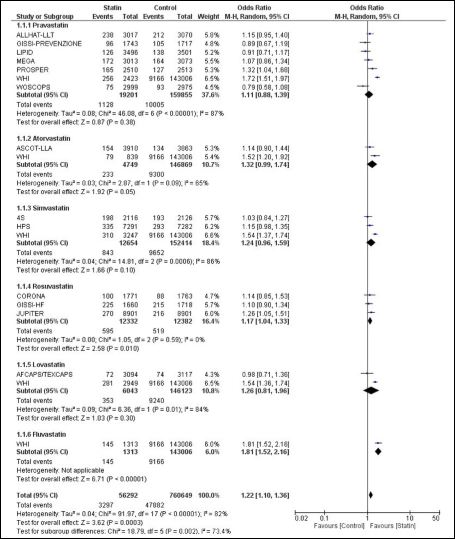
Figure 1: Association between Statin therapy and Incident Diabetes Mellitus.
The proposed mechanism with regard to hyperglycemia includes inhibition of isoprenoid synthesis and down regulating C/EBPα pro- duction [20]. This leads to down regulation of GLUT4 expression on adipocytes, decreasing insulin-mediated glucose uptake and intoler- ance to glucose. In addition, overproduction of nitric oxide can lead to beta cell apoptosis and decrease in insulin secretion. Statins by in- hibiting HMG-CoA reductase cause decrease production of Farnesyl Pyrophosphate (FPP), hence suppression of ubiquinone (CoQ10) synthesis resulting in decreased ATP production and decrease insu- lin secretion (Figure 2) [20]. Inhibition of HMG-CoA reductase also impair post-receptor insulin and IGF (Insulin Growth Factor-1) phos- phorylation and signaling by decrease Mevalonate and other metabo- lites. Decreased dolichol production cause decrease glycosylation and membrane translocation of mature insulin receptors. Decrease Gera- nylgeranyl Pyrophosphate impair GLUT-4 expression on adipocytes resulting in insulin resistance. Statins by decreasing adiponectin levels may result in higher tissue IL-6, TNF-α leading to beta cell apoptosis and increased insulin resistance. Other possible mechanisms is, statins inhibiting leptins levels negatively affects ß-cell proliferation and insu- lin secretion. All these mechanisms culminate to development of DM with the use of statins.
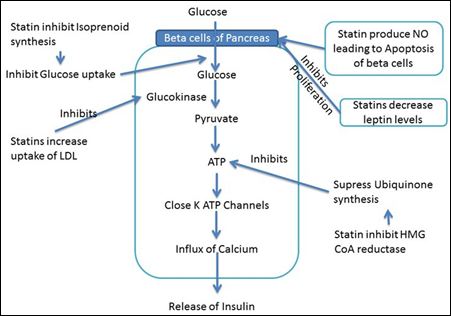
Figure 2: Mechanisms of Action of Statins on beta cells of pancreas resulting in Increase in HbA1c.
Decrease beta cell proliferation - Statins decrease leptin levels which inhibits the proliferation of beta cells of pancreas; Decrease in Insulin release - Statins inhibit isoprenoid synthesis resulting in decreased glucose uptake into beta cells; Statins increases LDL uptake leading to inhibition of glucokinase; Statins by inhibiting ubi- quinone synthesis results in decrease ATP generation.
HMG-CoA: 3-hydroxy-methylglutaryl coenzyme A; NO: Nitric oxide; LDL: Low-density lipoprotein
Recommendation for use of Statins in Diabetes
The risk-benefit assessment is necessary for recommendation of use of statins in diabetes patients. In DM patients with CVS risk factors, statins prevent CVS event 8 times more likely than causing a case of incident diabetes [21]. Therefore, a modest increase in blood glucose is not a cause of concern as they decrease morbidity and mortality due to macrovascular and microvascular complications. In patients with low CVS risk factors, lifestyle modifications should be considered and use of statins should be used for less aggressive management of LDL-cholesterol. Meta-analysis by Mihaylova et al., of 27 randomized trials in patients with low risk of vascular disease, statins reduce risk of CVS events and all-cause mortality by 15% and 9% respectively [22].
Study done by Bertolotti, M et al., showed that any king of lipid lowering drugs used in age > 65 years was not associated with the increased risk of diabetes [23]. Besides drugs, fibres, phytosterols and red yeast rice has a consistent effect on LDLcholesterol reduction with good level of evidence [24].
Factors like old age, increased weight, metabolic syndrome and higher blood glucose increase the risk of diabetes and in subjects with the above risk factors, risk-benefit ratio further increases with the use of statin. Improved outcome has been observed in patients undergoing cardiac surgery, therefore guidelines recommend use of statins in patients undergoing coronary artery bypass graft. Although there has been decrease in atrial fibrillation and MI after surgery but poor glycemic control may lead to increase infections and renal complications.
Conclusion
- Based on epidemiological data from the published literature, clin- ical trial meta-analyses and Forrest plot analysis of all 14 clinical studies (Figure 1), we have observed that there is increase in risk of development of diabetes with the use of statins. This increased risk appears to be a class effect as it is observed in all the statin
- Considering, the favorable risk-benefit ratio, statin use is recom- mended in patients with high CVS risk, low CVS risk as well as patients undergoing cardiac
References
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021366s016l- pdf
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, et al. (2008) Rosuvastatin to prevent vascular events in men and women with elevated C-reactive N Engl J Med 359: 2195-2207.
- Culver AL, Ockene IS, Balasubramanian R, Olendzki BC, Sepavich DM, et (2012) Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med172: 144-152.
- Liew SM, Lee PY, Hanafi NS, Ng CJ, Wong SSL, et al. (2014) Statins use is associated with poorer glycaemic control in a cohort of hypertensive patients with diabetes and without Diabetol Metab Syndr 6: 53.
- Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, et al. (2010) Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin Lancet Lond Engl 375: 735–742.
- Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, et al. (2009) Statin therapy and risk of developing type 2 diabetes: a meta-analy- Diabetes Care 32: 1924-1929.
- Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, et al. (2011) Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 305: 2556-2564.
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. (2002) Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihy- pertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALL- HAT-LLT). JAMA 288: 2998-3007.
- Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JG, et al. (2007) Ro- suvastatin in older patients with systolic heart failure. N Engl J Med 357: 2248-2261.
- Freeman DJ, Norrie J, Sattar N, Neely RD, Cobbe SM, et al. (2001) Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 103: 357-362.
- Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, et al. (2003) Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled Lancet 361: 1149-1158.
- Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, et (2008) Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372: 1231-1239.
- [No authors listed] (2000) Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico). Ital Heart J 1: 810-820.
- Collins R, Armitage J, Parish S, Sleigh P, Peto R, et al. (2003) MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 361: 2005-2016.
- Keech A, Colquhoun D, Best J, Kirby A, Simes RJ, et al. (2003) Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose: results from the LIPID trial.. Diabetes Care 26: 2713-2721.
- Scandinavian Simvastatin Survival Study Group (1994) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344: 1383-1389.
- Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto (2006) Prima- ry prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 368: 1155-1163.
- Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM. (2002) Pravas- tatin in elderly individuals at risk of vascular disease (PROSPER): a random- ized controlled trial. Lancet 360: 1623-1630.
- Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR. (1998) Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention JAMA 279: 1615-1622.
- Chogtu B, Magazine R, Bairy KL (2015) Statin use and risk of diabetes mel- World J Diabetes 6: 352-357.
- Bhatia L, Byrne CD (2010) There is a slight increase in incident diabetes risk with the use of statins, but benefits likely outweigh any adverse effects in those with moderate -to-high cardiovascular risk. Evidence Based Medicine 15: 84-85.
- Cholesterol Treatment Trialists’ (CTT) Collaborators, Mihaylova B, Emberson J, Blackwell L, Keech A, et (2012) The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 380: 581-590.
- Bertolotti M, Franchi C, Rocchi MB, Miceli A, Libbra MV, et al. (2017Preva- lence and Determinants of the Use of Lipid-Lowering Agents in a Population of Older Hospitalized Patients: the Findings from the REPOSI (REgistro PO- literapie Società Italiana di Medicina Interna) Study. Drugs Aging 34: 311-319.
- Pirro M, Vetrani C, Bianchi C, Mannarino MR, Bernini F, et al. (2017) Joint position statement on “Nutraceuticals for the treatment of hypercholesterol- emia” of the Italian Society of Diabetology (SID) and of the Italian Society for the Study of Arteriosclerosis (SISA). Nutr Metab Cardiovasc Dis 27: 2-17.
Citation: Sivashunmugam L, Ansari RM (2017) The Prevalence of Pre-Diabetes Among Year Two Students in a Malaysian Medical School and Their Knowledge of the Relationship of Obesity with Pre-Diabetes. J Diab Meta Syndro 1: 003.
Copyright: © 2017 Sivashunmugam L. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


