*Corresponding Author:
Gunapathy Devi,
Department of Zoology, Nehru Memorial College, Tamil Nadu, India
Tel: +91 4327234 327
Fax: +91 4327234 638
Email: gunapathydevi@gmail.com
Abstract
The present study was investigating the immunomodulatory effect of Hygrophila spinosa enriched diet on immune response and disease resistance in freshwater prawn, Macrobrachium malcolmsonii against Vibrio alginolyticus. The total heamocyte count (THC) and prophenoloxidase (pro PO) activity of M. malcolmsonii fed with H.spinosa enriched diet at 1.0 g showed significant level between weeks 1 and 4, but 0.01 g and 0.1 g diets shown low level. The respiratory burst (RB) activity and superoxide dismutase (SOD) activity significantly enhanced when prawn fed at 0.1 g and 1.0 g herbal extract enriched diets; however, it did not significantly enhanced in prawn fed at 0.01 g herbal diets during the experimental period. The phagocytic (PC) activity was significantly low when prawn fed at 0.01 g and 1.0 g diets whereas 1.0 g diet was obtained significantly enhanced. The percentage (%) of clearance efficiency (CE) was significantly enhanced in prawn fed at 0.1 g or 1.0 g diets against pathogen. The mortality was low in prawn fed at 0.1 g and 1.0 g herbal enriched diets than fed with 0.01 g diet against pathogen. This study concluded that H. spinosa extract enriched diet can positively modulate the immune system and decreased mortality in M. malcolmsonii against V. alginolyticus infection.
Keywords
Hygrophila spinosa, Immune response, Macrobrachium malcolmsonii, Supplementation diet, Vibrio alginolyticus
Introduction
Freshwater prawn farming is popular in South East Asian countries recently because of their high price and market demand in both domestic and export markets. In India, the largest species of prawns are of interest for aquaculture such as Macrobrachium rosenbergii, M. malcolmsonii, and M. gangeticum, respectively in monoculture and in polyculture with compatible carps [1,2]. Among, Indian River prawn, M. malcolmsonii, is the second largest fast-growing freshwater prawn after M. rosenbergii and one of the most economically important species in the aquaculture industry. M. rosenbergii is widely distributed throughout India, especially in the river draining into the Bay of Bengal. M. malcolmsonii culture was suffered due to viral disease such as Infectious hypodermal and hematopoietic necrosis virus (IHHNV), Macrobrachium muscle virus (MMV), Appendage deformity syndrome (ADS), Slow growth syndrome (SGS), Branchiostegal blister disease (BBD), and Idiopathic muscle necrosis (IMB); infected prawn is weakened swimming ability, reddish discoloration of cuticles, muscular atrophy, growth retardation and deformities [3]. Several bacterial disease outbreaks have been occurred such as Vibrio spp.,Aeromonas spp., Pseudomonas spp., and Lactococcus garviae which caused high mortalities in Macrobrachium hatcheries [4-11].
In decapods crustaceans, hyaline cells are generally involved in phagocytosis, which is very important process of eliminating micro-organisms or foreign particles of the host [12,13]. A series of reactive oxygen species (ROS) are produced during phagocytosis. Starting this process, the membrane-bound enzyme complex, NADPH oxidase, assembles after binding of a foreign particle to the cell, and reduces molecular oxygen to superoxide anion (O -), subsequently leading to the production of hydrogen peroxide (H2O2), singlet oxygen (1O2) and hydroxyl radicals (OH·) [14]. Superoxide anion is the first product released during this process known as respiratory burst (RB), and plays an important role in anti-bacterial activity [15]. A number of natural and herbal based immuno stimulants have been reported to enhance the innate and adaptive immune system in prawns and shrimps [5,10,11,16,17].
Hygrophila spinosa T. (Acanthaceae), commonly known as Gokulakanta /Talmakhana, is a well-known medicinal plant widely distributed throughout India, Srilanka, Burma, Malaysia, and Nepal. The leaf, root, and seed of the plant are being traditionally used for the treatment of rheumatism, inflammation, jaundice, hepatic obstruction, urinary infection, oedema, gout, diabetes, bacterial infection, etc. [18]. It was contains apigenin 7-o-glucuronide, alkaloids, flavonoids, and steroids in the chloroform extract, as well as alkaloids, flavonoids, tannins, and steroids in the alcoholic extract of the roots, leaves, and flowers of H. spinosa [19-21]. The anti-inflammatory and antipyretic potentials of alkaloids, steroids, and flavonoids have been reported [22-26]. There has been no information concerning the immune response of H. spinosa on M. malcolmsonii against diseases. Accordingly, the purpose of the present study was examining the effect of H. spinosa enriched diet on immune response and disease resistance in M. malcolmsonii against V. alginolyticus.
Material and Method
Plant extract and herbal diet preparation
H. spinosa plant was collected from locally and the identification was done by Plant Science Department. The roots were collected from the plants, washed thoroughly with tap water to rid them of dirt. After washing, the roots were dried under shade to make them suitable for grinding and the dried plant roots were grounded in a mechanical grinder. After it was sieved then stored in an air tight sterile container for further use. One hundred grams of coarsely powdered was successively extracted with 85% ethanol and then filtered. The successive extraction was performed by a cold maceration process for seven days with daily agitation twice following Cooper and Gunn [27] and Singh et al. [28]. The solvent was evaporated using a rotary vacuum evaporator (Buchi, Flawil, Switzerland). The residues obtained after evaporation were stored at -20°C until used for the experiment.
Supplementation diet
The formulated diet and the ingredients are shown in (Table 1). The ingredients of the experimental diet were well mixed and extruded by a pellet extruder (EX 920, Matador, Denmark). Four experimental diets prepared of the pellet with 0 g (control), 0.01 g, 0.1 g, and 1.0 g of H. spinosa extracts were sprayed to the basal diet slowly, mixing evenly in a drum mixer, after which it was air dried under sterile conditions for 12 hrs. The control basal diet was added the same volume of solvent without the extracts. The pellets were dried in an oven at 30°C for 18 hrs, packed, and stored in a freezer at -20°C until used. The proximate composition of the diets were quantified following AOAC method comprised 48.9% crude protein, 8.1% crude lipid, 7.1% crude ash, and 13.6% crude carbohydrate.
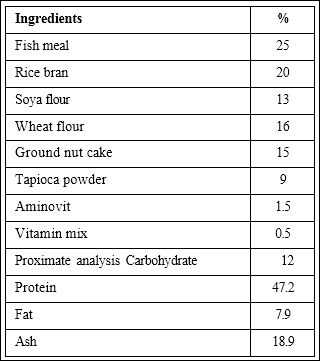
Table 1: Feed composition for prawn.
Vibrio alginolyticus
Diseased shrimps were collected from local prawn farms and the muscle was dissected out and culture on tryptic soy agar (TSA, Difco) plates incubated at 28°C for 18 hrs, subsequently they were incubated on thiosulfate-citrate-bile sucrose (TCBS, Difco). One dominant colony was selected and re-streaked onto TSA to obtain pure cultures. The biochemical characteristics were carried out with commercial API 20E Kits (ATB System, bioMerieux) and the biochemical reactions were compared with the reference strain V. alginolyticus ATCC (American Type Culture Collection). The genomic DNA was purified by DNA purification Kit (No. A1120, Promega) and the polymerase chain reaction (PCR) tests were carried out using specific PCR primers for identification of V. alginolyticus 16S rDNA according Ruimy et al. [17].
Experimental animal
M. rosenbergii were obtained from a commercial farm, reared in 500 mg-l round holding tanks for 2 weeks, and fed formulated diet (Table 1) until they were used for the experiment. The water quality such as temperature 26-32°C, water transparency 30-60 cm, pH 7.0- 8.5, dissolved oxygen > 5 mg l-1, free CO2 < 8 mg l-1, hardness 100-50 mg l-1, total alkalinity 80-150 mg l-1, NH4 +-N 0.02-0.20 mg l-1, calcium 30-80 mg l-1, phosphorus 0.01-0.90 mg l-1, and nitrogen 0.05-90.5 mg l-1 were maintained during the experiment.
Experimental design
After 2 weeks of acclimatization, prawn were divided into four groups of 25 prawns in 250 l tanks and fed with 0 g, 0.01 g, 0.1 g, and 1.0 g of H. spinosa extract supplementation diets at the rate of 10% of body weight twice a day. There were three replicate tanks per treatment were maintained. After 30 days of feeding, all groups except control were injected intraperitoneally (i.p.) the ventral sinus of the cephalothorax with 50 µl PBS containing V. alginolyticus at 3.7 x 107 cfu ml-1 whereas control group injected with same volume of PBS. On weeks 1, 2, and 4 post-infection, six prawns randomly collected from each tank to hemolymph blood samples for hematological and immunological assays.
Sample collection
Haemolymph (500 µl) was withdrawn from the ventral sinus of each prawn into a 1 ml sterile syringe (25 gauge) containing 0.9 ml anticoagulant solution (trisodium citrate 30 mM, sodium chloride 0.34 mM, EDTA 10 mM, pH 7.55, osmolality adjusted with 0.115 Mglucose to 780 mOsm kg-1). A drop of the anticoagulant-haemolymph mixture was placed on a haemocytometer to measure THC (Leica DMIL, Leica Microsystems,Wetzlar GmbH, Germany) and the remaining of the haemolymph mixture was used for immunological assays.
Prophenoloxidase (proPO) activity
The proPO activity was measured spectrophotometrically by recording the formation of dopachrome produced from L-dihydroxyphenylalanine (L-DOPA). The details of measurements were described previously [29,30]. The optical density (OD) of the prawn PO activity was expressed as dopachrome formation per 50 µl haemolymph.
Respiratory Burst (RB) activity
The respiratory burst (RB) of haemocytes was quantified using the reduction of nitroblue tetrazolium (NBT) to formazan as a measure of superoxide anion (O -) according Liu and Chen [29]. The OD at 630 nm was measured using a microplate reader (Model VERSAmax, Molecular Devices, Sunnyvale, CA, USA) and the RB was expressed as NBT-reduction per 10 µl haemolymph.
Superoxide Dismutase (SOD)
Superoxide dismutase (SOD) activity was measured by its ability to inhibit superoxide radical dependent reactions using the Ransod kit (Randox, Crumlin, UK). The details of measurements were described previously [31]. A reference standard for SOD was supplied with the Ransod kit. One unit of SOD was defined as the amount required inhibiting the rate of xanthene reduction by 50% and the specific activity was expressed as SOD unit’s ml-1 [32].
Phagocytic (PC) activity
Briefly, 200 µl of haemolymph was mixed with 200 µl of sterile anticoagulant for PC activity. The methods for the measurements of PC activity were described previously [29]. Two hundred haemocytes were counted and the PC activity, defined as percentage phagocytosis (PR) was expressed as:
PR = [(phagocytic haemocytes)/(total haemocytes)] x100
Clearance Efficiency (CE)
The CE was measured following the method of Adams [33] and the CE to V. alginolyticus, defined as percentage inhibition (PI), was calculated as:
PI=100- [(cfu in test group)/(cfu in control group)] x100
Cumulative mortality
There are twenty prawn were used in each group. All groups were used three replicate groups. The preparation of bacterial culture, challenge study, and disease were same in previous section.
Statistical analysis
Data was conducted to compare the significant differences among treatment using the SAS computer software (SAS Institute Inc., Cary, NC, USA). For statistically significant differences, it was required that P < 0.05.
Results
Hematology
The THC was significantly low when M. malcolmsonii fed with herbal enriched diet at 0.01 g and 0.1 g against V. alginolyticus. However, the THC did not significant change when prawn fed at 1.0 g herbal enriched diet against pathogen (Figure 1).
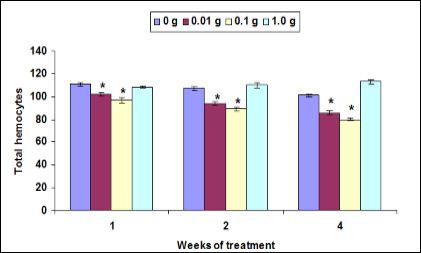
Figure 1: Changes in total haemocytes counts (THC) of M. malcolmsonii (mean ± S.E; n = 6) fed with herbal enriched diet against V. alginolyticus. Data at the same exposure time with asterisks are significantly different (P < 0.05) among treatments.
Prophenoloxidase (proPO) activity
The proPO activity significantly low when prawn feeding at 0.01 g and 0.1 g herbal enriched diets during the experimental period when compared to control against pathogen. However, prawn feeding with 1.0 g herbal enriched diet did not significantly change against pathogen from weeks 1 to 4 (Figure 2).

Figure 2: Prophenploxidase (proPO) activity M. malcolmsonii (mean ± S.E; n = 6) fed with herbal enriched diet against V. alginolyticus. Data at the same exposure time with asterisks are significantly different (P < 0.05) among treatments.
Respiratory Burst (RB) activity
The RB activity did not significantly enhanced at any time when prawn feeding with 0.01 g herbal enriched diet against pathogen. However, the RB activity significantly enhanced in prawn feeding with 0.1 g and 1.0 g herbal enriched diets against pathogen as compared to control (Figure 3).

Figure 3: Prophenploxidase (proPO) activity M. malcolmsonii (mean ± S.E; n = 6) fed with herbal enriched diet against V. alginolyticus. Data at the same exposure time with asterisks are significantly different (P < 0.05) among treatments.
Superoxide Dismutase (SOD) activity
The SOD activity significantly enhanced when prawn fed with 0.1 g diet but did not found significant at 0.01 g or 1.0 g diets against pathogen when compared to control on first week. Prawn feeding with 0.1 g and 1.0 g herbal enriched diets significantly enhanced the SOD activity on weeks 2 and 4 when compared to control against pathogen. However, the activity was not significant when fed with 0.01 g diet on weeks 2 and 4 (Figure 4).
Phagocytic (PC) activity
The PC activity significantly low in prawn fed at 0.01 g and 0.1 g herbal enriched diets from weeks 1 to 4 when compared to control against pathogen. However, prawn fed at 1.0 g diet significantly increased the enhanced the PC activity during the experiment (Figure 5).

Figure 4: Superoxide dismutase (SOD) activity of M. malcolmsonii (mean ± S.E; n = 6) fed with herbal enriched diet against V. alginolyticus. Data at the same exposure time with asterisks are significantly different (P < 0.05) among treatments.

Figure 5: Phagocytic (PC) activity of M. malcolmsonii (mean ± S.E; n = 6) fed with herbal enriched diet against V. alginolyticus. Data at the same exposure time with aster- isks are significantly different (P < 0.05) among treatments.
Clearance Efficiency (CE)
The CE did not significant change when prawn fed at 0.01 g herb- al diet on weeks 1 to 4 when compared to control against pathogen. However, the CE significantly increased when prawn fed at 0.1 g and 1.0 g herbal diets when compared to control. All diet supplementary doses of herbal extract enriched diet significantly increased the CE on second week against pathogen (Figure 6).
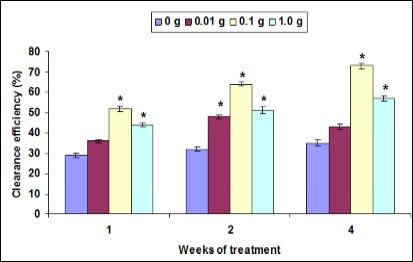
Figure 6: Clearance efficiency (%) of M. malcolmsonii (mean ± S.E; n = 6) fed with herbal enriched diet against V. alginolyticus. Data at the same exposure time with aster- isks are significantly different (P < 0.05) among treatments.
Disease resistance
The cumulative mortality was found 40% and 35% in groups feed- ing with 0.1 g and 1.0 g herbal extract enriched diet against pathogen. The mortality was found 55% in prawn feeding with 0.01 g diet against pathogen. Prawn fed with basal diet without herbal extract was found 90% mortality against pathogen (Figure 7).
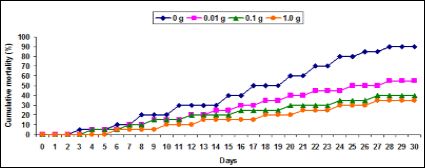
Figure 7: The percentage (%) of cumulative mortality (CM) of M. malcolmsonii fed with herbal enriched diet against V. alginolyticus for 30 days.
Discussion
In the present study, higher protection in terms of mortality was found 40% and 35% in M. malcolmsonii feeding with 0.1 g and 1.0 g H. spinosa extract enriched diets against V. alginolyticus. The mortality was found higher at 55% when prawn fed at 0.01 g diet. In a previous study indicated that in L. vannamei increased its susceptibility to V. alginolyticus infection by decrease in salinity, as well as the presence of ammonia, nitrite, and copper sulphate in the rearing water and when treated with noradrenaline against the same pathogen [29,31,34-36]. In this study prawn fed with basal diet and challenged with V. algi- nolyticus higher mortality. Pacific oyster C. gigas that had been chal- lenged with pathogen V. splendidus when subjected to mechanical stress increased the mortality [37]. C. gigas injection with noradrena- line, a key component of the neuroendocrine stress response system, also caused higher mortality [38]. Therefore, the present result was suggested that V. alginolyticus involved in the physiological changes in M. malcolmsonii.
In the present study, the THC significantly decreased when M. malcolmsonii fed with herbal enriched diet at 0.01g and 0.1g against V. alginolyticus. The circulating THC in L. vannamei displayed high- er THC and proPO activities [34,35,39,40]. In the present study, the THC did not significant change when fed at 1.0g herbal enriched diet against pathogen. The circulating THC was affected by extrin- sic factors like temperature and salinity variations, as well as nitrite and Cu2C in L. vannamei and L. stylirostris [34,35,40,41]. In this study, proPO activity significantly low when prawn feeding with 0.01 g and 0.1 g herbal enriched diets during the experimental period against V. alginolyticus. Both THC and proPO activity in M. rosenbergii were sig- nificantly higher at pH 7.5-7.7 and 30-31°C [42]. In this study, prawn feeding with 1.0 g herbal enriched diet did not significantly change against pathogen from weeks 1 to 4. Exposure of common shrimp, Crangon crangon to polychlorinated biphenyl 15 (PCB 15) resulted in significantly decreased THC and PO activity [43]. The PO activity was significantly decreased in both L. vannamei and M. rosenbergii when exposure to ammonia-N at a concentration of 0.55 mg l-1 [29,44] and nitrite-N at 9.87 mg l-1 and Cu2C 10 mg l-1 [34,35]. In the present study, prawn feeding with 1.0% herbal enriched diet significantly enhanced against pathogen, as indicating that herbal diet modulate the immune response in M. rosenbergii.
In this study, the RB activity significantly enhanced in prawn feeding with 0.1 g and 1.0 g herbal enriched diets against pathogen. The releases of superoxide anion (O2 -) and hydrogen peroxide (H2O2) were considered to play a more important role in shrimp microbicidal activity than hypochlorites (OCl-) and myeloperoxidase (MPO) [45]. In L. vannamei injection with fungicide propiconazole induced an increase of O2 at day 6, but caused a dose-dependent decrease in O2 - at day 13 [41]. It was proposed that the decreased production of O2 - in hypoxic P. stylirostris was due to the decrease of THC, and that the activity of NADPH oxidase responsible for the production of O2 - was not affected under hypoxia [40]. Similarly, L. vannamei decreased its release of O2 - when exposure of to 11.10 mg l-1 ammonia-N for 48 hrs, to 9.87 mg l-1 nitrite for 96 hrs, or to 20 mg l-1 Cu2C [29,34]. The SOD significantly enhanced when prawn fed with 0.1 g and 1.0 g herbal enriched diets on weeks 2 and 4 when compared to control against pathogen. However, the activity was not significant when fed with 0.01 g diet on weeks 2 and 4. This fact suggests that the activity of NADPH oxidase responsible for the release of O2 - decreased with decrease in the activity of SOD responsible for scavenging O2 -. The fact that the SOD activity recovered later than that of the RB suggests that the prawn received herbal enriched diet causes immunomodulation to scavenge O2 - to other reactive oxygen intermediates (ROIs) including (H2O2).
The PC activity significantly low in prawn fed at 0.01 g and 0.1 g herbal enriched diets from weeks 1 to 4 whereas fed at 1.0 g diet significantly increased during the experiment in this study. The CE significantly increased when prawn fed at 0.1 g and 1.0 g herbal diets and all diet supplementary doses of herbal extract diet on second week against pathogen. Phagocytosis is an important cellular defence mechanism, whereas CE is an important humoral defence mechanism in molluscs and crustaceans [46,47]. A significant reduction of PC activity and CE against V. alginolyticus was observed in L. vannamei following exposure to Cu2C [35] and ammonia-N [29]. A significant reduction in phagocytosis of Bacillus cereus was also observed in the C. maenas when exposure to Cd2C [48]. The PC activity and CE decreased in P. monodon against for V. harveyi following exposure to O2 - [49]. However, noradrenaline had a dose-dependent inhibitory effect on phagocytosis in C. gigas [38]. In conclusion, the present study documented that M. malcolmsonii feeding with H. spinosa extract enriched diet experienced an increase in susceptibility to V. alginolyticus. In addition H. spinosa plays an important role in immune modulation by decreasing THC, proPO activity, and PC activity, and CE in M. malcolmsonii against V. alginolyticus infection.
References
- Kanaujia DR, Mohanty AN (1996) Prospects of both mono and mixed culture of Macrobrachium malcolmsonii. Fish Chimes 15: 33-35
- Kanaujia DR, Mohanty AN, Tripathi SD (1997) Growth and production of Indian river prawn Macrobrachium malcolmsonii (H. Milne Edwards) under pond conditions. Aquaculture 154: 79-85.
- Sahoo PK (2008) Diseases in freshwater prawns and their management. In: National training course manual on seed production and grow out culture of freshwater prawns, CIFA 61-67.
- Chen SC, Lin YD, Liaw LL, Wang PC (2001) Lactococcus garvieae infection in the giant freshwater prawn Macrobrachium rosenbergii confirmed by polymerase chain reaction and 16S rDNA Dis Aquat Org 45: 45-52.
- Harikrishnan R, Jawahar S, Heo MS (2012) Effect of an Andrographis paniculata Enriched Diet on Immunomodulation of Andrographis paniculata enriched diet on Macrobrachium malcolmsonii against Lactococcus garviae. Isr J Aquac 1-5.
- Phatarpekar PV, Kenkre VD, Sreepada RA, Desai UM, Achuthankutty CT (2002) Bacterial flora associated with larval rearing of the giant freshwater prawn, Macrobrachium rosenbergii. Aquaculture 203: 279-291.
- Tonguthai K (1995) Diseases of the freshwater prawn, Macrobrachi- um rosenbergii. Aquat Ani Heal Res Ins Newslett 4: 1-4.
- Delves-Broughton J, Poupard CW (1976) Disease problems of prawns in recirculation systems in U.K. Aquaculture 7: 201-217.
- Sung HH, Hwang SF, Tasi FM (2000) Responses of giant freshwa- ter prawn (Macrobrachium rosenbergii) to challenge by two strains of Aeromonas J Invert Pathol 76: 278-284.
- Harikrishnan R, Balasundaram C, Jawahar, Heo MS (2012) Immu- nomodulatory effect of Withania somnifera supplementation diet in the giant freshwater prawn Macrobrachium rosenbergii (De Man) against Aeromonas hydrophila. Fish Shellfish Immunol 32: 94-100.
- Musthafa MS, Ali RJ, Ali RH, Mohamed MJ, War M, et (2016) Ef- fect of shilajit enriched diet on immunity, antioxidants, and disease resistance in Macrobrachium rosenbergii (de Man) against Aero- monas hydrophila. Fish Shellfish Immunol 57: 293-300.
- Johansson MW, Soderhall K (1989) Cellular immunity in crusta- ceans and the proPO system. Parasitol Today 5: 171-176.
- Soderhall K, Cerenius L, Johansson MW (1996) The prophenolox- idase activating system in invertebrates. Invertebrate Immunology 46-66.
- Munoz M, Cedeno R, Rodriguez J, Mialhe E, Bachere E, et al. (2000) Measurement of reactive oxygen intermediate production in haemocyte of the penaeid shrimp, Penaeus vannamei. Aquaculture 191: 89-107.
- Bell KL, Smith VJ (1993) In vitro superoxide production by hyaline cells of the shore crab Carcinus maenas (L.). Dev Comp Immunol 17: 211-219.
- Harikrishnan R, Balasundaram C, Heo MS (2011) Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 317: 1-15.
- Ruimy R, Breittmayer V, Elbaze P, Lafay B, Boussemart O, et al. (1994) Phylogenetic analysis and assessment of the genera Vib- rio, Photobacterium, Aeromonas, and Plesiomonas deduced from small-subunit rRNA sequences. Int J Syst Bacteriol 44: 416-426.
- Chopra RN, Nayar SL, Chopra IC (1986) Glossary of Indian Medic- inal Plants. CSIR.
- Patra A, Jha S, Murthy PN, Roy D, Sahu AN (2008) Analgesic and antimotility activities of Hygrophila spinosa Anders. Pharmacolo- gy 2: 821-828.
- Usha K, Kasturi GM, Hemlatha P (2007) Hepatoprotective effect of Hygrophila spinosa and Cassia occidentalis on carbon tetrachloride induced liver damage in experimental Indian J Cli Biochem 22: 132-135.
- Balraj P, Nagaraj S (1982) Apigenin 7-oglucuronide from the flowers of Asteracantha longifolia Nees. Indian Drugs 19: 150-152.
- Mossa JS, Tariq M, Mohsin A, Ageel AM, Al-yahya MA, et (1991) Pharmacological studies on aerial parts of Calotropis procera. Am J Cli Med 19: 223-231.
- Patra A, Jha S, Murthy PN, Aher Vaibhav D, Chattopadhyay P, et (2009) Anti-inflammatory and antipyretic activities of Hygrophila spinosa T. anders leaves (Acanthaceae). Trop J Pharmaceu Res 8: 133-137.
- Singh RK, Acharya SB, Bhattacharya SK (2000) Pharmacological activity of Elaeocarpus sphaericus. Phytother Res 14: 36-39.
- Al-said MS, Tariq M, Al-yahya MA, Rafatullah S, Ginnawi OT, et (1990) Studies on Ruta chalepensis, an ancient medicinal herb still used in traditional medicine. J Ethnopharmacol 28: 305-312.
- Mazumdar UK, Gupta M, Maiti S, Mukherjee D (1997) Antitumor activity of Hygrophila spinosa on Ehrlich ascites carcinoma and sar- coma -180 induced mice. Indian J Exp Biol 35: 473-477.
- Cooper, Gunn (2005) Tutorial in extraction method. CBS Publishers & Distributors 257-259.
- Singh R, Singh S, Kumar S, Arora S (2007) Free radical scavenging activity of acetone extract/fractions of Acacia auriculiformis Cunn. Food Chem 103: 1403-1410.
- Liu CH, Chen JC (2004) Effect of ammonia on the immune re- sponse of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus. Fish Shellfish Immunol 16: 321-334.
- Hernandez-Lopez J, Gollas-Galvan TS, Vargas-Albores F (1996) Activation of the prophenoloxidase system of the brown shrimp (Pe- naeus californiensis Holmes). Com Biochem Physiol Part C 113: 61-66.
- Wang LU, Chen JC (2005) The immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus at different salinity levels. Fish Shellfish Immunol 18: 269-278.
- Biagini G, Sala D, Zini I (1995) Diethyldithiocarbamate, a superox- ide dismutase inhibitor, counteracts the maturation of ischemic-like lesions caused by endothelin-1 intrastriatal injection. Neurosci Lett 90: 212-216.
- Adams A (1991) Response of penaeid shrimp to exposure to Vibrio species. Fish Shellfish Immunol 1: 59-70.
- Tseng IT, Chen JC (2004) The immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus under nitrite stress. Fish Shellfish Immunol 17: 325-333.
- Yeh ST, Liu CH, Chen JC (2004) Effect of copper sulfate on the im- mune response and susceptibility to Vibrio alginolyticus in the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 17: 437-446.
- Cheng W, Chieu HT, Ho MC, Chen JC (2006) Noradrenaline mod- ulates the immunity of white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 21: 11-19.
- Lacoste A, Jalabert F, Malham SK, Cueff A, Poulet SA (2001) Stress and stress-induced neuroendocrine changes increase the suscep- tibility of juvenile oyster (Crassostrea gigas) to Vibrio splendidus. Appl Environ Microbiol 67: 2304-2309.
- Lacoste A, Malham SK, Cueff A, Poulet SA (2001) Nor adrenaline modulates oyster hemocyte phagocytosis via a β-adrenergic recep- tor - cAMP signaling Gen Comp Endocrinol 122: 252-259.
- Liu CH, Yeh ST, Cheng SY, Chen JC (2004) The immune response of the white shrimp Litopenaeus vannamei and its susceptibility to Vibrio infection in relation with the moult Fish Shellfish Immu- nol 16: 151-161.
- Le Moullac G, Soyez C, Saulnier D, Ansquer D, Avarre JC, et al. (1998) Effect of hypoxic stress on the immune response and the resistance to vibriosis of the shrimp Penaeus stylirostris. Fish Shell- fish Immunol 8: 621-629.
- Le Moullac G, Haffner P (2000) Environmental factors affecting im- mune responses in Crustacea. Aquaculture 191: 121-131.
- Cheng W, Chen JC (2000) Effects of pH, temperature and salini- ty on immune parameters of the freshwater prawn Macrobrachium rosenbergii. Fish Shellfish Immunol 10: 387-391.
- Smith VJ, Johnston PA (1992) Differential haemotoxic effect of PCB congeners in the common shrimp, Crangon crangon. Com Biochem Physiol Part C 101: 641-649.
- Cheng W, Chen JC (2002) The virulence of Enterococcus to fresh- water prawn Macrobrachium rosenbergii and its immune resistance under ammonia stress. Fish Shellfish Immunol 12: 97-109.
- Song YL, Hsieh YT (1994) Immunostimulation of tiger shrimp (Pe- naeus monodon) hemocytes for generation of microbicidal sub- stances: analysis of reactive oxygen species. Dev Comp Immunol 18: 201-209.
- Ratcliffe NA, Rowley AF, Fitzgerald SW, Rhodes CP (1985) Inverte- brate immunity: basic concepts and recent Int Rev Cytol 183-350.
- Fryer SE, Bayne CJ (1989) Opsonization of yeast by the plasma of Biomphalaria glabrata (Gastropoda): a strain specific, time-depen- dent process. Parasite Immunol 11: 269-271.
- Truscott R, White KN (1990) The influence of metal and tempera- ture stress on the immune system of Funct Ecol 4: 455-461.
- Direkbusarakom S, Danayadol Y (1998) Effect of oxygen depletion on some parameters of the immune system in black tiger shrimp (Penaeus monodon). In: Flegel TW(ed). Advances in shrimp bio- technology. National Center for Genetic Engineering and Biotech- nology 147-149.
Citation: Devi G, Balasundaram C, Ramasamy H (2019) Immunostimulatory effect of Hygrophila spinosa enriched diet in freshwater prawn, Macrobrachium malcolmsonii against Vibrio alginolyticus. J Aqua Tech Deve 2: 002.
Copyright: © 2019 Devi G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


