*Corresponding Author:
Sebastião David Santos-Filho,
Biosciences Department, Universidade Federal do Rio Grande do Norte, UFRN, Campus Lagoa Nova, 59072-970, Natal, RN, Brazil
E-mail: sdavidsfilho@gmail.com
Abstract
Introduction: Researches observations show that SARS-CoV-2 is adapted to eliminate immune responses at the initial stage of in- fection. In China reviews summarized all the potential interventions for COVID-19 infection according to previous treatments of SARS and MERS. As both SARS-CoV and SARS-CoV-2 have the same receptor for virus entry, potential therapeutics to combat SARS could be used for SARS-CoV-2. The use of some drugs that were effec- tive to treat other diseases were implemented in a tentative to ame- liorate the symptoms or to reach the cure for coronavirus disease. Researchers in all world used in a randomized control experiments such medicine to discover a way to stop this pandemic.
Objective: to evaluate publications about immunology and immuno- therapy for COVID-19, and the use of drugs, to answer the question of the vaccine production and this application in future.
Methods: The search was done in PubMed, Scielo, and Cochrane Library. It was used the terms: SARS-CoV-2 or COVID-19 or coro- navirus or Immunotherapy, to obtain 57 free full texts, randomized controlled trial, for the last year, done with humans, and written in English.
Results: Those 57 free full texts obtained, 27 are about treatment, 20 are the use of drugs, 13 about the use of oral drugs, and only 7 elected the hydroxychloroquine as a good medicine to fight against the coronavirus infection.
Conclusions: As is evident from this systematic review, immunother- apy is an efficient therapeutic option intervention against COVID-19 and the main methods in this regard such as using Hydroxychlo- roquine therapy have no improved clinical outcomes in COVID-19 infected patients. We hope that a vaccine tools will be the next step to prevent this virus disease in all the World.
Keywords
Coronavirus; COVID-19; Hydroxychloroquine; Immunotherapy; SARS-CoV-2; Vaccine
Introduction
Researches observations show that SARS-CoV-2 is adapted to eliminate immune responses at the initial stage of infection. Mechanisms linked to type 1 IFN responses, immense production of cytokine and a defect in NK-cell functions. Data also suggest that evasion of the adaptive immune, as indicate by T Lymphocytes exhaustion. Scientific evidence must indicate that the Th1-type answer is the key to successful control of this pandemic disease, with the presence of specific neutralizing antibodies. Some patient’s still viral positive, while others even relapse, suggesting that full control of this agent by immune system could be difficult to induce at some patients. This fact could have an impact on the second wave of the disease. The use of a vaccine is the best way to counter this epidemic. Parameters of the vaccine efficacy will be defined to better monitor T/B cell responses of patients that it will recover and to better understand the impact on the immune responses in aging patients, including the protection of younger people. Although, the epitopes among different human virus can be identified, this could aid new design of vaccines that could protect individuals against several pandemic in the future [1].
In China reviews summarized all the potential interventions for COVID-19 infection according to previous treatments of SARS and MERS. They founded that the treatments enhance host immune response fight RNA viral infection. The immune response has shown being weak because the inadequate nutrition in some systems used as a model system as well as in humans. The nutritional status of the host has not been considered as a factor that contribute to the viral infectious diseases. They also founded that specific treatments for coronavirus were useful for the treatment of SARS and MERS. To have a complete eradication of virus infection the development of vaccines is very important [2].
As both SARS-CoV and SARS-CoV-2 have the same receptor for virus entry, potential therapeutics to combat SARS could be used for SARS-CoV-2. Monoclonal antibodies are useful due to specificity, purity, low risk contamination and safety when compared to serum therapy and preparations of Immunoglobulins to be intravenous administration. The use of protein in SARS-CoV and MERS-CoV by monoclonal antibodies encourages researchers to use them in fighting against coronavirus. These monoclonal antibody cocktails of different monoclonal antibodies that fight against different epitopes on the virus increase the virus neutralizing. The use of cytokines, still a promising target in immunotherapy for COVID-19, this specificity to IL-6 in COVID-19 is primordial for inflammatory responses. Immunotherapy show to be an efficient therapeutic option against COVID-19 and the principal methods in to obtain an improvement in clinical outcomes [3].
The use of some drugs that were effective to treat other diseases were implemented in a tentative to ameliorate the symptoms or to reach the cure for coronavirus disease. Researchers in all world used in a randomized control experiments such medicine to discover a way to stop this pandemic [1].
The objective of this work is to evaluate publications about immunology and immunotherapy for COVID-19, and the use of drugs, to answer the question of the vaccine production and this application in future.
Methodology
The search was done in PubMed (www.pubmed.com), in Scielo (https://www.scielo.br), and in Cochrane Library (https://www. cochranelibrary.com) in October, 26th, 2020. It was used the terms: SARS-CoV-2 or COVID-19 or coronavirus or Immunotherapy, to obtain free full texts, randomized controlled trial, for the last year, done with humans, and written in English. That were obtained 57articles. Only 27 works had presented any aspects about treatment or properties for coronavirus in the immune system.About those, only 20 were obtained by a randomised clinical trial study using drugs. These only 13 works covered the aspects of some orally therapy to treat the disease.Only 7 elected the hydroxychloroquine as a good medicine to fight against the coronavirus infection. See all the process in Figure 1 (flowchart).
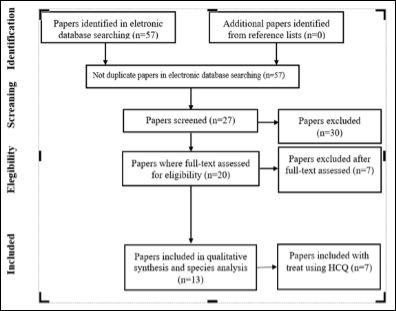
Figure 1: The way of the research flowing with the articles founded.

Table 1: Characteristics of the use of hydroxychloroquine to treat coronavirus disease.
Results
The works resulted from this research are presented in the following tables with their characteristics and conclusions. The works of Akram, Borba, Boulware, Lother, Niriella, Tang, and Vijayaraghavan, both produced in 2020, were agreed in the use of Hydroxychloroquine in the treatment of COVID-19 in association with other drugs or not. The works of Cao, Hung, and Xi Liu, both of 2020, indicate the treatment using an association of Lopinavir–Ritonavir. Coenen (2020) indicates Oseltamivir, Yeming Wang (2020) indicates Remdesivir, and Cao Y (2020) indicates Ruxolitinib, to the treatment of COVID-19. The comparison of this 7 hydroxychloroquine selected works was presented at the Table 1.
Discussion
As the works of Abram, Niriella and Vijayraghavan [4] are registered protocols, or are in preparation for publication the results, we didn’t discuss them in this article. The others [5-9] referred to the use of hydroxychloroquine alone or in association with other medicine, we presented a discussion with others works that argue with the results of the use of drugs against the coronavirus disease.
In all world researches in October, 26th, 2020, about the use of some medicine were done to discover a manner of treatment for COVID-19, one of this way is the use of immunotherapy against the virus [10].
In this review work, AminJafariand Ghasemi [11] referred to a possible immunotherapy for COVID-19 as a suitable option of treatment. Methods such as the use of immunoglobulins and plasma therapy have improved clinical outcomes in COVID-19 infected patients. The use of polyclonal antibody by plasma therapy, polypeptide hormone for maturation of T cells, immunoglobulins, ACE2 immunoadhesin and monoclonal antibody against the interleukin-6, viral-vectors, nanoparticles, inactivated whole virus, and DNA as vaccines have been described to be used for SARS-CoV that holds the promise in the future for using in this disease[12,13]. The vaccines and ACE2 immunoadhesin have not been tested yet. Zhang [14] showed that other diverse treatment was tested in China and they will be promisors for this pandemic. They suggested that all potential interventions be implemented to control COVID-19 infection if it is uncontrollable.
The use of some drugs associated or not with hydroxychloroquine were implemented in all world to treat the coronavirus disease as a way of treatment that Government accepted as effective.
Researcher proved that it seems are not effective against the coronavirus disease symptoms. Borba[15], no recommended the use of high dosages of chloroquine for clinical ill patients because this potential safety hazards in/or not associated with other drugs. Boulware[16] also no recommended the use of hydroxychloroquine because it was not effective to prevent illness compatible with COVID-19.Lother[17], although using a higher dose of hydroxychloroquine, concluded that “The clinical data are limited to small clinical trials and uncontrolled case series and cohorts’’. Thang[18,19], at his time, showed that the doses of hydroxychloroquine were not effective for a conversion the COVID-19 in patients admitted to hospitals mainly with moderate coronavirus disease.
As we can see the use of some medicine were not appropriate to treatment of coronavirus disease symptoms in acute or chronical steps of this illness. We hope that the development of a vaccine in the next days, months or year, could take care of this pandemic and immunize the population of the Earth[20].
Conclusions
As is evident from this systematic review, immunotherapy is an efficient therapeutic option intervention against COVID-19 and the main methods in this regard such as using Hydroxychloroquine therapy have no improved clinical outcomes in COVID-19 infected patients. We hope that a vaccine tools will be the next step to prevent this virus disease in all the World.
Acknowledgments
Author thanks to Biosciences Department of the UFRN for the support to this review.
References
- Guihot A, Litvinova E, Autran B, Debré P, Vieillard V (2020) Cell-Mediated immune responses to COVID-19 infection. Front Immunol 11: 1662.
- Zhang L, Liu Y (2020) Potential interventions for novel coronavirus in China: A systematic review. J Med Virol 92: 479-490.
- AminJafaria A, Ghasemi S (2020)The possible of immunotherapy for COVID-19: A systematic review. IntImmunopharmacol 83: 106455.
- Alram J, Azhar S, Shahzad M, Latif W, Khan KS (2020) Pakistan randomized and observational trial to evaluate coronavirus treatment (PROTECT) of hydroxychloroquine, oseltamivir and azithromycin to treat newly diagnosed patients with COVID-19 infection who have no comorbidities like diabetes mellitus: A structured summary of a study protocol for a randomized controlled trial. Trials 21: 702.
- Borba MG, Val FFA, Sampaio WS, Alexandre MAA, Melo GC, et al. (2020) Effect of high vs low doses of chloroquinediphosphate as adjunctive ther- apy for patients hospitalized with severe acute respiratory syndrome coro- navirus 2 (SARS-CoV-2) A randomized clinical trial. Jama Net- work Open 3: e208857.
- Boulware DR, Pullen MF, Banqdliwala AS, Pastick KA, Lofgren SM, et (2020) A randomized trial of hydroxychloroquine as postexposure prophy- laxis for Covid-19. N Engl J Med 383: 517-525.
- Lother SA, Abassi M, Agostinis A, Bangdiwala AS, Cheng MP, et (2020) Post-exposure prophylaxis of pre-emptive therapy for severe acute respi- ratory syndrome coronavirus 2 (SARS-CoV-2): Study protocol for a prag- matic randomized-controlled trial. Can J Anesth 67: 1201-1211.
- Niriella NA, Ediriweeras DS, De Silva AP, Premarathne R, Balasoori- ya P, et al. (2020) Hydroxychloroquine for post-exporure prophylaxis of COVID-19 among naval personnel in Sri Lanka: Study protocol for a ran- domized, controlled trial. Trials 21: 748.
- Tang W, Cao Z, Han M, Wang Z, Chen J, et (2020)Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ 369: m1849.
- Vijayaraghavan BKT, Jha V, Rajbhandari D, Myatra SN, John O, et (2020) Hydroxychloroquine plus personal protective equipment versus standard personal protective equipment alone for the prevention of COVID-19 in- fections among frontline healthcare workers: The HydrOxychloroquine Prophylaxis Evaluation (HOPE) trial: A structure summary of a study pro- tocol for a randomized controlled trial. Trials 21: 754.
- Jafari AA, Ghasemi S (2020) The possible of immunotherapy for COVID-19: A systematic review. Int Immuno pharmacol 83: 106455.
- Zhang L, Liu Y (2020) Potential interventions for novel coronavirus in Chi- na: A systematic review. J Med Virol 92: 479-490.
- Chen Z, John Werry E (2020) T cell responses in patients with COVID-19. Nat Rev Immunol 20: 529-536.
- Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, et (2020) Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vac- cine in healthy adults aged 18 years or older: A randomised, double-blind, placebo controlled, phase 2 trial. Lancet 396: 479-488.
- Gustafson CE, Kim C, Weyand CM, Goronzy JJ (2020) Influence of im- mune aging on vaccine J Allergy ClinImmunol 145: 1309-1321.
- Briguglio M, Pregliasco FE, Lombardi G, Perazzo P, Banfi G (2020) The malnutritional status of the host as a virulence fator for now coronavirus SARS-CoV-2. Front Med (Lausanne) 7: 146.
- McGonagle D, Sharif K, Oregan A, Bridegewood C (2020) The role of cyto- kines including interleukin-6 in COVID-19 induced pneumonia and mac- rophage activation syndrome-like disease. Autoimmun Rev 19: 102537.
- Costela-Ruiz V, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Mel- guizo-Rodriguez L (2020) SARS-CoV-2 infection: The role of cytokines I n COVID-19 disease. Cytokine Growth Factor Rev 54: 62-75.
- Grifoni E, Valoriani A, Cei F, Lamanna R, Gelli AMG, et (2020)Interleu- kin-6 as prognosticator in patients with COVID-19. J Infect 81: 452-482.
- Florindo HF, Kleiner R, Vaskovich-Koubi D, Acúrcio RC, Carreira B, et al. (2020)Immune-mediated approaches against COVID-19. Nat Nanotech- nol 15: 630-645.
Citation: Santos-Filho SD (2020) Immunology and Immunotherapy of Coronavirus: The Science RealityJ Immuno Immunothe 3: 008.
Copyright: © 2020 Santos-Filho SD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.


