*Corresponding Author:
Harikrishnan Ramasamy,
Department of Zoology, Pachai- yappa’s College for Men, Tamil Nadu, India
Tel: +91-4362227937
E-mail: rhari123@ yahoo.com
Abstract
Dietary administration of Rhizophora mucronata (RM) enhance the growth response, innate immunity for example the activity of lysozyme (LYZ), alternative complement (ACP), respiratory burst (RB), and phagocytosis (PC), and the protective mechanisms like survival rate and mortality; antioxidant activities such as catalase (CAT), glutathione (GSH), malondialdehyde (MDA), nitric oxide (NO), hydrogen peroxide (H2O2), and glutathione peroxidase (GPx) are reported in clownfish, Amphiprion sebae against Vibrio alginolyticus. There are five experimental groups: (1) uninfected or (2) infected groups fed basal control diet without RM extract; infected or challenged fish fed at (3) 1 g kg-1, (4) 5 g kg-1, and (5) 10 g kg-1 RM extracts enriched diets used in this study. After dietary administration with 5 and 10 g kg-1 of RM extract the weight gain (WG), feed conversion ratio (FCR), and growth rate (SGR) increased significantly on weeks 4 and 8. The LYZ, RB, ACP, and PC activities increased significantly in infected fish treated at 5 and 10 g kg-1 RM extract diets on weeks 4 and 8. A high rate survival or cumulative mortality was like 80% and 85% (20% and 15%) were found in the infected fish treated at 10 and 5 g kg-1 diets, although the survival rate was 15% and 70% with 1 g kg-1 or 0 (control) diets. This study confirm that 5 or 10 g kg-1 of RM extract as dietary supplementation provide positive growth, boost the antioxidant and non-specific immune response in A. sebae to V. alginolyticus infection.
Keywords
Disease resistance, Innate immune parameter, Mortality, Rhizophora mucronata, Vibrio alginolyticus
Introduction
In 2008, the ornamental fish industry was export and import valued were US$337 and US$287 million. Among the various marine ornamental species, the fishes have a tremendous economic potential with a trade value of US$43.8 million in 2006 [1]. Among, Amphiprion sebae, also known as the sebae clownfish originate from the northern Indian Ocean of Java to the Arabian Peninsula in association with sea anemones. This ornamental fish hit high market price and valuable foreign exchange than most other pomacentrids in the marine aquarium fish trade. Recently, the ornamental fish industry has been hit by out breaks of various diseases; among these, the most common infections are caused by Gram-negative organisms and are challenging in aquaculture production [1]. Antibiotics traditionally used to control or reduced the growth of bacteria long enough for the immune system. However, the use of antibiotics and chemotherapeutics under intensive aquaculture as prophylaxis and treatment has been criticized negative effects such as tissue residues, drug resistance pathogens, and immunosuppression [2], besides the adverse side effects on the host [3]. Alternatively, for some diseases against specific pathogens, vaccines have been provided with varying degrees of accomplishment. The prevalence of a wide range of pathogens in fish farms also restrictions the efficacy of vaccines [4]. Hence, to promote sustainable fish culture there is a serious necessity to look into novel and eco-friendly disease defensive measures. Due to these reasons, in recent years, considerable attention has been attentive to find out alternatives, such as herbal and new traditional therapies in fish disease management.
Innate (non-specific) immune response in fish is considered initial defense mechanisms against pathogens. Additionally, pollutants are adversely affects the fish ability to protect them against pathogens. In this line, the use of herbal medicines, as immunostimulants in aquaculture has expected increasing attention last few decades, not only as immunomodulators but also enhancing the growth circumventing the side effects of traditional chemotherapy [5]. Herbal immunostimulants enhance immunity and decrease mortality against parasitic, bacterial, viral, and fungal infections in fishes [5-7]; herbs have the potential to enhance the proliferation of leukocyte cells and trigger phagocytic activity which is the most significant cellular mechanisms of the innate immune system of fish [8]. The phagocytic activity is a primeval defense mechanism and constitutes an essential characteristic of the non-specific immune system [5].
In mangrove ecosystem, Rhizophora mucronata is one of the common trees. Phytochemical analyses of the plant bark and other parts of this tree reveal content of alkaloids, hydrolysable tannins, terpenoids, sterols, saponins, flavonoids, proteins, anthocyanidins, carbohydrates, carotenoids, gibberellins, inositols, lipids, minerals, polysaccharides, polyphenols, procyanidins, and phenol acid. These bioactive compounds exhibit anti-viral, anti-bacterial, anti-inflammatory, anti-oxidant, activities, boot immune system, etc. R. mucronata has been used in the treatment of heamaturia, diabetes, diarrhea and has anti-HIV activity. Further, it presents the following propertics astringent, anti-oxidant, anti-septic, hemostatic with anti-ulcerogenic, hepatoprotective, anti-bacterial, and anti-inflammatory activities [9-15].
Clownfish, Amphiprion sebae has been affected by bacterial disease like Vibrio alginolyticus leading to mass mortality [16,17]. Till date, there was no data available on the effect of dietary administration of R.mucronata on growth response, antioxidant activity, immune response, and disease resistance in aquatic species. Hence, the present study investigated first time on the effect of dietary augmentation of R.mucronata on growth response, non-specific response, antioxidant activity, and disease control in A. sebae to V. alginolyticus.
Material and Methods
Preparation of plant extraction
Rhizophora mucronata (RM) leaf were collected around the Vellar estuary mangroves, Parangipettai, Tamil Nadu, India (Latitude. 11° 29’N. Longtitude. 79° 46’E). The leaves were cleaned with double distilled water and shadow desiccated for one week. The dried leaf were powdered in a automatic grinder, separated, and then preserved in an air tight vessel until used. A 100 g of dried leaf powdered sample were consecutively excavated with 85% ethanol. Then, it was filtered and performed by a cold marinating process with agitation twice a day for a period of seven days. The ethanol solvent was vaporized by a rotary vacuum evaporator (Buchi, Flawil, Switzerland). The residues were collected and stored at -20 °C until used for feed formulation.
Vibrio alginolyticus
Vibrio alginolyticus (AUMOFP2) strain was isolated from infected Amphiprion sebae and maintained on Zobell Marine Agar (ZMA, Himedia, Mumbai) in slopes at 5 ºC [18]. The pathogenesis of V. alginolyticus was regularly confirmed by inoculation into A. sebae. The stock culture was preserved on Zobell Marine Broth (ZMB, Himedia, Mumbai) at -70 ºC in 0.85% NaCl with 20% glycerol (v/v) which affords constant inoculate throughout the experiment. For challenge study, V. alginolyticus culture was centrifuged at 1000 x g for 10 min at 4 ºC and then supernatant removed. The bacterial pellet was sterilized with phosphate-buffered saline (PBS) at pH 7.4 three times and re-suspended with PBS. The optical density (O.D.) of the bacterial solution was adjusted to 0.5 at 600 nm which is equal to 2.7 x 107 cells ml-1. The identification of V. alginolyticus was done by growing onto Tiosulphate Citrate Bilesalt Sucrose (TCBS, DifcoTM) agar plates, selective to vibrionaceae for 24 h at 30 °C and then isolated on Tryptone Soy Agar (TSA) to obtain a pure culture for identification using API 20E kit (Biomeriux®). The morphological features of bacteria and biochemical tests were confirmed by Bergey’s manual of systematic bacteriology [19].
Formulated diet preparation
The formulated diets and the proximate composition of the basal control diet were described in Table 1. All ingredients were evenly mixed with oil and then added required water until rigid dough, and then extruded through a pellet extruder. From the basal diet, there are four experimental diets were prepared namely: (i) 0g (control basal diet without RM extract), and dietary enriched at (ii) 1, (iii) 5, and (iv) 10 g kg-1 of RM extracts into the basal diet. The diets were shadow-dried, broken up, sieved into pellets, and stored in plastic bags at 4 °C until use. The control basal diet was added with the same volume of ethanol solvent without RM extract.

Table I: Dietary ingredients of the formulated fish feed for Amphiprion sebae.
Experimental fish
Clownfish (22.6 ± 2.3 g in weight) both sex have been purchased from Centre of Advanced Study in Marine Biology fish hatchery, Annamalai University of Parangipettai, India. The fish health were immediately check and stocked in 5000 l cement tanks containing 2500 l of UV treated seawater and provided aeration by an air stone throughout the experiment. Fish were acclimatization two weeks before experiment commencement. Fish were provided ad libitum twice daily at 5% formulated basal control diet (Table 1) at 09:00 h and 16:00 h. About 70% of water was exchanged once in two days during the experimental period. During the acclimatization period was observed no clinical signs. The water quality parameters such as temperature 28.8 oC ± 1.6, pH 8.3 ± 0.6, salinity 28.7 ± 1.8 ppt, dissolved oxygen concentration 5.7 ± 0.6 mg l-1, and photoperiod 14 h light : 10 h dark were recorded during the experiment. The fecal matter and unfed feed were tapped out daily to reduce ammonia content in water.
Experimental design and challenge study
Fish were randomly distributed into five groups of 25 fish each in three replicate (5 x 25 x 3 = 375) such as: (1) uninfected or (2) infected groups fed basal control diet without RM extract; infected or challenged fish fed at (3) 1 g kg-1, (4) 5 g kg-1, and (5) 10 g kg-1 RM extract enriched diets. After two weeks post-supplementation feeding, all groups except control group, were injected intramuscularly (i.m.) with 0.1 ml PBS containing V. alginolyticus (2.7 x 107 cells ml-1) whereas the control group were received the same volume of PBS alone. The LD50 of the pathogen was accomplished from our earlier study [18]. The use of goldfish for this study was approved by an official Ethics Committee of Annamalai University, Tamil Nadu, India.
Growth performance
Fish were collected from each experimental tank at the end of weeks 1, 2, 4, and 8 and weighed out individually. The growth response and survival were calculated using the following formula: specific growth rate (SGR) = 100 x (Final weight Initial weight) / (Total duration of experiment); feed conversion ratio (FCR) = Feed given (dried weight) / Weight gain (wet weight); survival rate (%) = (Total number of fish in experimental group) / (Total number of fish in control group) x 100.
Preparation of samples
Six fish from each tank were randomly sampled at 1, 2, 4, and 8 weeks of post-supplementation feeding. About 0.5 ml of blood was collected from each fish through caudal vein puncturing using a 1 ml syringe with 23-gauge needle after euthanized buffered tricaine (200 mg l-1 tricaine + 400 mg l-1 sodium bicarbonate) and equally divided into sterile microtubes (Eppendorf) containing with or without anticoagulant. The samples without anticoagulant were allowed to clot for 1 h at room temperature (RT) and than incubated 4 h at 4 °C. Then, it was centrifuged at 1500 x g for 5 min at 4 °C and collected serum to preserve at -20 °C until used for immunological tests.
Hematology
The blood samples with anticoagulant were diluted and incubated 5 min with Hayem’s fluid for complete haemolysis of white blood cells (WBCs) then loaded on the improved Neubauer haemocytometer by holding the pipette at 45o angle and the cover slip was placed. The blood cells were calculated and conveyed as cells ml-1.
Preparation of leukocytes
The leukocytes were separated from the peripheral blood according to MacArtain et al. [20]. The blood samples were mixed with 2 ml of Roswell Park Memorial Institute (RPMI) 1640 medium (RPMI 1640, Gibthai) and then prudently placed onto 3 ml of Histopaque (Sigma) in a sterile 15 ml tube. After, the tubes were centrifuged at 400 x g for 30 min at RT, then the white buffy coat of leukocytes cells floated on the top of the Histopaque. Impervious interfaces were discreetly extracted with a Pasteur pipette and transferred into a new sterile clean 15 ml tubes and added PBS in each tube to attain 6 ml then gently mixed by aspiration. Then, it was centrifuged three times at 250 x g for 10 min to remove any residual of Histopaque. The isolated leukocytes cells were then re-suspended with PBS and adjusted to the required cell numbers for phagocytic and respiratory burst activities.
Immunological assays
The lysozyme (LYZ) activity was examined by Parry et al. [21] and the phagocytic (PC) activity assay was studied after improved from Yoshida and Kitao [22]. The phagocytic index (PI) was calculated as: Average number of beads per cell divided by number of phagocytizing cells. The respiratory burst (RB) activity is estimated in the peripheral blood leucocytes by the improved method of Secombes [23]. The alternative complement pathway (ACP) and bactericidal activity were investigated by Yanno [24] and Kampen [25].
Antioxidant parameters
Liver and gills were dissected out and immediately washed in icecold saline. About 200 mg of liver and gills were taken in each fish and add cold PBS (pH 7.4) for grounded in glass homogenizer (pellet pestle motor). The homogenate samples were then centrifuged at 4000 x g for 15 min at 4 °C. The obtained supernatant samples were aliquot and preserved at -80 °C for oxidative stress and antioxidant enzyme study. Serun total antioxidant capacity (TAC) was estimated by the following method of Koracevic et al. [26] which accomplished by the reaction of antioxidants in the samples with a newly prepared exogenous H2O2 residual measured calorimetric by enzymatic reaction. The glutathione peroxidase (GPx), malondialdehyde (MDA), and nitric oxide (NO) levels were determined in the tissue homogenate by spectrophotometric which enunciated as U/g tissue, nmol/g tissue, and µmol l-1. Catalase (CAT) level was measured by the decrease of H2O2 concentration at 240 nm [27]. The reduced glutathione (GSH) is analysis by Beutler et al. [28] and the malondialdehyde (MDA) concentration (nmol ml-1 of extract) measured by Ohkawa et al. [29]. The hydrogen peroxide (H2O2) is analysis by Aebi [27].
Cumulative mortality
The bacteria culture, cell count, challenge study were same mentioned previously. To conduct the cumulative mortality, a group of 20 fish in each experimental group was injected i.m. with 0.1 ml of PBS containing V. alginolyticus (2.7 x 107 cells ml-1) except control group. The control group received 0.1 ml of PBS alone to each fish. The post-survival rate was calculated for 30 days of injection.
Statistical analysis
The obtained results in this study were subjected to one-way analysis of variance (ANOVA) using the SPSS software (Windows version 17.0). Differences between means of control and experimental groups were calculated by Duncan’s Multiple-Range Test and effects with a probability of P < 0.05 were considered significant.
Results
Total white blood cell (WBC) counts
The WBCs did not increase significantly in infected fish feeding with any RM enriched diet on weeks 1 and 2 when compared with control. On the other hand, it was statically significant when infected fish were feeding with 5 or 10 g kg-1 RM diets between weeks 4 and 8, but not with 1 g kg-1 diet at any time (Figure1).

Figure 1: White blood cell (WBC) counts in Amphiprion sebae (n = 6, mean ± S.D.) fed diets containing 0g (control); 1, 5, and 10 g kg-1 of R. mucronata against V. alginolyticus. Statistical differences (P < 0.05) between control and treatment groups are indicated by asterisks. C: control, I: infected.
Growth
The MWG and SGR did not statically significant in infected fish when feeding with any RM diet between weeks 1 and 2 whereas it significantly augmented with 5 and 10 g kg-1 RM enriched diet on weeks 4 and 8. In contrast, the FCR progressively increased in infected fish feeding with all diets from weeks 1 to 4. But it was statistically significant with only 5 and 10 g kg-1 RM diets as compared with control on weeks 4 and 8 (Table 2).
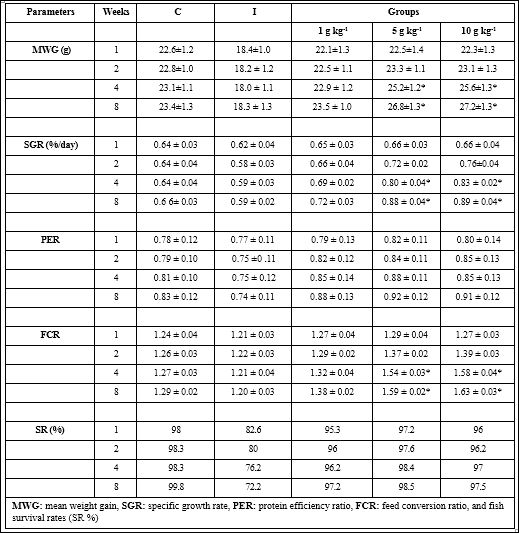
Table 2: Nutritional parameters of Amphiprion sebae fed with 0, 1, 5, and 10 g kg-1 R. mucronata supplementation diets.
Immune protection
The PC activity did not significant when infected fish feeding with any RM enriched diet on weeks 1 and 2, except with 5 g kg-1 diet where the increase manifested only after fourth week. However, the PC activity statistically significant with 5 and 10 g kg-1 RM diets on weeks 4 and 8 as compared with control diet, but it did not with 1 g kg-1 diet (Figure 2).

Figure 2: The phagocytic index in Amphiprion sebae (n = 6, mean ± S.D.) fed diets containing 0g (control); 1, 5, and 10 g kg-1 of R. mucronata against V. alginolyticus. Statistical differences (P < 0.05) between control and treatment groups are indicated by asterisks. C: control, I: infected.
The RB activity did not statistically enhance on weeks 1 and 2 in the infected fish feeding with any RM diet except with 5 g kg-1 diet on second week. Likewise, dietary administration of 5 and 10 g kg-1 diets significantly increased the RB activity on weeks 4 and 8, but not with 1 g kg-1 diet (Figure 3).

Figure 3: The respiratory burst (RB) activity in Amphiprion sebae (n = 6, mean ± S.D.) fed diets containing 0g (control); 1, 5, and 10 g kg-1 of R. mucronata against V. alginolyticus. Statistical differences (P < 0.05) between control and treatment groups are indicated by asterisks. C: control, I: infected.
The ACP did not increase statistically with any RM enriched diet on weeks 1 and 2, but it was statistically significant with 5 and 10 g kg-1 RM diets (Figure 4).
The LYZ activity did not increase significantly between weeks 1 and 2 in the infected fish feeding with any RM diet except with 5 g kg-1 diet on second week when compared to control. On the other hand, all the enriched diets significantly increased the LYZ activity between weeks 4 and 8 as compared to control diet (Figure 5).
Bactericidal activity
The bactericidal activity did not statistically high with any RM diet from weeks 1 to 2, but did not shown with 1 g kg-1 diet, while with any RM diet the bactericidal activity was found significantly better on weeks 4 and 8 in related to control diet (Figure 6).

Figure 4: The alternate complement (ACP) activity in Amphiprion sebae (n = 6, mean ± S.D.) fed diets containing 0 g (control); 1, 5, and 10 g kg-1 of R. mucronata against V. alginolyticus. Statistical differences (P < 0.05) between control and treatment groups are indicated by asterisks. C: control, I: infected.

Figure 5: The lysozyme (LYZ) activity in Amphiprion sebae (n = 6, mean ± S.D.) fed diets containing 0 g (control); 1, 5, and 10 g kg-1 of R. mucronata against V. alginolyticus. Statistical differences (P < 0.05) between control and treatment groups are indicated by asterisks. C: control, I: infected.
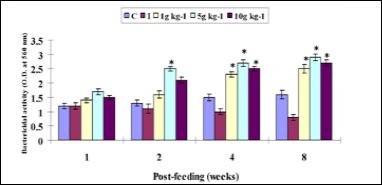
Figure 6: Bactericidal activity in Amphiprion sebae (n = 6, mean ± S.D.) fed diets containing 0g (control); 1, 5, and 10 g kg-1 of R. mucronata against V. alginolyticus. Statistical differences (P < 0.05) between control and treatment groups are indicated by asterisks. C: control, I: infected.
Antioxidant activity
The TAC showed no significant difference between treatment and control on first week. However, it was enhanced with 5 and 10 g kg-1 RM diets, but did not shown with 1 g kg-1 diet from weeks 2 to 4. On the other hand, all RM diets significantly high the TAC on week 8 (Figure 7).
The NO activity in the liver and gills did not get significantly enhanced with any diet on weeks 1 and 2; but with 5 and 10 g kg-1 diets it increased significantly on week 4 and all supplementation diet significantly increase on week 8 (Table 3). The CAT activity level in the liver and gills was moderately or did not significantly increased with all RM diet as compared with control diet (Table 3). The GSH and GPx levels did not increase significantly with any diet on weeks 1 and 2 whereas it was significantly enhanced with 5 and 10 g kg-1 RM diets on weeks 4 or 8 (Table 3). The MDA and H2O2 activities did not increased on first week with any RM enriched diet. However, it was significantly enhanced with all RM enriched diets on weeks 2, 4, and 8 in related to control diet (Table 3).
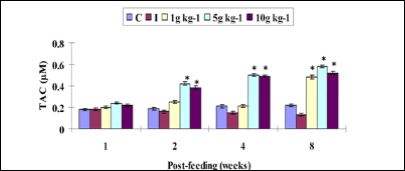
Figure 7: Total antioxidant capacity (TAC) activity in serum of Amphiprion sebae (n = 6, mean ± S.D.) fed diets containing 0g (control); 1, 5, and 10 g kg-1 of R. mucronata against V. alginolyticus. Statistical differences (P < 0.05) between control and treatment groups are indicated by asterisks. C: control, I: infected.
Survival rate and cumulative mortality
A high survival percentage was observed in the infected fish feeding with 5 and 10 g kg-1 RM enriched diets, i.e. 85% and 80%, respectively. It came down to 70% with 1 g kg-1 diet. However, the survival rate was found 15% when infected fish after feeding with basal control diet (Figure 8).

Figure 8: The survival rate in Amphiprion sebae (n = 6, mean ± S.D.) fed diets containing 0g (control); 1, 5, and 10 g kg-1 of R. mucronata against V. alginolyticus. Statistical differences (P < 0.05) between control and treatment groups are indicated by asterisks. C: control, I: infected.
Similarly, the cumulative mortality was found 15% and 20% in the infected groups were feeding with 5 and 10 g kg-1 diets. It was shown 85% mortality in the infected un-treated group fed with control diet, while there was no mortality in un-infected group fed with basal control diet (Figure 9).

Figure 9: The cumulative mortality of Amphiprion sebae (n = 20) fed diets containing 0g (control); 1, 5, and 10 g kg-1 of R. mucronata against V. alginolyticus for 30 days. C: control, I: infected.
Discussion
Aquaculture is one of the fast expanding income generating ventures providing many job opportunities in government and non-government sectors worldwide. Intensive aquaculture farms sometimes suffer sudden mass mortality of the cultivated organisms due to various adverse environmental factors and biological agents including microorganisms.
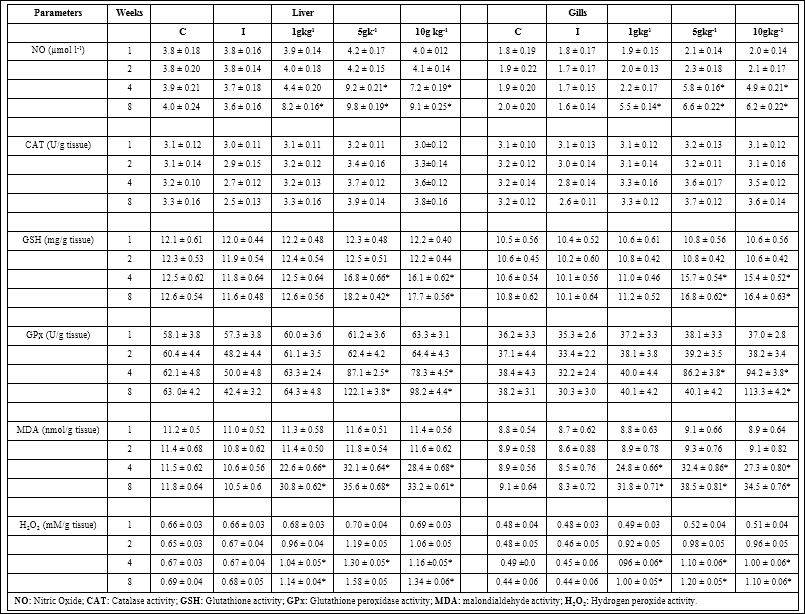
Table 3: Antioxidant activity in Amphiprion sebae fed diets containing 0, 1, 5, and 10 g kg-1 R. mucronata supplementation diets (n = 6, mean ± S.D.) against V. alginolyticus. C: control, I: infecred.
Traditional control measures were used with antibiotics and chemotherapy of fish diseases management creates resistant bacterial strains and aggravates immunosuppression in the host [2]. Very recently, herbal immunostimulants have been considered a promising option for fish disease management in aquaculture [2,5,7]. The dietary inclusion of herbs can modulate disease resistance by triggering up-regulation of the non-specific or specific immune defense mechanisms of the host against pathogens. In this regard, the non-specific immune defense mechanism is very important to boost immune system because aquatic animals are continually vulnerable to several opportunistic pathogens [8].
The WBCs gives protection in fish against infections and chemo toxicants [30]. This study, the WBC level slightly increased, but not statistically in the infected fish after dietary administration of any RM diet on weeks 1 and 2; on the other hand, 5 and 10 g kg-1 RM diets increased significantly on weeks 4 and 8. Observing these results and collecting information on the profile of leucocytes can reveal on the overall immune prominence of fish. However, the poor relationship between differential composition of leucocytes and increase in the percentage of neutrophiles in fish is correlated with viral or other infections [31].
The present study indicate that administration of 5 or 10 g kg-1 enriched diet could better growth rate and feed effectiveness than with 1g kg-1 RM diet; it appears that the effect of R. mucronata is dose independent. Further research is needed to address this issue. In fish, the non-specific defense system is most effective than the specific defense system, since the later required a longer time for antibody development and specific cellular stimulations [32].
In fish the phagocytes are regard a most significant constituent in the non-specific defense system which play an essential roles in both the initiation and regulation of immunity like in other vertebrates [33]. Further, it was early stage of fish innate immune response and plays an vital role in the non-specific defence mechanism of vertebrates against pathogens [34,35]; it establishes the key characteristic of innate immunity and first-line cellular defence mechanism. A number of immunostimulants are known to escalation the phagocytic activity in fish [36,37]. The present study indicate that the phagocytic activity did not statistically significant when infected fish were feeding with any enriched diet on weeks 1 and 2, but it was significant on weeks 4 and 8 when feeding with 5 or 10 g kg-1 RM enriched diets.
RB an key indicator of innate defence mechanism in fish has been extensively used to assess the defence ability to pathogens [38,39]. The respiratory burst participates in the degradation of internalized microorganisms during phagocytosis, which is extensively used to estimate the host defence against pathogens. The respiratory burst activity did not statistically increase on 1 and 2 weeks with any RM enriched diet, but with 5 and 10 g kg-1 RM diets it significantly better on weeks 4 and 8. This outcome is in contrast with earlier studies [6,7,40], which found that this activity was regulated by herbal supplementation diets against pathogens. Our results show that this activity did not increase with administration of R. mucronata, but no synergistic effect was observed.
Complement is a another key humoral constituent of the innate defence responses, which plays an critical role in alerting the host immunity in the existence of potential pathogens as well as in their clearance; it was possessed of more than 35 soluble plasma proteins that play key roles in innate and acquired immunity [41]. The complement is triggered by one or a combination of three pathways, i.e. alternative, lectin, and classical. Unlike in mammals, the complement in teleosts is active at very low temperatures; in several fish species the alternative complement pathway titers are relatively high and the ability to facilitate the lysis of target erythrocytes as suggesting their better capability to distinguish a wider range of foreign surfaces. Furthermore, the complement activity in fish is inclined by external factors including nutrition.
This study indicate that the infected fish dietary administration with 5 and 10 g kg-1 RM enriched diets significantly enhanced the complement activity on weeks 4 and 8 but it did not with any RM enriched diet between weeks 1 and 2. The oral administration of individual or mixed herbal extract enriched diets can modulate ACP in fish to pathogens [32,42,43]. However, some of the herbal extract supplemented diets did not enhance the complement activity during the initial phases of experiment (weeks 1 to 2). The increasing some immunological parameters following the administration of RM diets did not relate with an enhancement in disease resistance. Moreover, the mortality in herbal-treated group was significantly higher than that of infected untreated group. The exact mechanism through which herbal extract exert their immunostimulant effects have not understood in fish.
Lysozyme is one very essential non-specific immune factor in fish that arising from neutrophils and macrophages. Lysozyme is secreted into blood and mucus for bacteriolytic effects [44]. Lysozyme is an cationic enzyme that degrade the peptidoglycan of bacterial cell walls which enables the lysozyme to lyse certain Gram-positive bacteria, and in conjunction with complement, even some Gram-negative bacteria. In this study, the infected fish feeding with 5 or 10 g kg-1 RM enriched diet statistically improved the LYZ activity between weeks 4 and 8 but it did not on weeks 1 and 2. Previous studies indicate that the lysozyme activity is positively correlated with the administration of mono or triherbal extract enriched diets in grouper and olive flounder against diseases [7,40,45].
The bactericidal activity was significantly boosted in this study in fish feeding with any RM enriched diet from 4 to 8 weeks. In fish exposed to arsenic had reported higher bacterial load along with decreased bacterial clearance reported in Clarias batrachus against A. hydrophila [46]. Together with these results suggested that the arsenic exposure can compromises the host defense and decreases the ability to clearing of pathogen loading with the consequently increase in the susceptibility to pathogenic infection [47,48].
The antioxidant enzymes are very essential key elements in the animal defense to oxidative stress made by xenobiotic [49]. In the present study, there was no significant difference in the TAC on first week while it was significantly enhanced with 5 and 10 g kg-1 RM enriched diets from weeks 2 to 8. A previous investigation on analyzing the effects of chemicals on zebrafish found a reduction of antioxidant competence [50]. In the liver and gills the NO activity was significantly enhanced with 5 and 10 g kg-1 RM enriched diets on week 4 while all enriched diets the increase manifested on week 8. This present results are in line with Saha et al. [51]; a similar result was found by Chakraborty et al. [52] who had reported a significant enhancement in NO generation in the gills of Lamellidens marginalis after exposure to arsenic.
The CAT activity level in the liver and gills increased slightly with all supplementation diets during the experimental period. These results are in line with Aruljothi [53] who have found a down-regulation in CAT level in the brain and gill tissues of L. rohita exposed to arsenic. On the contrary, in zebrafish the CAT levels had no significant change when exposed to this chemical [54].
The GSH and GPx significantly enhanced with 5 and 10 g kg-1 RM diets on 4 and 8 weeks. These results agree with Aruljothi [53] who had found a decrease of GSH and GPx levels in gill and brain tissues of L. rohita exposed to arsenic. A similar result was obtained in a previous work in goldfish [55]. GSH content may showed both increases and declines trends in fish tissues exposed to chemical due to their organ-specific responses; whereas, decreasing of GPx activity indicate that direct effects of metal ions on the active site of enzyme molecules [56]. The MDH and H2O2 activities were significantly enhanced with all diet on weeks 2 to 8. LPO regards a biomarker for heavy metal toxicant or microbial infections, causing cell membrane structure damage or function with consequent imbalance between synthesis and degradation of enzyme protein [57,58]. Our results had shown a higher level of gills and liver MDA and H2O2 in treated groups coincided with previous study showed that lead exposure to induction of LPO in the kidney, liver, and gills [59]. The LPO had been reported to elevate in gills and brain tissues of L. rohita after exposure arsenic [53]. In the present study, H2O2 level was found a similar pattern to the MDA level, increasing in fish feeding with herbal diet compared to control diet. These results was confirm our present results; CAT enzyme cata-
lyzes the removal of H2O2 with production of H2O and O2 [60,61]; in this study a decreasing level of CAT would have prevented the formation of radical intermediates into water and oxygen. It was probable through above results; chemical exposure can cause modifications in the innate immune response and antioxidant defense system in fish [62].
The aggregate mortality was 15% and 20% in the infected groups that were feeding with 5 and 10 g kg-1 RM diets but it was 85% in the infected un-treated group feeding with control diet. This study was confirm the infected fish treated with R. mucronata extract enriched diet at 5 and 10 g kg-1 dose can improved immune status and antioxidant ability to V. alginolyticus after 4th week. To our knowledge, this is the first investigation showing that RM extract can be used as a feed additive in fish, to enhance the growth parameter, immune system, and decrease the mortality. The detailed mechanism of action for enhanced growth response and involvement of the immune system with R. mucronata extract remains unclear. Our results have revealed that the potential of R. mucronata as an immunostimulant for fish, although further extensive research concerning the consequences and effects on immune system stimulating against pathogens, especially its protective ability against specific diseases. Further detailed immunology and molecular research works are needed to address the relation between growth performance and involvement of the immune system in other fish against diseases.
Acknowledgement
Authors thank Prof. T. Balasubramanian, Former Dean, Faculty of Marine Science and the Annamalai University for providing facilities. First and second authors are thankful to the University Grants Commission (UGC), New Delhi for providing the financial assistance. Reference No. U.G.C. No. 33-384/2007 (SR).
References
- Choudhury S Sree1 A, Mukherjee SC, Pattnaik P, Bapuji M (2005) In vitro Antibacterial Activity of extracts of selected marine algae and mangroves against fish pathogens. Asian Fisheries Sci 18: 285-294.
- Harikrishnan R, Balasundaram C (2005) Modern trends in Aeromonas hydrophila disease management with fish. Reviews in Fisheries Sci 13: 281-320.
- Sugahara KE, Fujiwara-Nagata E, Eguchi M (2010) Dynamics of the bacterial cold-water diseases pathogen, Flavobacterium psychrophilum, in infected fish organs and rearing water after warmed water treatment. Fish Pathol 45: 58-65.
- Robertsen B (1999) Modulation of the non-specific defence of fish by structurally conserved microbial polymers. Fish Shellfish Immunol 9: 269-290.
- Harikrishnan R, Balasundaram C, Heo MS (2011a) Impact of plant products on innate and adaptive immune system of cultured finfish and Aquaculture 317: 1-15.
- Harikrishnan R, Kim MC, Kim JS, Balasundaram C, Heo MS (2012) Effect of Coriolus versicolor supplementation diet on innate immune response and disease resistance in kelp grouper Epinephelus bruneus against Listonella anguillarum. Fish Shellfish Immunol 32: 339-344.
- Wu CC, Liu CH, Chang YP, Hsieh L (2010) Effects of hot-water extract of Toonasin ensis on immune response and resistance to Aeromonas hydrophila in Oreochromis mossambicus. Fish Shellfish Immunol 29: 258-263.
- Magnadottir B (2010) Immunological control of fish Marine Biotechnology 12: 361-379.
- Bandaranayake WM (2001) Bioactivities, bioactive compounds and chemical constituents of mangrove plants.Wetl Ecol Manag 10: 421-452.
- Das AK, Rohini R, Hema A (2009) Evaluation of anti-diarrhea activity of Rhizophora mucronata bark extracts. Intet J Alt Med 7.
- Basak UC, Das AP, Das P (1996) Chlorophyll, carotenoids, proteins and secondary metabolites in leaves of 14 species of mangroves. Bull Marine Sci 58: 654-659.
- Rohini RM, Das AK (2009) A Comparative evaluation of analgesic and anti-inflammatory activities of Rhizophora mucronata Pharmacologyonline 1: 780-791.
- Rahmi N, Firdaus M, Prihanto AA (2012) Dept. Fishery Product Technology, Faculty of Fisheries and Marine Science, Malang. Indo J Basic Sci Tech 1: 27-29.
- Ramanathan T (2000) Studies on medicinal plants of Parangipettai coast South East Coast of India) D.Thesis, Annamalai University: Parangipettai.
- Premanathan M, Kathiresan K, Yamamoto N, Nakashima H (1999) In vitro anti-human immunodeficiency virus activity of polysaccharide from Rhizophora mucronata Biosci Biotechnol Biochem 63: 1187-1191.
- Dhayanithi NB, Ajith Kumar TT, Kathiresan K (2010) Effect of neem extract against the bacteria isolated from marine fish. J Environ Biol 3: 409-412.
- Dhayanithi NB, Ajith Kumar TT, Valsala H, Balasubramanian T (2011) Studies on the effect of Vitex negundo leaves against the bacteria isolated from marine ornamental fish. Int J Biol Sci 3: 11-16.
- Dhayanithi NB, Ajith Kumar TT, Balasubramanian T (2012) In vitro and experimental Screening of mangrove herbal extract against Vibrio alginolyticus in marine ornamental fish. World Acad Sci Eng Technol 68: 1310-1314.
- Elizabeth KM (2005) Antimicrobial activity of Terminalia bellerica. Indian J Clin Biochem 20: 150-153.
- Mac Artain P, Gill CIR, Brooks M, Campbell R, Rowland IR (2007) Nutritional value of edible sea weeds. Nutr Rev 65: 535-543.
- Parry RM, Chandan RC, Shahani KM (1965) A rapid and sensitive assay of Exp Biol Med 119: 384-386.
- Yoshida T, Kitao T (1991) The opsonic effect of specific immune serum on the phagocytic and chemiluminescent response in rainbow trout, Oncorhynchus mykiss Indo J Basic Sci Tech 26: 29-33.
- Secombes J (1990) Isolation of salmonid macrophage and analysis of their killing ability. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, Van Muiswinkel WB ) Techniques in fish immunology. New Jersey: SOS Publication: 137-152.
- Yano T (1992) Assays of hemolytic complement activity. In: Techniques in Fish Immunology, Stolen JS, Fletcher TC, Anderson DP, Kaattari SL, Rowley Eds.), SOS Publication, Fair Haven: 131-142.
- Kampen AH, Tollersrud T, Lund A (2005) Staphylococcus aureus capsular polysaccharide types 5 and 8 reduce killing by bovine neutrophils in vitro. Infect Immun 73: 1578-1583.
- Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001) Method for the measurement of antioxidant activity in human J Clin Pathol 54: 356-361.
- Aebi H (1984) Catalase in vitro. Methods Enzymol 105: 121-126.
- Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882-888.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analyt Biochem 95: 351-358.
- Ranzani Paiva MJ, Rodrıguez EL, Veiga ML, Eiras AC, Campos BE (2003) Differential leucokocyte counts in “dourado”, Salminus maxillosus Valenciennes, 1840, from the Mogi-Guac¸u River, Pirassununga, Braz J Biol 63: 517-525.
- Ellis AE (2001) Innate host defense mechanisms of fish against viruses and Dev Comp Immunol 25: 827-839.
- Harikrishnan R, Kim JS, Kim MC, Balasundaram C, Heo MS (2011b) Lactuca indica extract as feed additive enhances immunological parameters and disease resistance in Epinephelus bruneus to Streptococcus iniae. Aquaculture 318: 43-47.
- Clem LW, Sizemore RC, Ellsaesser CF, Miller NW (1985) Monocytes as accessory cells in fish immune responses. Dev Comp Immunol 9: 803-809.
- Olivier G, Eaton CA, Campbell N (1986) Interaction between Aeromonas salmonicida and peritoneal macrophages of brook trout Salvelinus fontinalis). Vet Immunol Immunopathol 12: 223-234.
- Sharifuzzaman SM, Austin B (2010) Development of protection in rainbow trout Oncorhynchus mykiss, Walbaum) to Vibrio anguillarum following use of the probiotic Kocuria SM1. Fish Shellfish Immunol 29: 212-216.
- Amar EC, Kiron V, Satoh S, Watanabe (2004) Enhancement of innate immunity in rainbow trout (Oncorhynchus mykiss Walbaum) associated with dietary intake of carotenoids from natural products. Fish Shellfish Immunol. 16: 527-537.
- Zhou X, Tian Z, WangmY, Li W (2010) Effect of treatment with probiotics as water additives on tilapia Oreochromis niloticus) growth performance and immune response. Fish Physiol Biochem 36: 501-509.
- Secombes CJ, Fletcher TC (1992) The role of phagocytes in the protective mechanisms of fish. Ann Rev Fish Dis 2: 53-71.
- Miyazaki T (1998) Influences of pH and temperature on Lysozyme activity in the plasma of Japanese flounder and Japanese char. Fish Pathol 33: 7-10.
- Harikrishnan R Balasundaram C, Kim MC, Kim JS, Han YJ, Heo MS (2010) Effect of a mixed herb enriched diet on the innate immune response and dis- ease resistance of Paralichthys olivaceus against Philasterides dicentrarchi J Aquat Anim Health 22: 235-243.
- Boshra H, Li J, Sunyer JO (2006) Recent advances on the complement system of teleost fish. Fish Shellfish Immunol 20: 239-262.
- Sharma A, Deo AD, Riteshkumar ST, Chanu TI, Das A (2010) Effect of Witha- nia somnifera Dunal) root as a feed additive on immunological parameters and disease resistance to Aeromonas hydrophila in Labeo rohita Hamilton) fingerlings. Fish Shellfish Immunol 29: 508-512.
- Yin G, Ardo L, Jeney Z, Xu P, Jeney G (2008) Chinese herbs Lonicera ja- ponica and Ganoderma lucidum) enhance non-specific immune response of tilapia, Oreochromis niloticus, and protection against Aeromonas hydrophila. Fish Shellfish Immunol 24: 140-145.
- Saurabh S, Sahoo PK (2008) Lysozyme: and important defence molecule of fish innate immune system. Aquacult Res 39: 223-239.
- Harikrishnan R, Balasundaram C, Heo MS (2011c) Korean mistletoe enriched diet enhances innate immune response in kelp grouper, Epinephelus bruneus against Philasterides dicentrarchi. Vet Parasitol 183: 146-151.
- Ghosh D, Datta S, Bhattacharya S, Mazumder S (2007) Long-term exposure to arsenic affects head kidney and impairs humoral immune responses of Clarias batrachus. Aquat Toxicol 81: 79-89.
- Phelan PE, Pressley ME, Witten PE, Mellon MT, Blake S et al. (2005) Char- acterization of Snakehead Rhabdovirus Infection in zebrafish (Danio rerio). J Virol 79: 1842-1852.
- Pressley ME, Phelan PE, Witten PE, Mellon MT, Kim CH (2005) Pathogene- sis and inflammatory response to Edwardsiella tarda infection in the zebraf- Dev Comp Immunol 29: 501-513.
- Ray S, Saha S (2011) Arsenic toxicity of estuarine Germany: LAP LAMBERT Academic Publishing GmbH & Co KG.
- Richetti SK, Rosemberg DB, Ventura-Lima J, Monserrat JM, Bogo MR, et (2011) Acetylcholinesterase activity and antioxidant capacity of zebrafish brain is altered by heavy metal exposure. Neurotoxicology 32: 116-122.
- Saha S, Ray M, Ray S (2010) Screening of phagocytosis and intrahemocy- totoxicity in arsenic exposed crab as innate immune response. Asian J Exp Biol Sci 1: 47-54.
- Chakraborty S, Ray M, Ray S (2010) Toxicity of sodium arsenite in the gill of an economically important mollusc of India. Fish Shellfish Immunol 29: 136-148.
- Aruljothi B, SamipillaiSS (2014) Effect of arsenic on lipid peroxidation and antioxidants system in fresh water fish, labeo rohita. Int J Mod Res Rev 2: 15-19.
- Bhattacharya A, Bhattacharya S (2007) Induction of oxidative stress by arsenic in Clarias batrachus: Involvement of peroxisomes. Ecotoxicol Environ Saf 66: 178-187.
- Bagnyukova TV, Luzhna LI, Pogribny IP, Lushchak VI (2007) Oxidative stress and antioxidant defenses in goldfish liver in response to short-term exposure to arsenite. Environ Mol Mutagen 48: 658-665.
- Orun I, Talas ZS, Ozdemir I, Alkan A, Erdogan K (2008) Antioxidative role of selenium on some tissues of Cd2+, Cr3+)-induced rainbow trout. Ecotoxicol Environ Saf 71: 71-75.
- Ochi T, Kaise T, Oya Ohta Y (1994) Glutathione plays different roles in the induction of the cytotoxic effects of inorganic and organic arsenic compounds in cultured BALB/c 3T3 cells. Experientia 50: 115-120.
- He X, Lin GX, Chen MG, Zhang JX, Ma Q (2007) Protection against chromium (VI)-induced oxidative stress and apoptosis by Nrf2. Recruiting Nrf2 into the nucleus and disrupting the nuclear Nrf2/Keap1 association. Toxicol Sci 98: 298-309.
- Allen T, Singhal R, Rana S (2004) Resistance to oxidative stress in a fresh- water fish Channa punctatus after exposure to inorganic arsenic. Biol Trace Elem Res 98: 63-72.
- Ferrari A, Lascano CI, Anguiano OL, D’Angelo AM, Venturino A (2009) Antioxidant responses to azinphos methyl and carbaryl during the embryonic development of the toad Rhinella (Bufo) arenarum Aquat Toxicol. 93: 37-44.
- Ma Q (2009) Transcriptional responses to oxidative stress, pathological and toxicological implications. Pharmacol Therapy 125: 376-393.
- Bosnir J, Puntaric D, Skes I, Klaric M, Simic S, et al. (2003) Toxic metals in freshwater fish from the Zagreb area as indicators of environmental Coll Antropol 27: 31-39.
Citation: Dhayanithi NB, Kumar TTA, Balasundaram C, Devi G, Ramasamy H (2020) Immuno-antioxidant Defense of Partially Purified Rhizophora mucronata in Clownfish (Amphiprion sebae) against Vibrio alginolyticus. J Cell Mol Biol 4: 009.
Copyright: © 2020 Dhayanithi NB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.