*Corresponding Author:
Timothy J Falla,
Rodan + Fields, 60 Spear Street, San Francisco, CA 94105, USA
Tel: +1 415 597 6328
E-mail: tfalla@rodanandfields.com
Abstract
Background: Topical moisturizers, such as humectants containing glycerin and hyaluronic acid (HA), are the first-line treatments for preventing and treating xerosis; they work by increasing the water content in the stratum corneum. Because humectants vary widely in their water-binding and skin-penetrating capabilities, an improved moisture delivery system is needed. The objective of this study was to determine if a novel super-humectant delivery system synthesized from 3 polymers into a 3-dimensional, interpenetrating polymer network that entraps HA (3D3P-IPN) is effective and safe in improving skin hydration levels and substantivity compared to a commercial moisturizer containing HA.
Methods: This single-center, double-blind study enrolled 44 female subjects 30-60 years old who had a Fitzpatrick skin type classification of I-VI, mild-to-moderate dry skin, global facial fine lines and skin dullness (Griffiths Skin Grading scores 3-6). Subjects were randomized to 1 of 3 groups (2 groups used Hydration serums with different concentrations of 3D3P-IPN; 1 group used a commercial moisturizer) and applied their respective test materials nightly for 8 days. Efficacy was assessed using clinical grading, subject self-rating of skin attributes, and bioinstrumentation (Corneometer, Skicon and Tewameter) measurements.
Results: All 3 test materials were effective and showed statistically significant improvements in clinical grading scores, subject self-ratings, and skin moisture content; however, the 2 Hydration serum groups demonstrated more consistent and sustained benefits.
Conclusion: Organizing humectants and water into a 3D3P-IPN through a simple synthetic cross-linking process can improve the way humectants increase and maintain water retention at the skin’s surface. Formulations containing 3D3P-IPN are effective and safe in improving the delivery of glycerin and HA to the skin, helping to maintain skin softness and elasticity. This may be a significant breakthrough in treating xerosis and age-related skin effects of dullness, sagging and rough texture.
Keywords
Humectants; Polymers; Skin hydration; Skin moisturizers; Xerosis treatment
Abbreviations
3D3P: 3-Dimensional 3-Polymer formation
3D3P-IPN: 3-Dimensional, 3-Polymer Interpenetrating Polymer Net- work
AE: Adverse Event
EDC: Electronic Data Capture
HA: Hyaluronic Acid
IPN: Interpenetrating Polymer Network SD: Standard Deviation
SE: Standard Error
TEWL: Transepidermal Water Loss VAS: Visual Analog Scale
Introduction
Xerosis, or abnormally dry skin, results from a decrease in water and sebaceous secretions from the stratum corneum–the outermost layer of the epidermis–which is composed of flattened dead cells called corneocytes. The stratum corneum provides a barrier against the external environment, helping to prevent water loss among many other functions [1-3]. Signs of xerosis include changes in differentiation-related keratins expressed by basal cells and keratinocytes. There is also degradation of corneodesmosomes, reducing skin elasticity and causing abnormal keratinocyte desquamation [1,4].
Heredity, psychological stress and environmental factors influence skin-barrier function precipitating dryness [5]. Usually temporary, xerosis more often occurs in the winter months due to climate changes producing low humidity coupled with use of forced air heating systems [6,7]. Other extrinsic factors impacting skin moisture levels include exposure to ultraviolet radiation, smoking, contact with chemical agents and air pollution [5,8,9]. Medical conditions, such as, atopic or contact dermatitis, psoriasis, thyroid disease and diabetes, perturb the skin’s barrier function, resulting in pervasive and chronic rough, itchy and flaky skin [2,10]. In addition, with age, the skin’s inherent moisture level becomes more mobile, resulting in increased Transepidermal Water Loss (TEWL), which visibly manifests as deepening of fine lines, wrinkles, dullness and flaking [11,12]. All these conditions create unique challenges for keeping the skin hydrated.
Regardless of the cause or duration, topical moisturizers (e.g., emollients, humectants, occlusives and protein rejuvenators) are the first-line approaches to preventing and treating xerosis and serve as important adjuncts to prescription topical corticosteroids for severe cases [2,10,13,14]. Moisturizers increase the water content in the stratum corneum and improve the skin’s superficial and deep layers by 1) filling the spaces between desquamated skin cells; 2) maintaining skin smoothness, softness and elasticity; and 3) contributing to the health of metabolic processes in the deeper epidermis [2,10].
Moisturizers with humectant agents hydrate the stratum corneum by attracting water from both the deeper dermis layer and the external environment [2,10,15]. Humectants vary widely in their water-binding capabilities and ability to penetrate the skin [2,10,15]. Glycerin and sodium hyaluronate (the sodium salt of HA) have superior water-binding capabilities, but because of their hydrophilic characteristics, they poorly penetrate the hydrophobic environment of the skin’s surface, thus failing to hydrate effectively [10,16,17]. Furthermore, in dry environmental conditions, humectants pull water from the dermis to the stratum corneum that then tends to evaporate, increasing TEWL just when moisture retention is most needed [10].
An improved system is needed to attract and retain skin moisture for generating and maintaining a well-hydrated stratum corneum-a key dermatological endpoint. We synthesized a hydrophobically modified Interpenetrating Polymer Network (IPN) from 3 polymers into a 3-Dimensional formation (3D3P) by interlacing highand low-acyl Gellan gums with a branched, hydrophobically modified cellulose and a linear sodium hyaluronate for entrapping the humectants glycerin and HA. This new molecular network consists of a more rigid polymer structure. During homogenization (agitation), the structure is broken into small, micron-sized particles, creating a suspension of individual hydrogel particles with greater waterand humecant-binding capabilities as compared to traditional HA-containing hydration serums. The result is improved delivery of glycerin and HA for increased moisture deposition at the stratum corneum and improved product substantivity for a prolonged hydration benefit.
To determine the effectiveness of this super-humectant polymer hydration serum (referred to as Hydration serum) in delivering water and restoring hydration levels, we studied 2 different concentrations of 3D3P-IPN and compared clinical grading and bioinstrumentation measurements against a commercially available moisturizer containing HA.
Synthesis and Characterization of a 3D3P-IPN
A 3D3P-IPN was created by interlacing high-acyl and low-acyl Gellan gums with a branched hydrophobically modified cellulose and a linear sodium hyaluronate. It was assembled by first unraveling dry-spooled Gellan gums in water at a hydration temperature of 80ºC under aggressive homogenizing agitation conditions. When the gums reached full hydration, a solution of hydrophobically modified cellulose and sodium hyaluronate in glycerin was introduced under slow agitation into the Gellan gum solution [18]. After the 3 polymers in soluble form at 80ºC were interlaced by agitation, a solution of divalent magnesium and calcium ions was added in the form of natural moisturizing factors (PCA salts) to initiate cross-linking of the low-acyl Gellan gum. Under homo-mixing agitation, the mixture was cooled to room temperature, allowing the cross-linking of the Gellan gums to form rigid hydrogel structures [19]. During the cooling process, the cross-linked gel structure was degraded by homogenization into a uniform and flowable consistency consisting of suspended micron-sized gel particles. Other cosmetic ingredients were added including ceramides, preservatives and a fragrance. The end result is a suspension of gelatinous hydrogel particles capable of binding humectants and water within the particle structures. The percentage of the 3 polymers in 3D3P-IPN is 0.12% sodium hyaluronate, 0.1% gellan gum, and 0.3% cellulose.
The soft gel particles size was not successfully characterized with laser particles size techniques due to the density of particles in suspension and instrumental limitations of limited light-scattering intensity. Therefore, a microscopy method was employed along with the HA staining reagent toluidine blue O to reveal distinct hydrogel particles suspended in solution and a 3D micro-scaffold (Figure 1). For comparison, a commercially available moisturizer also containing hydrogels including HA, xanthan gum and cellulose did not exhibit the same 3D scaffold-structure organization under the same microscopy conditions (Figure 1).

Figure 1: Microphotographs of 3D3P-IPN (A,C,D) and a commercially available moisturizer with similar ingredients (B). Note the globular, 3D network structure of 3D3P-IPN (A,C) which is absent in the non-cross-linked moisturizer (B). The globular structures of 3D3P-IPN are aligned with thin scaffolding structures (see arrows) visible in a different focal plane (D), absent in the commercially available moisturizer. Magnification: X200.
Viscosity measurements were carried out using a TA instruments AR-G2/AR2000ex Rheometer with a 40 mm two-degree cone. The rheological flow characteristics under mechanical shear demonstrated almost no yield stress, a very low viscosity and only minor pseudoplastic properties. For comparison, traditional hydrogels and gums expanded in water exhibit a greater viscosity, yield stress and significant shear thinning properties [20]. This is corroborated by the commercial moisturizer sample in the rheology diagram demonstrating significant viscosity and yield stress with respect to shear (Figure 2). The rheology characteristics are consistent with the microscopy findings of 3D3P-IPN hydrogel particles that no longer exist as a continuous viscous hydrogel but as suspended, gelatinous particles with low viscosity and shear thinning properties [21].
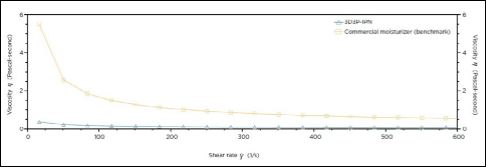
Figure 2: Viscosity of 3D3P-IPN compared to a commercially available moisturizer with similar water content and polysaccharide hydrogel ingredients. 3D3P-IPN lacks a continuous gel structure, thus imparts little viscosity under shear.
Methods
Population and Study Design
This single-center, double-blind clinical trial was designed to assess the Hydration serum’s efficacy in improving the condition and moisturization levels of facial skin. Eligible females were blinded and randomized to 1 of 3 groups each using different test materials. Groups 1 and 2 received the Hydration serum in different concentrations composed of a 3-dimensional, gelatinous, globular-particle suspension. Group 1 received 0.53% 3D3P-IPN (% solids of 3 polymers includes 0.12% HA) + 30% glycerin and group 2 received 0.053% 3D3P-IPN (% solids of 3 polymers includes 0.012% HA) + 11% glycerin. As the benchmark (control) group, group 3 received a commercially available moisturizer consisting of glycerin, HA, hydroxyethylcellulose and xanthan gum that is not organized into an IPN cross-linking structure and exists in a soluble polymer state. Subjects were instructed to apply their assigned test material every evening, after cleansing and toning, for 8 days.
Females were eligible to participate if they were 30-60 years old, had a Fitzpatrick skin type classification of I-VI, and had mild to moderate facial dryness, global facial fine lines and facial skin dullness according to the modified Griffiths Skin Grading Scale (score 3-6, where 0 = none and 9 = severe) [22,23]. Eligible subjects could not have had any facial treatments (e.g., injectable fillers, microdermabrasion, peels, facials or laser treatments) for 6 months prior to enrollment. Subjects were ineligible if they had known skin allergies, a history of skin cancer, self-described normal-to-oily facial skin, any dormant dermatological conditions, or if they had routinely used any anti-aging/anti-wrinkle treatments within 30 days before enrollment. All subjects were advised to avoid applying any topical moisturizing products to the face for 1-2 days prior to their baseline visit and to remove their makeup at least 30 minutes before arriving to each visit. All subjects gave their written informed consent before enrollment. This study was conducted in accordance with the acceptable standards for Good Clinical Practice and the International Conference on Harmonization.
Efficacy was assessed through clinical grading of efficacy parameters (radiance, fine lines and overall dryness) globally on each subject’s face and around the perioral, cheek and eye areas at baseline/Day 1 (pre-application, 30 minutes post-application and 8 hours post-application) and on Day 8 (pre-application and 30 minutes post-application). Independent evaluators completed clinical grading assessments using the modified Griffiths scale. At each time point, all subjects conducted self-rating of specific facial skin attributes (e.g., skin feel, appearance and fine lines) according to a 10-point Visual Analog Scale (VAS) where 1 = worst condition and 10 = best condition. Subjects completed a daily diary to monitor their study compliance, use of the test materials, and adverse events.
As the benchmark for comparison, a commercially available skin-hydrating product was selected from a leading skin care brand. The benchmark formulation was robust in humectants and osmolytic ingredients. Similar to the ingredient listing of the Hydration formulas, the commercial product contained glycerin along with 3 polysaccharide polymers including sodium hyaluronate, hydroxyethyl cellulose and xanthan gum. In addition, the benchmark formulation contained a variety of osmolytic ingredients including betaine, hydrolyzed wheat protein, mannitol, beta glucan, zinc, calcium and magnesium salts, making it a high standard for comparison.
Bioinstrumentation Measurements
Bioinstrumentation assessments of efficacy included Corneometer and Skicon measurements of moisture content in the stratum corneum, performed at baseline/Day 1 (pre-application, 30 minutes post-application and 8 hours post-application) and on Day 8 (pre-application and 30 minutes post-application). Tewameter assessments of TEWL in the stratum corneum were performed pre-application at baseline/Day 1 and on Day 8 (pre-application).
Corneometer measurements: The Corneometer® CM 825 (CM 825) (Courage+Khazaka Electronic GmbH; Cologne, Germany) measures moisture content in the stratum corneum by an electrical capacitance method; results are proportional to the dielectric constant of the surface layers of the skin. The measurement has no units and increases as the skin becomes more hydrated. The readings, in picofarads, are directly related to the skin’s electrical capacitance. CM 825 measurements were taken on the center of each subject’s cheek (at the intersection of lines extending down from the outer corner of the eye and horizontally across the bottom of the nose) to measure product hydration effects on the skin surface. Triplicate measurements were averaged and reported as mean. The CM 825 validity and sensitivity to hydration changes in the skin have been widely documented [24,25].
Skicon measurements: Triplicate Skicon 200EX (IBS Inc.; Osaka, Japan) measurements were taken on the center of each subject’s cheek (at the intersection of lines extending down from the outer corner of the eye and horizontally across the bottom of the nose) to measure test material effects on the moisture content of the stratum corneum using high-frequency conductance methodology. Data are collected in microsiemens (μS), and measurements increase with skin hydration.
Tewameter measurements: A single Tewameter TM300 (Courage+Khazaka) measurement was taken on the center of each subject’s cheek (at the intersection of lines extending down from the outer corner of the eye and horizontally across the bottom of the nose) to assess passive water transport through the stratum corneum TEWL. The measurement of this water evaporation is based on the diffusion principle in an open chamber, and the density gradient is measured indirectly by 2 pairs of sensors located inside the hollow cylinder probe. Data are analyzed by a microprocessor and reported in g/m2/h. A decrease in TEWL values reflects an improvement in the barrier properties of the skin.
Safety
Adverse Events (AEs) were monitored through physical examination, direct questioning at study visits and review of completed diary entries. An AE was defined as any unwanted medical occurrence or unfavorable or unintended sign or symptom of disease or condition, whether or not it was considered related to the test materials. Severity of AEs was determined by the investigator to be mild (awareness of a sign or symptom that was easily tolerated), moderate (discomfort sufficient to cause interference with the subject’s usual activities or to affect clinical status), or severe (incapacitating, leading to the subject’s inability to do usual activities or significantly affecting clinical status). The investigator also indicated AEs’ relationship to the test materials as unlikely, possible, probable, definite or unknown.
Statistical Analysis
For continuous variables, descriptive statistics including number of subjects (N), mean, and standard deviation (SD) values are presented. For categorical variables, the frequency and percentage of each category are listed. A descriptive statistical summary is provided for clinical grading of efficacy parameters, subjects’ self-rating of skin attributes and bioinstrumentation (Corneometer, Skicon and Tewameter) measurements.
Comparisons between the treatment groups were made in terms of changes from baseline. The null hypothesis was the mean change from baseline (Day 1 pre-application) being equal among the 3 treatment groups at post-baseline time points and was tested using Kruskal-Wallis one-way Analysis Of Variance (ANOVA) followed by Wilcoxon rank-sum test for clinical grading and rating of skin attributes, and using ANOVA, with change from baseline as the response variable and treatment as a factor, for bioinstrumentation measurements.
All statistical tests were 2-sided at significance level alpha = 0.05. P values were reported to 3 decimal places (0.000). SAS software version 9.30 series (SAS Institute, Inc.; Cary, North Carolina) was used for statistical analyses. Clinical grading and bioinstrumentation measurements were recorded using Stephens Electronic Data Capture (EDC) system (Stephens & Associates; Texas, Colorado, Japan). The Stephens EDC is a computerized system designed for the collection of clinical data in electronic format. Data review and analysis were performed by an independent data committee consisting of representatives from quality assurance, clinical services and the statistical department of Stephens & Associates.
Results
Study population
A total of 44 subjects met the eligibility criteria and were enrolled (group 1: n = 15, group 2: n = 15, group 3: n = 14), with 39 subjects (group 1: n = 14; group 2: n = 13; group 3: n = 12) completing the study. A total of 5 subjects discontinued the study: 3 subjects (1 from each group) requested withdrawal; 1 subject from group 2 was non-compliant, having used acne treatment during the study; and the investigator decided to withdraw 1 subject in group 3 due to high baseline Skicon readings.
Mean (SD) age was 48.8 (7.7) years with the majority having a Fitzpatrick skin type III (41.0%) and of non-Hispanic/Latino ethnicity (87.2%; Table 1).
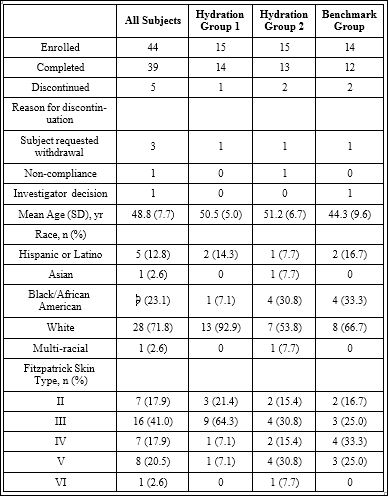
Table 1: Subject demographics and disposition.
Efficacy
Clinical grading: For all 3 treatment groups, there was a statistically significant (P < 0.05) improvement in clinical grading scores for radi-ance and fine lines at each post-baseline time point: 30 minutes and 8 hours post-application on Day 1 and pre-application and 30 minutes post-application on Day 8 when compared with baseline (Day 1 pre-application) scores (Figure 3). The baseline fine-line scores for Hydration groups 1 and 2 were 4.5 and 4.38, respectively, and 4.17 for the benchmark group; the scores decreased after 8 days of use (pre-application scores) to 3.25, 3.38 and 3.42, respectively, resulting in mean changes of -1.25, -1.00 and -0.75, respectively (Figure 3).
Comparisons among the treatment groups, based on the mean change from baseline for each parameter, indicated that the benchmark group had a significantly greater improvement in radiance and fine lines 30 minutes post-application on Day 1 compared to both Hydration groups and 8 hours post-application versus Hydration group 2 in radiance only. By Day 8, Hydration group 1 had significantly greater improvement in fine lines scores (preand post-application) compared to the benchmark group (Figure 3).
Subject ratings: Rating of skin attributes showed a statistically significant improvement in moisturization (skin feel and appearance), radiance (appearance), suppleness (elastic snapback), softness (to touch), smoothness (to touch), bouncy skin (to touch), dryness (appearance), overall appearance and overall comfort for all treatment groups at all time points. The rating of fine lines (appearance) was significant for all excluding Group 3 at Day 1 (30 minutes post-application).

Figure 3: Mean score in clinical grading of A) radiance and B) fine lines and mean change from baseline in C) radiance and D) fine lines. All data are mean change from baseline significant (P < 0.05) versus baseline for all. Error bars indicate standard deviation.
P < 0.05 mean difference versus *Hydration group1, versus ƗHydration group 2, and versus ǂBenchmark at corresponding time points.
Bioinstrumentation Measurements
Corneometer measurements: All treatment groups had statistically significant improvements in the moisture content of the stratum corneum as assessed by corneometer measurements at each post-baseline time point (30 minutes and 8 hours post-application on Day 1; pre-application and 30 minutes post-application on Day 8) compared with baseline pre-application values (P < 0.05). Mean change from baseline comparisons among the treatment groups was significant between Hydration group 2 and the benchmark group on Day 1, 8 hours post-application (P = 0.022), with a difference (Standard Error [SE]) of 10.92 (4.53). Hydration group 1 had a significantly greater difference versus the benchmark group on Day 8, post-application (P = 0.20), with a difference (SE) of 14.11 (5.77; Figure 4). The 2 Hydration formulations (groups 1 and 2) had greater mean changes from baseline compared to the benchmark, non-cross-linked structure (group 3) at all post-application time points (Figure 4). Hydration group 1 had a percentage mean change of 95.9% by Day 8 post-application compared to 73.9% and 68.5% for Hydration group 2 and the benchmark group, respectively. The group 2 Hydration serum with 0.053% 3D3P-IPN had a greater mean change in moisture content on Day 1 (30 minutes and 8 hours post-application), but by Day 8 the group 1 Hydration serum with 0.53% 3D3P-IPN had a greater mean change from baseline preand post-application on Day 8 (Figure 4).
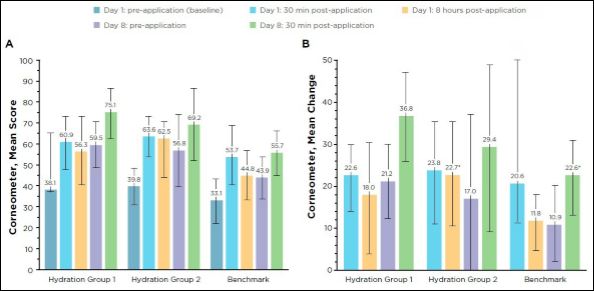
Figure 4: A) Mean score and B) mean change from baseline in moisture content as measured by the Corneometer.
Mean change from baseline is significant (P < 0.05) versus baseline for all. Error bars indicate standard deviation. P < 0.05 mean difference versus Benchmark at corresponding time points.
Skicon measurements: All treatment groups experienced a statistically significant increase in moisture content of the stratum corneum as assessed by Skicon measurements at each post-baseline time point compared with baseline pre-application values (Figure 5). The 0.53%
3D3P-IPN formulation (group 1) had a greater mean change compared to all other groups at all time points (Figure 5). Mean change from baseline improvements was significant in favor of Hydration group 1 versus Hydration group 2 and the benchmark group on Day 1 at both post-application time points. The Hydration group 1 change was also significant versus the benchmark group on Day 8 post-application (Figure 5).
Tewameter measurements: The TEWL decreased (improved) for all 3 treatment groups at both time point measurements (baseline pre-application and Day 8 pre-application). Mean change from baseline on Day 8 was -0.41, -0.86 and -2.67 for Hydration groups 1 and 2 and the benchmark group, respectively, which was significant for the benchmark group only versus baseline (P = 0.04). There were no significant differences between groups.
Adverse events
No serious AEs were reported or detected; only 2 non-serious AEs were reported, both from Hydration group 2. One patient reported acne on the face that was deemed mild and possibly related to the test materials. Petechiae were detected on another patient that were considered moderate and unlikely to be related to the test materials. Both conditions resolved without further exacerbation or intervention.
Discussion
Commercially available skin care moisturizers and over-the counter skin protectants often contain high concentrations of humectants including glycerin, HA and various osmolytes. However, not all formulations with humectants and osmolytes offer an adequate level of hydration performance. Pure humectant formulations are not well known for retaining and building hydration over time without the concomitant use of barrier ingredients.
Delivering humectants to the skin surface requires vehicles that can facilitate topical delivery of these hydrophilic substances to the hydrophobic skin surface. To achieve this, we did not chemically alter HA but instead entrapped it within a hydrophobically modified IPN creating a super-humectant scaffold to restore and sustain hydration levels in the stratum corneum.
Our investigation has demonstrated that organizing humectants and water into a 3D3P-IPN through a simple synthetic cross-linking process can improve the way humectants increase and maintain water retention at the skin surface. Although all 3 test materials were effective in improving facial skin conditions and moisturization over the course of 8 days under the conditions of this study, the Hydration serums had greater mean changes in skin moisture content compared to the benchmark, a non-cross-linked formulation, and demonstrated more consistent and sustained benefits. Our research has shown that advances are still possible for improving the topical delivery of humectant agents for prevention and treatment of xerosis and age-related skin changes.
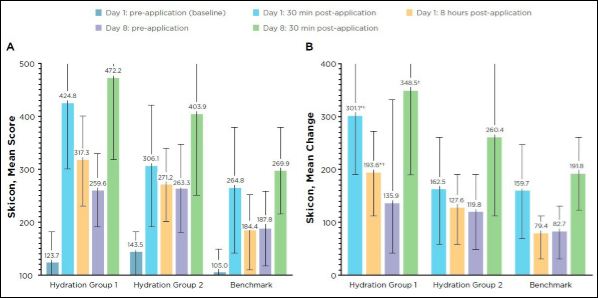
Figure 5: (A) Mean score and (B) mean change in Skicon measurements.
Mean change from baseline is significant (P < 0.05) versus baseline for all. Error bars indicate standard deviation. P < 0.05 mean difference versus *Hydration group 2 and ƗBenchmark at corresponding time points.
All groups showed improvement in radiance and reduction in the appearance of fine lines immediately following the first product application. Those in the Hydration groups had steady improvement and retention of these benefits without reduction or loss of gained benefits between applications. However, results in the benchmark group appeared to be more variable, with scores increasing (worsening) 8 hours post-application on Day 1. This suggests the Hydration groups may have greater long-term, consistent and sustained benefits.
Similar observations were noted in hydration levels. The Hydration groups experienced a more rapid and sustained improvement while the benchmark group had more variable hydration levels. Mean change from baseline 30 minutes and 8 hours post-application Day 1 and pre-application Day 8 were greater in the Hydration groups compared to the benchmark group, indicating better sustained-hydration levels.
The changes in hydration of the stratum corneum were measured using both capacitance (Corneometer® CM 825) and conductance (Skicon 200EX) techniques. The benchmark product was compared to the Hydration serum containing 0.53% 3D3P-IPN (group 1) and also against a diluted form in which the Hydration serum contained 0.053% 3D3P-IPN (group 2). On Day 8 (preand post-application), both hydration measurement techniques showed an increase from baseline across all 3 study groups. The Hydration serum at full strength (0.53% 3D3P-IPN; group 1) demonstrated the most significant increase in surface hydration on Day 8 in both the pre-and post-application measurements of capacitance and conductance. Interestingly, the mean Corneometer values of the 0.53% 3D3P-IPN serum (group 1) produced Day 8 pre-application measurements that were nearly equivalent to the 30-minute post-application values measured on Day 1. The same Day 1/Day 8 comparison in the other 2 test groups was less meaningful. This data suggests that the Hydration serum at full strength (0.53% 3D3P-IPN) may be capable of improving hydration retention at the skin surface immediately and sustaining this improvement through Day 8.
The Corneometer capacitance data of both Day 1 post-applications and Day 8 pre-application did not compare consistently with the nearly equivalent measurements made with the Skicon conductance technique. In trying to understand which technique may be most informative for assessing the retention of water in skin, it is important to realize the technical capabilities of each device. First, conductance measurements are influenced by electrolytes, while capacitance measurements are not. Second, the capacitance method carries information from deeper layers (up to 45 µm) compared with the conductance instrument (up to 15 µm) [24]. Considering that all 3 test products contained electrolytes, and conductance measurements occur closer to the probe, the different skin contact points would explain why the measurements taken on Day 1 (30 minutes post-application) may be less useful in representing the saturation of water within the surface of the skin for comparison over the 8-day period. This rationale supports the capacitance observations, in which full strength Hydration serum (0.53% 3D3P-IPN concentration) has shown the ability to improve hydration retention using a purely hydrophilic humectant-based formulation containing no hydrophobic barrier function.
The single-center location means that our results cannot be extrapolated to apply in other geographical areas with different climates and populations. Other limitations of this study include the short duration, small number of patients and limited xerosis of the subjects. (We intentionally selected a small and short study design to safely evaluate the efficacy of this first-in-humans trial of the Hydration serum.) Longer-term studies in multiple populations of subjects with moderate to severe xerosis, a variety of skin types and more prevalent age-related skin attributes will further expand our knowledge of the Hydration serum’s benefits and limitations. This study lacked a placebo group. However, a high-standard over-the-counter product was selected for comparison, offering a more complete picture of the unmet needs for adequate hydration.
Overall, results from this single-center, double-blind clinical trial indicate that the Hydration serum increases and maintains hydration and substantivity in the stratum corneum with more consistent and sustained benefits than a leading commercial moisturizer. Formulations containing 3D3P-IPN are effective in improving the delivery of glycerin and HA to the skin and helping to maintain skin softness and elasticity, which may be a significant breakthrough in treating xerosis as well as age-related skin concerns.
Funding Source and Acknowledgment
This study was funded by Rodan + Fields. Medical writing support was provided by Stephanie Eide and was funded by Rodan + Fields.
Conflicts of Interest
All authors are employees of Rodan + Fields, San Francisco, CA.
References
- Engelke M, Jensen JM, Ekanayake-Mudiyanselage S, Proksch E (1997) Effects of xerosis and ageing on epidermal proliferation and differentiation. Br J Dermatol 137: 219-225.
- Lodén M (2003) Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol 4: 771-788.
- Farage MA, Miller KW, Elsner P, Maibach HI (2013) Characteristics of the Aging Skin. Adv Wound Care 2: 5-10.
- Rawlings A, Harding C, Watkinson A, Banks J, Ackerman C, et al. (1995) The effect of glycerol and humidity on desmosome degradation in stratum Arch Dermatol Res 287: 457-464.
- Hashizume H (2004) Skin aging and dry J Dermatol 31: 603-609.
- Sunwoo Y, Chou C, Takeshita J, Murakami M, Tochihara Y (2006) Physiological and subjective responses to low relative J Physiol Anthropol 25: 7-14.
- Sunwoo Y, Chou C, Takeshita J, Murakami M, Tochihara Y (2006) Physiological and subjective responses to low relative humidity in young and elderly J Physiol Anthropol 25: 229-238.
- Laurent TC, Fraser JR (1992) FASEB J 6: 2397-2404.
- Simpson RM, Meran S, Thomas D, Stephens P, Bowen T, et al. (2009) Age-related changes in pericellular hyaluronan organization leads to impaired dermal fibroblast to myofibroblast differentiation. Am J Pathol 175: 1915-1928.
- Sethi A, Kaur T, Malhotra S, Gambhir M (2016) Moisturizers: The slippery Indian J Dermatol 61: 279-287.
- Mayrovitz HN, Singh A, Akolkar S (2016) Age-related differences in tissue dielectric constant values of female forearm skin measured noninvasively at 300 MHz. Skin Res Technol 22: 189-195.
- Wilhelm KP, Cua AB, Maibach HI (1991) Skin aging. Effect on transepidermal water loss, stratum corneum hydration, skin surface pH, and casual sebum Arch Dermatol 127: 1806-1809.
- Lucky AW, Leach AD, Laskarzewski P, Wenck H (1997) Use of an emollient as a steroid-sparing agent in the treatment of mild to moderate atopic dermatitis in children. Pediatr Dermatol 14: 321-324.
- Bikowski J (2001) The use of therapeutic moisturizers in various dermatologic disorders. Cutis. 68: 3-11.
- Simon M, Bernard D, Minondo AM, Camus C, Fiat F, et (2001) Persistence of both peripheral and non-peripheral corneodesmosomes in the upper stratum corneum of winter xerosis skin versus only peripheral in normal skin. J Invest Dermatol 116: 23-30.
- Williams AC, Barry BW (2004) Penetration enhancers. Adv Drug Deliv Rev 56: 603-618.
- Pham QD, Topgaard D, Sparr E (2017) Tracking solvents in the skin through atomically resolved measurements of molecular mobility in intact stratum Proc Natl Acad Sci. 114: E112-E121.
- Matricardi P, Cencetti C, Ria R, Alhaique F, Coviello T (2009) Preparation and characterization of novel gellan gum hydrogels suitable for modified drug Molecules 14: 3376-3391.
- Tako M, Kitajima S, Yogi T, Uechi K, Onaga M, et (2016) Structure-function relationship of a gellan family of polysaccharide, S-198 Gum, produced by Alcaligenes ATCC31853. Adv Biol Chem 6: 55-69.
- Gibbs DA, Merrill EW, Smith KA, Balazs EA (1968) Rheology of hyaluronic Biopolymers 6: 777-791.
- Chun C, Lee DY, Kim JT, Kwon MK, Kim YZ, et (2016) Effect of molecular weight of hyaluronic acid (HA) on viscoelasticity and particle texturing feel of HA dermal biphasic fillers. Biomater Res 20: 24.
- Fitzpatrick TB (1988) The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 124: 869-871.
- Griffiths CE, Wang TS, Hamilton TA, Voorhees JJ, Ellis CN (1992) A photonumeric scale for the assessment of cutaneous photodamage. Arch Dermatol 128: 347-351.
- Clarys P, Clijsen R, Taeymans J, Barel AO (2012) Hydration measurements of the stratum corneum: comparison between the capacitance method (digital version of the Corneometer CM 825®) and the impedance method (Skicon-200EX®). Ski Res Technol 18: 316-323.
- Hashimoto-Kumasaka K, Takahashi K, Tagami H (1993) Electrical measurement of the water content of the stratum corneum in vivo and in vitro under various conditions: comparison between skin surface hygrometer and corneometer in evaluation of the skin surface hydration Acta Derm Venereol. 73: 335-339.
Citation: Majewski G, Rodan K, Fields K, Ong D, Falla TJ (2017) Hydrating the Skin with a Novel Interpenetrating Polymer Network. J Clinic Exper Cosme Derma 1: 003.
Copyright: © 2017 Majewski G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


