*Corresponding Author:
Asami Satoh,
Department of Frontier Surgery, Chiba University Graduate School of Medicine, Chiba, Japan
Tel: +81 432262110
Email: samasami@gmail.com
Abstract
Background
Changes in blood flow of the liver have been shown to be associated with the presence of distant metastases from colorectal cancers. We assessed the potential of Perfusion computed tomography to identify patients at risk for future appearance of metastases using preoperative liver perfusion parameters.
Methodology
Perfusion CT was performed on 58 patients who underwent surgery for colorectal cancer, and liver blood flow along with hepatic arterial fraction was measured and compared with histopathological characteristics of the primary tumor. The short-term outcome of the patients without synchronous overt metastases was also evaluated.
Results
There was a significant increase in liver blood flow and hepatic arterial fraction in patients with advanced tumor depth and lymph node metastases. Cases with distant metastases had increased liver blood flow and hepatic arterial fraction compared to cases with early-stage colorectal cancer. As for cases without synchronous metastases, both parameters measured preoperatively were significantly increased in those with postoperative metastases.
Conclusion
Increased liver perfusion parameters acquired with Perfusion computed tomography may reflect an advanced or metastatic state of colorectal cancer. Perfusion computed tomography could be a method for detecting the smallest changes in liver perfusion and determine the risk for future appearances of distant metastases.
Keywords
Distant metastases; Liver perfusion; Perfusion parameters
Abbreviations
CT: Computed Tomography,
mg: milligram (s)
dl: decilitre (s)
ml: millilitre (s)
sec: second (s)
cm: centimetre (s)
kVp: kilo Volt peek (s)
mA: milliampere (s)
Introduction
Colorectal cancer is the third most common malignant neoplasm, as well as the fourth leading cause of cancer-related deaths worldwide [1]. Colorectal cancer metastasizes to various organs, with lymph nodes being the most frequent, followed by liver and lungs [2]. Overall survival of patients with metastatic colorectal cancer, with the 5-year survival at almost 90% for Dukes A and 15% for Dukes D tumors [3].
Distant metastases, especially the lesions becoming apparent early in the follow-up period, may already be present at the time of resection of the primary tumor because small lesions are unable to be detected on preoperative diagnostic images. Visual analysis is largely based upon evaluating morphological information, and there is always a threshold size and contrast below which differentiation between normal and abnormal tissue is impossible. If we were able to obtain more direct evidence of a high possibility of distant metastasis in individual cases, treatment with potentially effective protocols could be initiated earlier, when these patients have a better chance of cure. The concept of the early detection of metastatic lesions has occupied many researchers in the search for more sensitive techniques.
Most published material on prediction of the appearance of metastatic lesions after resection of colorectal cancer has focused on findings in the primary tumors of patients who subsequently developed metastases [4,5], with controversial results. Recently, reports have indicated a shift of attention to the metastatic organ itself. Color Doppler ultrasonography has been shown to associate the presence of liver metastasis too small to be detected on CT images with an increased ratio of hepatic arterial to total liver blood flow [6]. The mechanism underlying this phenomenon is unclear, but there are suggestions of a circulating vasoactive agent [7] with background evidence of a change in hepatic perfusion due to increased splanchnic vascular resistance [8]. However, this method using Doppler ultrasonography is strongly operator-dependent, and other groups could not reproduce the same results [9-11].
Perfusion Computed Tomography (Perfusion CT) is a relatively new technology that was primarily developed for brain perfusion, and it has now also found its way into perfusion of different organs [12-14]. It uses the enhancement pattern of contrast media over time to determine perfusion parameters, which have provided effective measurements in humans and animal experiments for evaluation of tumor angiogenesis and antivascular chemotherapy [12,13,15]. The functional information acquired with this new modality can be useful for liver imaging as well [16]. Alterations in hepatic contrast enhancement in morphologically normal livers using multidetector row CT have been shown to indicate the subsequent development of metastases and identify those patients with reduced survival [17,18]. There have been no reports on the prediction of appearance of metastatic lesions using preoperative liver perfusion calculated with perfusion CT.
By revealing the perfusion differences between the livers of patients with and without synchronous metastases, this study examined the potential of Perfusion CT to identify those patients with occult metastasis. The short-term outcome of those patients without synchronous overt metastases was also evaluated to determine liver perfusion parameter levels at risk for the future appearance of distant metastases.
Methodology
Patient population
The study was approved by the ethics committee and written in- formed consent was obtained from the patients before their study participation. Patients were included in this study if they had histo- pathologically proven colorectal cancer and pathological stage could be determined after surgical resection. All patients underwent Perfu- sion CT scans prior to surgical treatment, incorporated right before the routine diagnostic exam using an additional 40 ml of contrast. None of the patients had any liver perfusion diseases such as cirrho- sis. Patients with inadequate renal function (serum creatinine over 2.0 mg/dl) were also excluded from the study. Between April 2006 and December 2008, 58 patients, 35 men and 23 women (mean age 62.94 ± 10.6 years, range 43-83 years), met the criteria to participate in this study. Sixteen patients had synchronous metastatic lesions, 13 to the liver, 2 to the lung, and 1 to both. Diagnosis of hepatic metastases was based on radiological findings from the portal phase of enhanced CT scans, supported by angiography, MRI, or sonography. Metastases to the lung was confirmed by surgical pathology in 1 patient, and diag- nosis was based on the morphological characteristic of lung metasta- sis by conventional CT in the other 2 patients.
All patients without evidence of metastatic disease underwent cu- rative resection together with regional lymphadenectomy. Fifteen of the patients with known synchronous distal metastases also under- went resection of the primary tumor. One patient with multiple metas- tases to both the liver and lung possessed an unresectable tumor due to invasion to the retroperitoneum. Standard pathologic analysis was performed on all resected surgical specimens, and biopsy specimen was used for pathological analysis in the unresectable case. Each tu- mor was staged according to the International Union Against Cancer (UICC) classification [19].
After surgery, patients with distant metastases either received sys- temic adjuvant chemotherapy or were prepared for subsequent resec- tion. All stage III and IV patients received adjuvant chemotherapy, if not contraindicated. Patients without synchronous distant metastases were followed regularly according to established guidelines, with routine physical examination, serum carcinoembryonic antigen, colo- noscopic surveillance, and CT scans. These patients were subjected to surveillance for at least one year, and none were lost to follow-up. The median follow-up period was 19 months (range 12-29 months).
Perfusion CT imaging protocol
A 20-gauge venous cannula was placed in the forearm prior to the scan. Perfusion CT was performed with a 16-row multidetector CT scanner (Light speed Ultra 16; GE Medical Systems, Milwaukee, WI). For initial localization of vessels, a non-enhanced scan of the upper abdomen was obtained during a breath-hold at the end of inha- lation. After localization, a 2-cm region including the slices passing through both the portal vein and aorta was selected and 4 sequential 5-mm slice scans were performed for each patient. Images were ob- tained 10 seconds after intravenous injection of 40 ml of Iomeprol (Iomeron 300; Bracco Diagnostics, Princeton, NJ) containing 300 mg of iodine per milliliter given at a rate of 5 ml/sec, followed by 40 ml of saline chaser, by means of a dual power injector. The scanning parameters were as follows: 5 mm reconstructed section thickness, 120 kVp, 60 mA. Seventy-five images per slice were acquired with a continuous scan of over 40 seconds. After completion of the perfusion scans, intravenous contrast was administered at 2 ml/kg and a routine abdominal study was obtained by using 1.25-mm contiguous sections (1-second gantry rotation time, 120kVp, 60mA). We set a delay time of more than 5 minutes to avoid the possibility that contrast enhanced liver from the previous perfusion scan could make less evident the metastases at the diagnostic scan. All perfusion images were reviewed by two or more members of the radiological team of our department, with more than 5 years of experience in gastrointestinal radiological imaging.
Image analysis
The data were transferred to an image processing workstation (Advantage Workstation 4.2, GE Medical Systems, Milwaukee, WI) and analyzed by using commercial deconvolution-based CT perfu- sion software (Functool 2.6; GE Medical Systems, Milwaukee, WI). The liver is an organ with a dual blood supply, and the following pharmacokinetic model was adopted to take both into account:
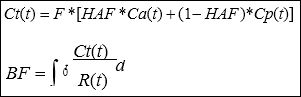
Where *denotes the convolution operator. When iodinated con- trast material can be considered as a purely intravascular tracer on dynamic CT, concentration of the contrast material within tissue Ct(t) can be obtained with the hepatic arterial input Ca(t) and portal input Cp(t). Hepatic Arterial Fraction (HAF) is the percentage ratio of in- puts. These inputs were determined by placing a Region of Interest (ROI) over the abdominal aorta, as a surrogate for the hepatic artery, and the portal venous trunk. A time-density curve for each input is generated by these ROIs. The change in contrast material volume is shown as the impulse residue function R (t), and when F is constant in time, we can calculate Ct(t), Ca(t), and Cp(t) from the ROIs. With the deconvolution method we can calculate F•R(t) and its height, to determine blood flow (BF, ml/min/100g tissue).
The hepatic tissue ROI was set over the whole liver, drawn free-hand along the peripheral margin, and care was taken to avoid in- cluding visible hepatic vessels. Lesions such as metastatic tumors and cysts were also excluded from the ROI area. Movements were corrected manually by adjusting the ROI position on each frame so as to encompass the same region for the whole dynamic sequence. This process was repeated 3 times for each scan.
The BF and HAF values were calculated in the workstation and a functional map was displayed in colors ranging from blue to red, with red being the higher range of display. The Perfusion CT param- eter values within the hand-drawn ROI area were averaged within the whole liver, and then across the 4 sequential slice. We then compared the parameters with the histopathological characteristics of the prima- ry colorectal tumor.
Statistical analysis
All statistical analyses were performed using Stat View-J Version 5.0 (SAS Institute Inc., Cary, NC). Nonparametric Mann-Whitney U test was used for comparisons of quantitative values and P values were calculated for each comparison. All P values were two-tailed, and P values of 0.05 or less were considered statistically significant.
Results
Perfusion CT parameters in various histopathological background
The demographic data and clinicopathologic characteristics of all patients are summarized in table 1. Of the 58 patients with colorectal cancer, 16 were shown to have synchronous distant metastasis. Thirteen possessed liver metastases, 2 had lung metastases, and 1 had both. The median total hepatic blood flow of all patients was 154 ml/min/100g, and the hepatic arterial fraction was 21.8%. Univariate analysis showed that 8 significant factors were correlated to the perfusion parameters: Sex, tumor size, depth, lymph node metastasis, stage, lymphovascular invasion, synchronous and postoperative metastasis. No significant correlation was found between BF or HAF and age, location or histopathological differentiation. There was a significant increase in hepatic blood flow with tumor size over 5cm in comparison to 5cm and under, and with T3/T4 compared to Tis/T1/T2. The presence of lymph node metastases also increased BF in comparison to their absence. Advanced cases of stages III/IV according to TNM staging had higher BF than the earlier staged cases of 0/I/II.
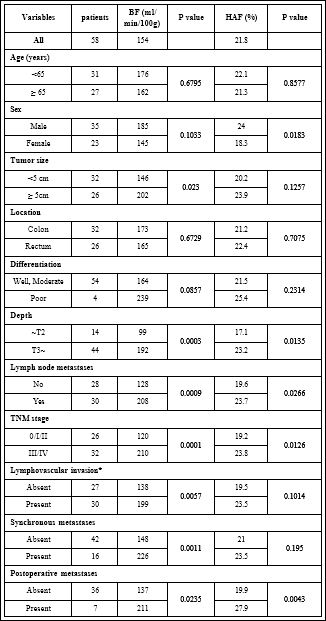
Table 1: Median values of Perfusion CT derived parameters and different variables.
Note: *Lymphovascular invasion was assessed in 57 patients whose cancer specimens were surgically resectable.
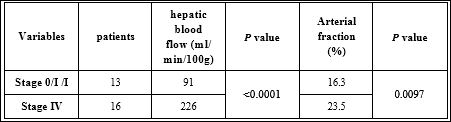
Table 2: Median values of hepatic blood flow and arterial fraction compared between patients with overt metastases and stage 0/I colorectal cancers.
Comparison of Perfusion CT parameters between livers with and without synchronous distant metastases
We also observed significant differences between the perfusion values of livers with and without metastatic lesions. BF in cases with overt distant metastasis significantly increased compared to cases without. There was no significant difference in HAF values between livers with and without metastases. However, when BF was compared between cases with overt metastases and stage 0/I cases, which are unlikely to have any risk for distant metastases and therefore can substitute for normal control, the difference became statistically significant (Table 2). Both BF and HAF were lower in stage 0/I cases compared to metastatic cases.
Perfusion CT and short-term outcome
In the 42 cases diagnosed as stage III and under, preoperative liver perfusion parameters were compared between cases which did and did not develop postoperative metastases. There was a significant increase in HAF in the 7 cases with postoperative metastases compared to those with no recurrence. The metastatic lesions appearing in the early periods of postoperative follow-up are summarized in table 3, 3 metastases to the liver, 3 to the lung, and 1 to both. There was no difference in BF or HAF in terms of metastasis location, liver, lung or both. The median BF and HAF values in the metastatic cases were 194 ml/min/100g and 29.4%, but there was no definite parameter level that would indicate a risk for future metastases.
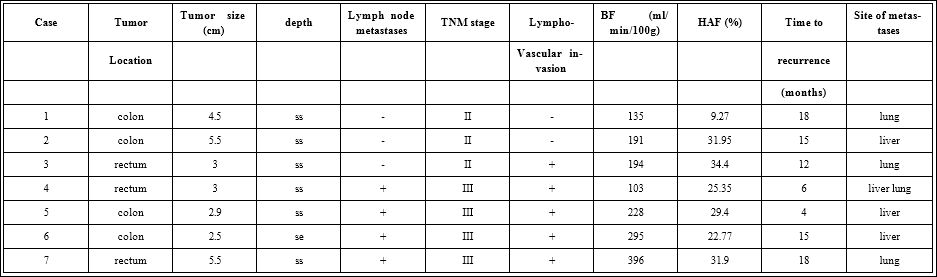
Table 3: Clinicopathological factors and perfusion parameters of 7 cases with postoperative metastases appearing during follow-up.
Discussion
The normal liver receives approximately 20-30% of its blood supply via the hepatic artery and the remainder by the portal vein. This proportion between hepatic artery and portal perfusion varies according to the pathologic status of the liver. In chronic liver diseases, the increase in intrahepatic vascular resistance decreases the fraction of portal hepatic blood flow. This decrease is partially compensated for by an increase in arterial inflow [20]. It is well documented that hepatocellular carcinomas are mainly supplied by the hepatic artery [21], and as for metastatic lesions, portal blood flow decreases over the evolution of the lesion and is replaced by the hepatic artery by a process of neoangiogenesis in late stages [22,23]. These observations suggest that quantitative measurement of hepatic arterial and portal blood flow may prove useful for identifying patients with hepatic tumors before they become visible by conventional imaging modalities. CT imaging of the liver has delivered promising results in this area. Quantification of liver perfusion parameters with contrast-enhanced CT enabled early detection of hepatocellular carcinoma in rats [24]. Micro metastases in a morphologically normal rat liver was shown to cause alterations in liver blood flow [25] (Figure 1).
As venous drainage of the colon is via the portal system, the first site of hematogenous spread of malignancy has always been regarded as the liver. Upon follow-up of the patients without synchronous overt metastases, we encountered 3 patients with postoperative metastases to the liver, 3 to the lung, and 1 to both, all with a significant change in hepatic flow. Among cases with metastases only to the lung, the possible existence of occult liver metastases that are yet to become apparent on near-future CT scans must be considered, although metastases bypassing the liver have been mentioned in several reports over the years, including to the lungs and thyroid [26,27]. An elevation of hepatic arterial flow during the early stage of metastases formation with minimal reduction in portal flow has been reported on the basis of Doppler ultrasound measurements [8]. These observations may suggest that changes in hepatic hemodynamics may not be purely local. Early flow alterations have been reported to be at least partly due to some kind of humoral tumor effect on several vascular targets, locally or more distally at the splanchnic network [7]. Whether this circulating agent is a tumor product or an endogenous agent remains unclear. Circulating tumor cells in peripheral blood coexisting with occult distant metastases of colorectal cancer patients have been detected [28,29], and such tumor cells may be associated with the vasoactive process. Another explanation for the change in hemodynamics prior to metastases could be the premetastic niche, or the change in the microenvironment as preparation for the metastases to grow [30]. There have been reports on the process of cancer metastases to depend not only on the existence of circulating products from the primary tumor, but also on the change in the environment of the metastatic organ itself [31,32]. Any of these speculations could lead to the possibility of detecting hepatic hemodynamic changes accompanying occult metastases as shown in our study with Perfusion CT.
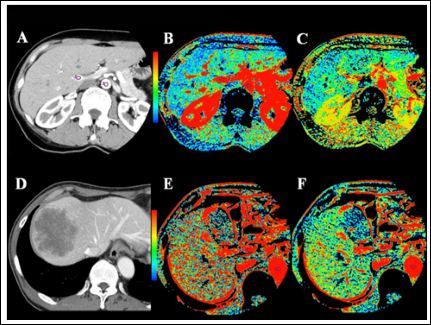
Figure 1:
Case 1: A) CT image of a 56 year old female with rectal cancer shows region of interest placed within the aorta and portal vein. B) Perfusion map of hepatic blood flow shows a blue-green liver, meaning a low degree of perfusion with an average BF of 92.4 ml/ min/100g. C) Perfusion map of hepatic arterial fraction shows the average HAF to be 23.1%. Although the resected cancer invaded the serosa, with lymph node metastases, the patient has been recurrence free for 2 years.
Case 2: Case 2: D) A 67 year old male with cancer to the cecum presented subserosal invasion with no lymph node metastases. Six months into postoperative follow-up, a metastatic lesion to the liver appeared on CT. E) The preoperative perfusion map of liver blood flow shows the liver to be mainly red, the average BF of 191 ml/min/100g. F) Preoper- ative assessment of HAF showed 32.0%.
We evaluated BF and HAF of the liver parenchyma at the time of diagnosis of the primary colorectal cancer. When comparing the perfusion parameters of the liver with and without metastases, there was no change in HAF. Livers possessing metastases large enough to become overt on conventional CT have been reported to change the blood flow within the organ due to mechanical obstruction [33]. Angiogenesis surrounding large tumors may become activated to the extent that total blood flow will increase, but at the same time mechanical tumor compression may result in the reduction of both arterial and portal flow. This hypothesis would only be true for metastases to the liver, but 15 out of the 16 known synchronous distant metastases were to the liver, an observation that may provide a reasonable explanation for the rise in BF of the whole liver despite unaltered HAF.
On the other hand, both parameters were statistically elevated in patients who later developed distant metastases compared to those who did not. These findings suggest that patients who subsequently develop metastases already have altered hepatic hemodynamics before lesions can be visualized on conventional helical CT scans. At the time of diagnosis, about 25% of patients are believed to possess occult liver metastases [34]. About 10% of colorectal cancer patients have lung metastases [35,36], with only 10% of these being isolated in the absence of liver metastases [37,38], indicating an extremely low incidence for any assessment of occult disease. Most of these occult metastases already present at the time of surgical resection of the primary tumor are said to become apparent during the first 2 years of follow-up [39]. If these occult metastases are able to be detected beforehand, patients in need of more aggressive adjuvant therapies and close follow-up could be distinguished. A biological marker for these occult metastases is urgently needed.
To date, the most useful criterion for predicting residual microscopic disease is the presence of lymph node metastases, although other criteria such as the presence of venous invasion [4], a deeper level of invasion, and less differentiation of the carcinoma [40] may also be important. To reduce the incidence of recurrent metastases, adjuvant systemic chemotherapy is recommended for all patients with node-positive colorectal cancer [41]. Up to 30% of patients with node-negative cancer will develop recurrent disease, including liver metastases [42,43], but adjuvant systemic treatment of all node-negative patients would lead to overtreatment and unnecessary complications since survival benefits have not yet been proven [44]. Node-negative colorectal cancer patients with higher risks of recurrence have been reported to include those presenting with bowel obstruction or perforation, T4 invasion, lymphovascular invasion, poorly differentiated histology, and inadequate lymph node sampling [45]. However, none of our stage II patients with distant recurrence presented any of these poor prognostic factors. Combined with the known risk factors, preoperative assessment of liver perfusion may be useful for identifying high-risk stage II patients who may benefit from adjuvant therapy. The earlier identification of these patients would also facilitate the use of other strategies such as antiangiogenetic therapies targeted against vascular endothelial growth factors. Promising results have been demonstrated as in the effective use of bevacizumab in colorectal cancer [46].
Another important factor of this study is that distant metastases could occur in colorectal cancer patients with a variety of histopathological backgrounds, and therefore traditional staging systems are insufficient to accurately predict outcome. The addition of hepatic perfusion assessment to the more traditional TNM staging may improve outcome prediction, as these parameters could be used to recognize those who should be considered for adjuvant therapy.
Our short-term follow-up of one year may be too short to make assumptions on predictive factors for metastases, and our assessments are preliminary. We were unable to clearly define a threshold for determining the level of perfusion parameters at risk for metastases, due to the number of patients with developing metastases. However, there have been a number of other reports on the prediction of short-term outcome with hepatic hemodynamic changes [23,47], and if our results along with those preliminary reports are subsequently confirmed, they will demonstrate a statistically significant advantage for postoperative chemotherapy.
This study has highlighted the fact that changes in hepatic perfusion are due to the appearance of metastatic lesions, and Perfusion CT may be a method for identifying the smallest changes in liver blood flow to detect the presence of metastases before visualization by any other imaging modality.
References
- 1.Shike M, Winawer SJ, Greenwald PH, Bloch A, Hill MJ, et (1990) Pri- mary prevention of colorectal cancer. The WHO collaborating centre for the prevention of colorectal cancer. Bull World Health Organ 68: 377-385.
- 2.Kumar V, Abbas A, Fausto N (2005) Robbins and cotran pathological basis of disease: 7th Pathology.
- 3.Figueredo A, Coombes ME, Mukherjee S (2008) Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev.
- 4.Yamazoe Y, Maetani S, Onodera H, Nishikawa T, Tobe T (1992) Histo- pathological prediction of liver metastasis after curative resection of col- orectal cancer. Surg Oncol 1: 237-244.
- 5.Ohji Y, Yao T, Eguchi T, Yamada T, Hirahashi M, et (2007) Evaluation of risk of liver metastasis in colorectal adenocarcinoma based on the com- bination of risk factors including CD10 expression: Multivariate analysis of clinicopathological and immunohistochemical factors. Oncol Rep 17: 525-530.
- 6.Leen E, Goldberg JA, Anderson JR, Robertson J, Moule B, et al. (1993) Hepatic perfusion changes in patients with liver metastases: Comparison with those patients with cirrhosis. Gut 34: 554-557.
- 7.Carter R, Anderson JH, Cooke TG, Baxter JN, Angerson WJ (1994) Splanchnic blood flow changes in the presence of hepatic tumour: Evi- dence of a humoral mediator. Br J Cancer 69: 1025-1026.
- 8.Nott DM, Grime SJ, Yates J, Day DW, Baxter JN, et (1989) Changes in the hepatic perfusion index during the development of experimental hepat- ic tumours. Br J Surg 76: 259-263.
- 9.Fowler RC, Harris KM, Swift SE, Ward M, Greenwood DC (1998) He- patic doppler perfusion index: Measurement in nine healthy volunteers. Radiology 209: 867-871.
- 10.Glover C, Douse P, Kane P, Karani J, Meire H, et (2002) Accuracy of investigations for asymptomatic colorectal liver metastases. Dis Colon Rectum 45: 476-484.
- 11.Roumen RM, Scheltinga MR, Slooter GD, van der Linden AW (2005) Doppler perfusion index fails to predict the presence of occult hepatic col- orectal metastases. Eur J Surg Oncol 31: 521-527.
- 12.Sahani DV, Holalkere NS, Mueller PR, Zhu AX (2007) Advanced hepato- cellular carcinoma: CT perfusion of liver and tumor tissue--initial experi- Radiology 243: 736-743.
- 13.Sahani DV, Kalva SP, Hamberg LM, Hahn PF, Willett CG, et al. (2005) Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: Initial observations. Radiology 234: 785-792.
- 14.Wang JH, Min PQ, Wang PJ, Cheng WX, Zhang XH, et (2006) Dynam- ic CT evaluation of tumor vascularity in renal cell carcinoma. AJR Am J Roentgenol 186: 1423-1430.
- 15.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, et al. (2004) Direct evidence that the VEGF-specific antibody bevacizumab has anti- vascular effects in human rectal cancer. Nat Med 10: 145-147.
- 16.Pandharipande PV, Krinsky GA, Rusinek H, Lee VS (2005) Perfusion im- aging of the liver: Current challenges and future goals. Radiology 234: 661-673.
- 17.Platt JF, Francis IR, Ellis JH, Reige KA (1997) Liver metastases: Early detection based on abnormal contrast material enhancement at dual-phase helical CT. Radiology 205: 49-53.
- 18.Sheafor DH, Killius JS, Paulson EK, DeLong DM, Foti AM, et (2000) Hepatic parenchymal enhancement during triple-phase helical CT: Can it be used to predict which patients with breast cancer will develop hepatic metastases? Radiology 214: 875-880.
- 19.Sobin LH, Fleming ID (1997) TNM Classification of Malignant Tumors, fifth edition (1997). Union internationale contre le cancer and the Ameri- can Joint Committee on Cancer. Cancer 80: 1803-1804.
- 20.Van Beers BE, Leconte I, Materne R, Smith AM, Jamart J, et al. (2001) Hepatic perfusion parameters in chronic liver disease: Dynamic CT mea- surements correlated with disease severity. AJR Am J Roentgenol 176: 667-673.
- 21.Materne R, Van Beers BE, Smith AM, Leconte I, Jamart J, et al. (2000) Non-invasive quantification of liver perfusion with dynamic computed tomography and a dual-input one-compartmental model. Clin Sci (Lond) 99: 517-525.
- 22.Voboril R (2005) Blood supply of metastatic liver tumors: An experimen- tal study. Int Surg 90: 71-77.
- 23.Miles KA, Leggett DA, Kelley BB, Hayball MP, Sinnatamby R, et al. (1998) In vivo assessment of neovascularization of liver metastases using perfusion CT. Br J Radiol 71: 276-281.
- 24.Fournier LS, Cuenod CA, de Bazelaire C, Siauve N, Rosty C, et (2004) Early modifications of hepatic perfusion measured by functional CT in a rat model of hepatocellular carcinoma using a blood pool contrast agent. Eur Radiol 14: 2125-2133.
- 25.Cuenod C, Leconte I, Siauve N, Resten A, Dromain C, et (2001) Early changes in liver perfusion caused by occult metastases in rats: Detection with quantitative CT. Radiology 218: 556-561.
- 26.Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Tanaka Y, et al. (2003) Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology 50: 1362-
- 27.Phillips JS, Lishman S, Jani P (2005) Colonic carcinoma metastasis to the thyroid: A case of skip metastasis. J Laryngol Otol 119: 834-836.
- 28.Guadagni F, Kantor J, Aloe S, Carone MD, Spila A, et al. (2001) Detec- tion of blood-borne cells in colorectal cancer patients by nested reverse transcription-polymerase chain reaction for carcinoembryonic antigen messenger RNA: longitudinal analyses and demonstration of its potential importance as an adjunct to multiple serum Cancer Res 61: 2523- 2532.
- 29.Lledo SM, Garcia-Granero E, Dasi F, Ripoli R, Garcia SA, et al. (2004) Real time quantification in plasma of human Telomerase Reverse Tran- scriptase (hTERT) mRNA in patients with colorectal cancer. Colorectal Dis 6: 236-242.
- 30.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, et (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438: 820-827.
- 31.Mimori K, Fukagawa T, Kosaka Y, Kita Y, Ishikawa K, et al. (2008) He- matogenous metastasis in gastric cancer requires isolated tumor cells and expression of vascular endothelial growth factor receptor-1. Clin Cancer Res 14: 2609-2616.
- 32.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, et al. (2008) Endothelial progenitor cells control the angiogenic switch in mouse lung Science 319: 195-198.
- 33.Civalleri D, Scopinaro G, Balletto N, Claudiani F, DeCian F, et (1989) Changes in vascularity of liver tumours after hepatic arterial embolization with degradable starch microspheres. Br J Surg 76: 699-703.
- 34.Finlay IG, McArdle CS (1988) The role of occult hepatic metastases in staging colorectal carcinoma. Scand J Gastroenterol Suppl 149: 150-154.
- 35.Rotolo N, De Monte L, Imperatori A, Dominioni L (2007) Pulmonary re- sections of single metastases from colorectal cancer. Surg Oncol 1: 141-
- 36.Goya T, Miyazawa N, Kondo H, Tsuchiya R, Naruke T, et (1989) Sur- gical resection of pulmonary metastases from colorectal cancer. 10-year follow-up. Cancer 64: 1418-1421.
- 37.Pihl E, Hughes ES, McDermott FT, Johnson WR, Katrivessis H (1987) Lung recurrence after curative surgery for colorectal cancer. Dis Colon Rectum 30: 417-419.
- 38.McCormack PM, Attiyeh FF (1979) Resected pulmonary metastases from colorectal cancer. Dis Colon Rectum 22: 553-556.
- 39.Stone MD, Kane R, Bothe AJr, Jessup JM, Cady B, et al. (1994) Intra- operative ultrasound imaging of the liver at the time of colorectal cancer Arch Surg 129: 431-435.
- 40.Adachi Y, Inomata M, Kakisako K, Sato K, Shiraishi N, et (1999) His- topathologic characteristics of colorectal cancer with liver metastasis. Dis Colon Rectum 42: 1053-1056.
- 41.Moertel CG (1994) Chemotherapy for colorectal cancer. N Engl J Med 330: 1136-1142.
- 42.Quah HM, Chou JF, Gonen M, Shia J, Schrag D, et (2008) Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum 51: 503-507.
- 43.Madbouly KM, Senagore AJ, Mukerjee A, Delaney CP, Connor J, et al. (2007) Does immunostaining effectively upstage colorectal cancer by identifying micrometastatic nodal disease? Int J Colorectal Dis 22: 39-48.
- 44.Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer (1999) International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IM- PACT B2) Investigators. J Clin Oncol 17: 1356-1363.
- 45.Benson AB, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, et al. (2004) American Society of clinical oncology recommendations on adju- vant chemotherapy for stage II colon J Clin Oncol 22: 3408-3419.
- 46.Fernando NH, Hurwitz HI (2003) Inhibition of vascular endothelial growth factor in the treatment of colorectal Semin Oncol 30: 39-50.
- 47.Leggett DA, Kelley BB, Bunce IH, Miles KA (1997) Colorectal cancer: Diagnostic potential of CT measurements of hepatic perfusion and impli- cations for contrast enhancement protocols. Radiology 205: 716-720.
Citation:Satoh A, Shuto K, Okazumi S, Ohira G, Natsume T, et al. (2021) Hepatic Blood Flow in Patients with Colorectal Carcinoma Assessed by Perfusion Ct May Represent a Marker of Early Metastases. J Case Repo Imag 5: 041.
Copyright: © 2021 Satoh A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


