*Corresponding Author:
Sara Giannoni,
Neurology Unit, San Giuseppe Hospital, Empoli, Italy
Tel: +39 3489313995
Email: giannonisara@gmail.com
Abstract
Cardiac myxoma is the most common benign heart tumor. Its complete resection is usually curative; however, sometimes recurs with metastases, exhibiting a malignant potential through mechanisms which remain unclear. The brain is the most frequent metastatic site. With a small number of cases reported in literature, there is currently no standard management for cerebral myxoma metastases, and most of the reported patients have been treated with cere- bral surgery, but postoperative chemotherapy and/or radiation have been attempted. We report a case treated for a benign atrial myxoma, which developed multiple brain metastases, in whom a “wait and see” strategy was adopted, delaying surgery approach with a favorable outcome over a prolonged follow-up period.
Keywords
Brain; Cardiac myxoma; Metastases
Case Presentation
A 72-year-old woman was admitted to the emergency room for acute weakness of the right arm and speech impairment.
Ten months before, she underwent resection of an inter-atrial sep- tal cardiac benign myxoma that has manifested embolizing the left upper limb.
Her past medical history also included resection of VIII right cranial nerve for schwannoma at the age of 52 years, with recurrence of this lesion at the age of 55, with hydrocephalus treated with ventriculoperitoneal shunt and complete resection of the lesion and subsequent radiotherapy.
At the neurological examination the patient was drowsy, with fluent aphasia, mild dysarthria, right hemianopsia and a mild weakness of the right arm. As a consequence of the previous neurosurgery, chronic right facial nerve perifer ical palsy and deafness in the right ear were also observed. The acute neurological deficits persisted only a few hours with subsequent complete remission. Transient neurological symptoms and electroencephalographic focal slowing in the left temporo-occipital regions led us to interpret the neurological symptoms as a focal limited epileptic seizure.
In addition to previous neuroradiological features related to the past neurosurgery, the brain CT scan revealed some hyperdense masses of round shapes in the right occipital and left parietal regions (Figure 1, section 1 a-b), interpreted as hemorrhagic metastatic lesions or, alternatively, as abscesses from opportunistic germs. The brain MRI defined these findings better, revealing one nodular lesion in the right occipital pole and a group of numerous and confluent lesions in the left parietal lobe, surrounded by a vasogenic edema, the largestone located in the left supramarginal gyrus and parieto-temporo-occipital carrefour (section 1 c-f). All lesions presented hemorrhagic components in subacute or chronic phases (section 1e-f, g-h), mildly enhancing gadolinium (section 1 i-l). There were also signs of leptomeningeal hemosiderosis (section 1 g-h).
In the absence of clinical and biohumoral signs of infection, the neuroradiological features supported the diagnosis of metastatic hemorrhagic lesions. Total body CT scans and F18-fluorodeoxyglucose positron emission tomography were both negative for malignan- cy, as well as esophagogastro-and colonoscopy. We have therefore assumed that cerebral lesions were secondary to myxoma. Transe- sophageal echocardiogram demonstrated no recurrence of the tumor.
Given the age of the patient, with an already complex neurosurgical history, the paucisymptomatic presentation of disease, and the site of lesions in eloquent areas, it was preferred not to proceed with surgical options, and to keep a “wait and see” strategy.
Thus, the patient was treated with Dexamethasone 8 mg/die per os for two weeks, which was then slowly tapered down until a low main- tenance dose of 0.5-1 mg/die. She was also treated with Clobazam as an antiepileptic drug.
A second total body CT-PET scan, performed 12 months after the first screening, remains negative.
During the follow-up (42 months since the removal of myxoma, 32 months since detection of cerebral involvement) the clinical situation remained stable. Only a brief epileptic seizure similar to the one described above occurred, and Carbamazepine was started while Clobazam stopped. The series of brain MRIs -performed at 2, 5, 12, 18, months - showed a favorable evolution of the neuroradiological picture with a reduction of nodular lesions, decreasing in both va- sogenic edema and enhancement (Figure 1, section 2 a-b, c-d, i-l). No more acute bleeding was detectable but persistent hemosiderin components both in the parenchymal and subarachnoid space were observed (section 2 g-h). CT scans progressively showed increased hyperdensity in some of the cortico-subcortical left parietal lesions consistent with calcifications (section 2, a-b).
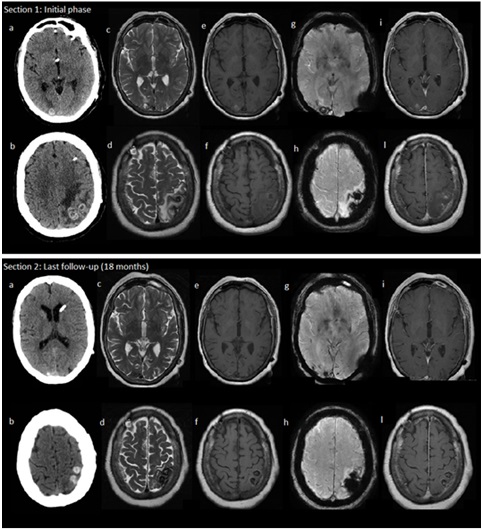
Figure 1: Section 1 shows neuroimaging findings in the initial phase: the brain CT scan shows haemorragic metastatic lesions in the right occipital and left parietal re- gions (a-b); the MRI shows haemorragic metastatic confluent lesions surrounded by vasogenic edema (c-f) with haemorragic components in subacute or chronic phases (g-h); Metastases were mildly and patchy enhancing gadolinium (i-l). Section 2 shows neuroimaging findings at the last follow up (18 months later - 28 months after com- plete resection of myxoma): we show a reduction of nodular lesions, in size, vasogenic oedema, contrast enhancement (a-b, c-d, i-l); any new haemorragic lesions was de- tected, while blood components were detectable as haemosiderine (g-h) Mixed with calcifications (a-b).
Discussion
After a complete curative treatment with local resection, myxo- mas can rarely cause metastatic disease to the brain [1-5]. In medical literature, including the present case, there only 35 cases of myxoma cerebral metastases (Table 1), that are described with variable out- comes [1-33], including fatal cases [5-11]. Although a standard man- agement of patients with cerebral myxoma metastases has not been established, the most reported treatment is surgery, with or without adjuvant radiotherapy or chemotherapy [2,5,11-16].
Our report shows a patient who has not been treated with brain surgery or adjuvant therapy that experienced a benign course of disease at prolonged follow-up. Over time, our patient had no substantial changes in the neurological status, while the MRI evidence of reduction of metastases size, blood components and vasogenic edema. In addition, we observed the evolution of some of the hemorrhagic lesions into calcifications, supporting the idea of a change of disease from an evolving to an inactive stage.
|
Author |
Year of report |
Age |
Sex |
Interval to recurrence (months) since myxoma diagnosis |
Other recur- rent sites |
Surgery for brain lesions |
Radiotherapy |
Chemotherapy |
Outcome follow-up (months) |
|
Our case |
2020 |
72 |
Female |
10 |
No |
No |
No |
No |
AWD42 |
|
Ghodasara et al. [16] |
2020 |
63 |
Female |
12 |
No |
No |
No |
No |
- |
|
Rajeshwari et al. [11] |
2020 |
56 |
Female |
-6 |
- |
No |
No |
No |
DOD6 |
|
Rajeshwari et al. [11] |
2020 |
17 |
Male |
18 |
- |
Yes |
Yes |
No |
- |
|
Wan et al. [18] |
2020 |
39 |
Female |
7 |
No |
Yes |
No |
No |
AWD18 |
|
Roque et al. [13] |
2020 |
48 |
Female |
7 |
No |
No |
Yes |
No |
AWD18 |
|
Maas JA et al. [12] |
2019 |
62 |
Male |
12 |
Fingertips |
Yes |
No |
No |
NED48 |
|
Asranna et al. [19] |
2017 |
57 |
Female |
12 |
No |
- |
- |
- |
- |
|
Rose et al. Case 15 |
|
|
|
|
|
|
|
|
|
|
2016 |
44 |
Male |
5 |
No |
No |
Yes |
No |
DOD17 |
|
|
Rose et al. Case 2 [5] |
2016 |
52 |
Female |
0 |
No |
No |
No |
Yes |
AWD6 |
|
Raza and Kamal [1] |
2012 |
47 |
Female |
4 |
No |
Yes |
No |
No |
- |
|
Wolf et al. Case 1 [20] |
2008 |
60 |
Male |
- |
No |
Yes |
No |
No |
- |
|
Wolf et al. Case 2 [20] |
2008 |
65 |
Female |
- |
No |
No |
No |
No |
- |
|
Moiyadi et al. [2] |
2007 |
35 |
Male |
48 |
No |
Yes |
Yes |
No |
AWD6 |
|
Altundag et al. [14] |
2005 |
41 |
Female |
15 |
No |
Yes |
Yes |
No |
AWD 63 |
|
Acikel et al. [21] |
2004 |
58 |
Female |
0 |
No |
No |
No |
No |
- |
|
Hirudayaraj et al. [22] |
2004 |
50 |
Female |
-1 |
No |
Yes |
No |
No |
- |
|
Hou et al. [6] |
2001 |
37 |
Female |
10 |
Bone |
No |
No |
No |
DOD 12 |
|
Bernet et al. [15] |
1998 |
31 |
Male |
2 |
Muscle, lung |
Yes |
Yes |
Yes |
NED 120 |
|
Scarpelli [23] |
1997 |
64 |
- |
144 |
No |
Yes |
No |
No |
- |
|
Samaratunga et al. [24] |
1994 |
60 |
Female |
-7 |
No |
Yes |
No |
No |
NED 21 |
|
Kanda et al. [25] |
1994 |
70 |
Male |
-7 |
No |
Yes |
No |
No |
NED 9 |
|
Wada et al. [26] |
1993 |
70 |
Male |
- |
No |
Yes |
No |
No |
- |
|
Todo et al. [7] |
1992 |
32 |
Female |
10 |
Jejenum |
No |
No |
No |
DOD 10 |
|
Chozick et al. [27] |
1992 |
61 |
Female |
- |
No |
Yes |
No |
No |
- |
|
Kotani et al. [8] |
1991 |
48 |
Male |
3 |
Soft tissue, aorta |
Yes |
No |
No |
DOD 53 |
|
Ng and Poon [3] |
1990 |
54 |
Male |
6 |
No |
Yes |
No |
No |
AWD 18 |
|
De Morais et al. [9] |
1988 |
73 |
Male |
0 |
Kidney, pan- crea, stomach |
No |
No |
No |
DOD 1 |
|
Kadota et al. [28] |
1987 |
44 |
- |
3 |
Skin |
Yes |
No |
No |
- |
|
Bazin et al. [29] |
1987 |
56 |
- |
48 |
No |
Yes |
No |
No |
- |
|
Morimoto [30] |
1986 |
44 |
Female |
- |
Skin |
Yes |
No |
No |
- |
|
Markel et al. [31] |
1986 |
18 |
Female |
30 |
Bone |
No |
No |
No |
AWD 39 |
|
Seo et al. [32] |
1980 |
36 |
Female |
96 |
Bone |
Yes |
No |
No |
AWD 120 |
|
Budzilovich et al. [10] |
1979 |
52 |
- |
0 |
No |
No |
No |
No |
DOD 1 |
|
Rankin and DeSousa [33] |
1978 |
44 |
Female |
96 |
No |
Yes |
No |
No |
AWD120 |
Table 1: Reported cases in literature of cardiac myxoma metastasizing to the brain whit relative treatment and outcomes.
Abbreviations: NED: No Evidence of Disease; DOD: Dead of Disease; AWD: Alive With Disease. Modified from Rose at al., Moiyadi et al., and Altundag et al.
The natural history of brain metastases of myxoma is extremely variable, as it may be stable over months, or manifesting with neurological deficits in a brief period of time with fatal outcome [2,5,12,14]. In the series of 27 cases collected by Maas and collegues [12], eight patients did not undergo brain surgery; among these, only two were still alive after a follow up of 63 and 39 months respectively, five died within 8 months on average, one was lost to follow up. The few heterogeneous cases reported so far does not allow to indicate possible factors for disease progression, although the worst prognosis has been observed in patients who had other recurrent sites of disease in addition to the brain, thus underlining a more aggressive disease in these patients [5,14].
Although the mechanisms by which myxoma may have malignant potential have yet to be elucidated, the presence of robust inflamma- tory and vasculitic changes associated with metastatic lesions suggest a strong focal inflammatory reaction induced by myxoma [14,17]. In our case, the good response to corticosteroids suggests a main role of inflammation produced by myxoma, more than a malignant local invasiveness of the tumor. However, this finding must be confirmed -or not- in additional cases.
In conclusion, a “wait and see” strategy with a conservative ap- proach may be considered in patients with myxoma brain metastases, as no strong evidence exists to treat these patients with surgery, radio- or chemotherapy. Anti-inflammatory treatments, monitoring clinical and neuroradiological pictures and seizure controls are appropriate options.
Multicenter studies with uniform multidisciplinary clinical and radiological criteria at the follow-up are needed to better characterized the natural history of brain metastases management in myxoma.
References
- 1. Raza E, Kamal AK (2012) Recurrent non-aneurysmal, metastatic intra- parenchymal haemorrhages following resection of atrial myxoma-Case report and literature review. BMJ Case Rep.
- 2. Moiyadi AV, Moiyadi AA, Sampath S, Kalpana SR, Mahadevan A, et al. (2007) Intracranial metastasis from a glandular variant of atrial myxoma. Acta Neurochir (Wien) 149: 1157-1162.
- 3. Ng HK, Poon WS (1990) Cardiac myxoma metastasizing to the Case report. J Neurosurg 72: 295-298.
- 4. Kierdaszuk B, Gogol P, Kolasa A, Maj E, Zakrzewska-Pniewska B, et al. (2014) Multiple metastatic intracranial lesions associated with left atrial Pol J Radiol 79: 262-267.
- 5. Rose D, Papa A, Tomao S, Greco E, Zacharias J (2016) Cerebral metasta- ses in patients with left atrial myxoma. J Card Surg 31: 289-293.
- 6. Hou YC, Chang S, Lo HM, Hsiao CH, Lin FY (2001) Recurrent cardiac myxoma with multiple distant metastasis and malignant change. J Formos Med Assoc 100: 63-65.
- 7. Todo T, Usui M, Nagashima K (1992) Cerebral metastasis of malignant cardiac Surg Neurol 37: 374-379.
- 8. Kotani K, Matsuzawa Y, Funahashi T, Nozaki S, Tarui S, et (1991) Left atrial myxoma metastasizing to the aorta, with intraluminal growth causing renovascular hypertension. Cardiology 78: 72-77.
- 9. de Morais C, Falzoni R, Alves VA (1988) Myocardial infarct due to a unique atrial myxoma with epithelial-like cells and systemic metastases. Arch Pathol Lab Med 112: 185-190.
- 10. Budzilovich G, Aleksic S, Greco A, Fernandez J, Harris J, et al. (1979) Malignant cardiac myxoma with cerebral Surg Neurol 11: 461- 469.
- 11. Rajeshwari M, Subramanian P, Suri V, Nambirajan A, Garg A, et (2020) Metastatic lesions of atrial myxoma. A pathologist can clinch them all. Neuropathology 40: 295-301.
- 12. Maas JA, Menes M, Siomin V (2020) Cardiac myxoma with cerebral me- tastases and chronic lymphocytic leukemia/small lymphocytic lymphoma: A case report and J Neurol Surg Reports 81: 1-6.
- 13. Roque A, Kimbrough T, Traner C, Baehring JM, Huttner A, et al. (2019) Somatic PRKAR1A mutation in sporadic atrial myxoma with cerebral pa- renchymal metastases: A case report. J Med Case Rep 13: 1-9.
- 14. Altundag MB, Ertas G, Ucer AR, Ucer AR, Durmus, DS, et al. (2005) Brain metastasis of cardiac myxoma: Case report and review of the litera- J Neurooncol 75: 181-184.
- 15. Bernet F, Stulz PM, Carrel P (1998) Irradiation of a Metastatic Myxoma. Published online 1-2.
- 16. Ghodasara SA, Balasubramanian R, Varadharajan S, Shobhanaa PS (2020) Cardiac phoenix in the brain-occult intracranial hemorrhagic metastases from completely resected atrial Surg Neurol Int 11.
- 17. Keeling IM, Oberwalder P, Anelli-Monti M, Schuchlenz H, Demel U, et (2002) Cardiac myxomas: 24 Years of experience in 49 patients. Eur J Cardio-thoracic Surg 22: 971-977.
- 18. Wan Y, Du H, Zhang L, Guo S, Xu L, et (2019) Multiple cerebral metas- tases and metastatic aneurysms in patients with left atrial Myxoma: A case report. BMC Neurol 19: 4-9.
- 19. Asranna AP, Kesav P, Nagesh C, Sreedharan SE, Kesavadas C, et (2017) Cerebral aneurysms and metastases occurring as a delayed complication of resected atrial Myxoma: Imaging findings including high resolution Vessel Wall MRI. Neuroradiology 59: 427-429.
- 20. Wolf M, Wibail A, De Jonghe P (2008) Delayed hemorrhagic cerebral me- tastases after atrial myxoma resection: Report of two cases and review of the Rev Esp Cir Ortop Traumatol 52: 75-79.
- 21. Acikel M, Yekeler I, Ates A, Erkut B (2004) A giant left atrial myxoma: An unusual cause of syncope and cerebral emboli. Int J Cardiol 94: 325-326.
- 22. Hirudayaraj P, Arya B, Suvarna SK, Payne G, Palaniswamy A (2004) Myxomatous meningeal tumour: A case of “metastatic” cardiac myxoma. Int J Cardiol 96: 471-473.
- 23. Scarpelli M, Montironi R, Ricciuti R, Vecchioni S PF (1997) Cardiac myx- oma with glandular elements metastatic to the brain 12 years after the re- moval of the original Clin Neuropathol 16: 190-194.
- 24. Samaratunga H, Searle J, Cominos D, Le Fevre I (1994) Cerebral metasta- sis of an atrial myxoma mimicking an epithelioid hemangioendothelioma. Am J Surg Pathol 18: 107-111.
- 25. Kanda T, Sakamaki T, Murata K (1994) A cardiac myxoma with interleu- kin-6 production and cerebral metastasis. Int J Cardiol 45: 144-146.
- 26. Wada A, Kanda T, Hayashi R, Imai S, Suzuki T, et (1993) Cardiac myx- oma metastasized to the brain: potential role of endogenous interleukin-6. Cardiology 83: 208-211.
- 27. Chozick BS, Ambler MW, Stoll JJ (1992) Malignant astrocytoma six years after the resection of a cerebral metastatic cardiac myxoma: Case report. Neurosurgery 30: 923-927.
- 28. Kadota T, Imakita S, Mitomo M (1987) Metastatic brain tumor of atrial Neuroradiology 29: 218.
- 29. Bazin A, Peruzzi P, Baudrillard JC, Pluot M RP (1987) Cardiac myxoma with cerebral metastases. Neurochirurgie 33: 487-489.
- 30. Morimoto K, Fujita T, Wakayama A (1986) Cardiac myxoma metastatic to the No to Shinkei 38: 865-869.
- 31. Markel ML, Armstrong WF, Waller BF, Mahomed Y (1986) Left atrial myxoma with multicentric recurrence and evidence of metastases. Am Heart J 111: 409-413.
- 32. Seo IS, Warner TF, Colyer RA, Winkler RF (1980) Metastasizing atrial Am J Surg Pathol 4: 391-399.
- 33. Rankin LI, DeSousa AL (1978) Metastatic atrial myxoma presenting as intracranial mass. Chest 74: 451-452.
Citation:Giannoni S, Pizzanelli C, Frosini D, Canovetti S, Mancuso M, et al. (2021) Hemorrhagic Cerebral Metastases Presenting After Complete Resection of Atrial Myxoma: A Case Report with a Favorable Outcome and Review of the Literature. J Case Repo Imag 5: 042.
Copyright: © 2021 Giannoni S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


