*Corresponding Author:
Saad Al Shareef,
Section of Allergy & Immunology, Depart- ment of Medicine. King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia
Tel: +966114427492
Fax: +966114427499
E-mail: saalshareef@kfshrc.edu.sa
Abstract
Introduction: Allergic reactions to insect stings like Bees, Wasp as well as Ant bites are well known worldwide. These reactions can be mild ones, or severe causing anaphlyaxis. Fire ants (Red ants) Pogonomyrmex barbatus have been studied thoroughly in the United States since considered responsible for most of allergic reactions due to ant bites. In the Middle East including the Arabian Gulf region another species called Pachycondyla sennaarensis (black samsum ant) is responsible for most of the ant bites allergic reactions.
Case Presentation: A 43-year-old female with few years’ history of multiple severe allergic reactions (Anaphylaxis) due to exposure to Samsum Ant bites. Allergic skin prick test, using 1:10 dilution of Samsum Ant whole body extracts showed +7mm wheal at the site of Samsum Ant injection. Rush immunotherapy for Samsum ant was done using our protocol, and the patient tolerated the procedure well.
Discussion: Insect bites including Samsum ant immunotherapy could be life saving for patients who cannot avoid the recurrent exposure to the insect bites. Samsum Ant immunotherapy was never attempted previously as this is mostly a regional problem and thus the extract is not available commercially.
Conclusion: Samsum Ant Immunotherapy was never done before, as all the published data were referring to Fire Ant Immunotherapy. This is of significant importance as our area “Middle East” is populated with this type of Ants, and an allergic reaction to their bites can be fatal.
Keywords
Anaphylaxis; Pachycondyla sennaarensis; Rush Immunotherapy; Samsum Ant; Venom Immunotherapy
Introduction
Allergic reactions to Ant bites have been reported worldwide, ranging from mild reaction hives, anaphylaxis and shock. Fire ants have been studied thoroughly and are considered responsible for most of allergic reactions secondary to ants in the United States [1]. In China and Korea another species of ant known as Bachycondayla chinensis is a major cause of allergic reactions. In addition, Myrmecia pilosula (jack jumper ant) in Australia is a known health hazard, whereas in the Middle East another species called Pachycondyla sennaarensis (Samsum ant) is the responsible for most of the ant bites allergic reactions [2,3].
Pachycondyla sennaarensis belongs to the subfamily Ponerinae, a species that is widely distributed in tropical and subtropical regions worldwide. It is believed to be indigenous to Southeast Asia but has been reported from all of the Arab Gulf countries including Kuwait, Qatar, the United Arab Emirates, Oman, Yemen and Saudi Arabia [4]. In Saudi Arabia it seems to prefer areas of human habitation. Although the ant is a scavenger in nature, it stings human beings as a defensive behavior [5]. Recently, several anaphylactic cases following Samsum ant stings were reported in local clinics in Saudi Arabia, some of them were critical [6,7]. In a case series in Riyadh, Saudi Arabia in year 2006-2007, allergic reactions secondary to Samsum ant stings, which were identified by certified entomologist, was ranging from mild to severe allergic reactions requiring epinephrine use [8].
Patients with past anaphylactic reactions to stings and positive skin tests have a 30 to 60 percent chance of a similar reaction to a future sting [9-11]. This signifies the importance of Venom Immunotherapy (VIT) in such patients. The decision to pursue VIT should be a shared decision between the clinician and the patient.
VIT should be firmly advocated for individuals who had life-threatening anaphylaxis, and these patients should be advised that insect avoidance, and access to epinephrine is not considered an adequate alternative to VIT [12,13]. Also, it is important to discuss the risks of VIT with patients when obtaining informed consent. Briefly, 3 to 12 percent of patients have treatment-induced systemic reactions, although the majority of these reactions were mild and easily treated [14,15].
The mechanism of action of VIT is only partially understood. Venom-specific immunoglobulin G (IgG) typically increases, peaking at two to four months after starting VIT and then remains fairly constant over five to six years of treatment. These IgG antibodies are referred to as “blocking” antibodies because they are capable of blocking in vitro mediator release from allergen-stimulated mast cells and basophils. The production of blocking antibodies has been considered the most likely therapeutic mechanism [16,17]. Also, VIT appears to shift the T cell phenotype away from the T helper type 2 (producing interleukin-4 and interleukin-5) and toward the T helper type 1 (interferon-gamma) or a regulatory type of T response (interleukin-10 and the production of immunoglobulin G4) [18-21]. With this introduction, we will proceed to the case report.
Case 1
A 43-year-old female patient, who is known to have Hashimoto’s disease “Hypothyroidism”, presented with few years’ history of multiple anaphylaxes after exposure to Samsum ant. Initially, she would have only skin itchiness and redness. However, these symptoms progressed to be more sever, in form of shortness of breath and loss of consciousness, requiring emergency room visit and epinephrine use after each exposure to Samsum ant. Upon presenting to our institution, Skin Prick Test (SPT) was done using 1:10 dilution of Samsum ant whole body extract. The patient developed 7mm wheal at the site of Samsum ant injection and started to have shortness of breath (Histamine was 3 mm). Her SPT to commercial Black Ant extract was negative.
Preparation of Samsum ant extract
Live Samsum ants were collected from a garden in Riyadh. Collected ants were frozen using liquid nitrogen at -70°C. The frozen Samsum Ants were grinded and extracted using 1:10 (w/v) in coca’s solution (0.5 % NaCl, 0.4% Phenol, 0.25 % NaHCO3) by shaking for 12 hours at 4°C. The supernatant was collected after centrifugation at 4°C for 30 minutes. Extract was sterilized using Millipore bacterial filtration through 0.8µm, 0.45 µm, and finally with 0.22 µm filters. Sterility and purity test were conducted using Blood Agar and Brain Heart Infusion Agar for 15 days at 37°C. For the Skin Prick Test, 50% Glycerin was added to keep the extract stable for up to a year period. For the rush immunotherapy, extract was also prepared in coca’s solution, but the amount of phenol crystal was reduced to half (0.2% only).
As Samsum ant allergy was greatly affecting her quality of life, the patient was eager to get desensitized. She was premedicated with antihistamine and one dose of Omalizumab (which was given one week prior to immunotherapy).
VIT was initiated at 1:1,000,000 dilution, on a monitored bed. Escalation of concentration and volume was done over 10 hours until we reached the maintenance concentration (1:100) (Table 1).
Apart from mild local reaction, the patient tolerated the procedure well, and was discharged the following day. Thereafter, she was given incremental volume of the maintenance concentration 1:100 on a weekly basis, until we reached 0.5 cc of volume weekly. She was given 4 doses of the same volume weekly, then every other week for 4 doses, then every 3 weeks for 4 doses, and then every month thereafter with very good tolerance. A repeated SPT to Samsum ant was done, while the patient on the maintenance monthly dose, and the patient developed a 2 mm wheal (Histamine was +3mm).
Unfortunately, after the patient has received 14 doses of the monthly maintenance dose, immunotherapy was stopped due to unavailability of extract. The patient was exposed to Samsum ant bite 4 months after stopping immunotherapy which resulted in only mild local reaction that responded well to antihistamine. Despite having only mild symptoms, her husband gave her Epipen injection, but he removed the injection immediately after he injected her, and he was sure that the drug was not administrated (the husband is familiar with Epipen as he had given her the injection several times before starting immunotherapy).
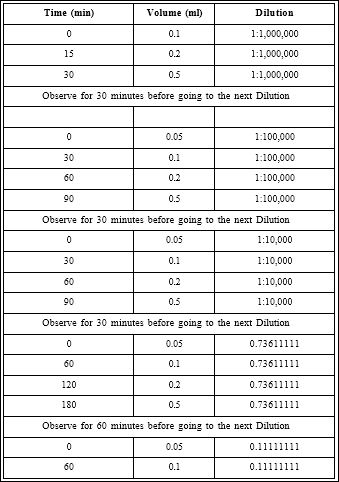
Table 1: Samsum ant Ultra-rush immunotherapy protocol. The immunotherapy was administered in a monitored setting in the intensive care unit over a period of 10 hours.
Discussion
Insect bites including Samsum ant immunotherapy could be life saving for patients who cannot avoid recurrent exposure to insect bites. Several Immunotherapy protocols have been suggested for different ant types. However, none was specific for Pachycondyla sennaarensis (black samsum ant). Research has shown that patients who have had an anaphylactic reaction to a Pachycondyla species “including Samsum” ant might not benefit from immunotherapy with an imported fire ant extract [7]. Immunotherapy with the extract of Pachycondyla species ants is expected to be highly effective.
Samsum Ant immunotherapy was never attempted previously as this is mostly a regional problem and thus the extract is not available commercially. A whole-body extract of confirmed Samsum ants was prepared in a in our Aerobiology laboratory. We created our own protocol for Samsum ant Rush immunotherapy, with the guidance of different protocols used for other types of insects and ants. The patient tolerated the procedure well with no significant complications. As our area is postulated with such ant, the availability of VIT for this type of ant is of significant importance as it could be lifesaving, especially when avoidance measures are ineffective.
Conclusion
We reported here a case of successful Samsum ant immunotherapy which was never done before, as all the published data were referring to Fire Ant Immunotherapy. This is of significant importance as our area “Middle East” is populated with this type of Ants, and an allergic reaction to their bites can be fatal.
References
- Bloom FL, DelMastro PR (1984) Imported fire ant death. A docu- mented case report. J Fla Med Assoc 71: 87-90.
- Hoffman DR, Dove DE, Moffitt JE, Stafford CT (1988) Allergens in Hymenoptera venom. XXI. Cross-reactivity and multiple reactivity between fire ant venom and bee and wasp venoms. J Allergy clin immunol 82: 828-834.
- Valentine MD (1992) Anaphylaxis and stinging insect hypersensitivity. JAMA 268: 2830-2833.
- Collingwood CA, Agoste D (1996) Formicidae (Insecta: Hymenoptera) of Saudi Arabia (Part 2). Fauna of Saudi Arabia 15.
- Collingwood CA (1985) Fauna of Saudi Arabia
- Al-Shahwan M, Al-Khenaizan S, Al-Khalifa M (2006) Black (samsum) ant induced anaphylaxis in Saudi Arabia. Saudi Med J 27: 1761-1763.
- Yun YY, Ko SH, Park JW, Hong CS (1999) Anaphylaxis to venom of the Pachycondyla species ant. J Allergy Clin Immunol 104: 879-
- Marzouqah AlAnazi, Mohammad AlAshahrani, Majid AlSalamah (2009) Black ant stings caused by Pachycondyla sennaarensis: A significant health hazard. Ann Saudi Med 29: 207-211.
- Hunt KJ, Valentine MD, Sobotka AK, Benton AW, Amodio FJ, et (1978) A controlled trial of immunotherapy in insect hypersensitivity. N Engl J Med 299: 151-61.
- Reisman RE (1992) Natural history of insect sting allergy: Relation- ship of severity of symptoms of initial sting anaphylaxis to re-sting J Allergy Clin Immunol 90:33-339.
- Van der Linden PW, Hack CE, Struyvenberg A, van der Zwan JK (1994) Insect-sting challenge in 324 subjects with a previous anaphylactic reaction: Current criteria for insect-venom hypersensitivity do not predict the occurrence and the severity of anaphylaxis. J Allergy Clin Immunol 94:151-159.
- Joint Task Force on Practice Parameters, American Academy of Al- lergy, Asthma and Immunology, American College of Allergy, Asth- ma and Immunology, Joint Council of Allergy, Asthma and Immunol- ogy (2007) Allergen immunotherapy: A practice parameter second update. J Allergy Clin Immunol 120: S25-85.
- Valentine MD (1993) Insect-sting Ann Intern Med 118: 225-226.
- Golden DB, Valentine MD, Kagey-Sobotka A, Lichtenstein LM (1980) Regimens of Hymenoptera venom immunotherapy. Ann In- tern Med 92: 620-624.
- Lockey RF, Turkeltaub PC, Olive ES, Hubbard JM, Baird-Warren IA, et al. (1990) The Hymenoptera venom study. III: Safety of venom J Allergy Clin Immunol 86: 775-780.
- Lessof MH, Sobotka AK, Lichtenstein LM (1978) Effects of passive antibody in bee venom Johns Hopkins Med J 142: 1-7.
- Golden DB, Lawrence ID, Hamilton RH, Kagey-Sobotka A, Valen- tine MD, et (1992) Clinical correlation of the venom-specific IgG antibody level during maintenance venom immunotherapy. J Allergy Clin Immunol 90: 386-393.
- McHugh SM, Deighton J, Stewart AG, Lachmann PJ, Ewan PW (1995) Bee venom immunotherapy induces a shift in cytokine re- sponses from a TH-2 to a TH-1 dominant pattern: comparison of rush and conventional immunotherapy. Clin Exp Allergy 25: 828- 838.
- Jutel M, Pichler WJ, Skrbic D, Urwyler A, Dahinden C, et (1995) Bee venom immunotherapy results in decrease of IL-4 and IL-5 and increase of IFN-gamma secretion in specific allergen-stimulated T cell cultures. J Immunol 154: 4187-4194.
- Pereira-Santos MC, Baptista AP, Melo A, Alves RR, Soares RS, et (2008) Expansion of circulating Foxp3+) D25bright CD4+ T cells during specific venom immunotherapy. Clin Exp Allergy 38: 291- 297.
- Nasser SM, Ying S, Meng Q, Kay AB, Ewan PW (2001) Interleu- kin-10 levels increase in cutaneous biopsies of patients undergoing wasp venom immunotherapy. Eur J Immunol 31: 3704-3713.
Citation: Shareef SA, Arnaout RK, Hasnain SM, Al Banyan M, Al-Masri W, et al. (2020) First Report of Rush Immunotherapy for Samsum Ant. J Immuno Immunothe 3: 005.
Copyright: © 2020 Shareef SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.


