*Corresponding Author:
Mesfin Fransua,
Department of Internal Medicine, Division of Infectious Disease, Morehouse School of Med- icine, 720, Westview Dr, Atlanta, GA, USA
Tel: +1 4047561366
Email: mfransua@msm.edu
Abstract
Cryptococcus meningitis needs high index of suspicion given its subtle presentation. Though the gold standard for diagnosis is culture of cerebrospinal fluid, given the delay in culture results, treatment is usually initiated with positive latex agglutination test or Indian ink staining. A 50 years old male with HIV/AIDS (Human immunodeficiency virus infection/ acquired immune deficiency syndrome) non compliant with antiretroviral therapy presented complaining of throbbing non radiating occipital headache. Physical examination is unremarkable and computerized tomography of head showed no evidence of mass lesions or raised intracranial pressure. Cryptococcal meningitis is suspected especially given CD4 count of 19 cells/ mcL. Cerebrospinal Fluid (CSF) Indian ink stains and Cryptococcus antigen latex agglutination test from pronase treated CSF sample was negative. Pt initiated on treatment with amphotericin B and flucytosine for Cryptococcemia as per infectious disease society of Amrericaguidelines given high serum antigen titers of >1.512. CSF cultures later came back positive for Cryptococcus neoformans. Agglutination was re performed on the initial CSF sample using dilution technique still resulted negative. Pt completed 2 weeks of induction therapy for culture proven Cryptococcal meningo-encephalitis, clinically improved and then discharged on oral fluconazole consolidation therapy. Our case report illustrates a false negative CSF cryptococcal latex agglutination test that was not corrected even with dilution techniques and pronase treatment in the setting of high serum antigen titers.
Keywords
Cryptococcal meningitis; False negative agglutination
Abbreviations
ART (Ant Retro Viral Therapy) CSF (Cerebro Spinal Fluid)
HIV/AIDS (Human Immunodeficiency Virus Infection and Acquired Immune Deficiency Syndrome)
LP (Lumbar Puncture)
CT scan (Computerized Tomography)
IDSA (Infectious Diseases Society of America) mCL (micro Liter)
Introduction
Cryptococcus neoformans is a ubiquitous soil saprophyte, usually spread by inhalation initially leading to pulmonary infection. Primary pulmonary infection can be eradicated or contained as granuloma. Mode of infection in HIV-seropositive cases is likely secondary to reactivation of latent infection [1]. Even though the incidence declined after the advent of Anti retro Viral Therapy (ART), Cryptococcal disease still remains the leading cause of mortality in the developing nations [2]. Mortality of cryptococcal meningitis remains as high as 10% to 30% even in developed countries [3]. The sensitivity of commercial available kits for detecting Cryptococcal polysaccharide antigenin Cerebrospinal Fluid (CSF) reaches up to >99% in Human Immunodeficiency Virus (HIV) infected cases, facilitatingearly diagnosis and treatment initiation. We hereby report a case of false negative Cryptococcal latex agglutination test that was not corrected even with dilution techniques and pronase treatment especially in the setting of high serum antigen titers in case with no preserved immune function.
Case Presentation
A 50 years old male with Human Immunodeficiency Virus infection/Acquired Immune Deficiency Syndrome (HIV/AIDS) diagnosed in 2009 presented to clinic complaining of two months’ history of fever, malaise and occipital headaches. Headaches were throbbing non radiating in nature, not associated with any blurry vision, nausea, vomiting, gait disturbances, incontinence, focal neurological defects, change in mentation and confusion or neck pain/stiffness. Patient has not been taking ART for the past 18 months secondary to chronic non adherence. Vitals on presentation include temperature 36.9 C, blood pressure-125 mm Hg systolic over 75 mm Hg diastolic with heart rate of 79/minute, respiratory rate of 18/minute, oxygen saturation of 99% on room air. Review of systems positive for headache and exertional dyspnea. Physical examination revealed normal appearing male who is alert and oriented to time, place and person and in no acute distress. Neurological examination revealed normal cranial nerve examination, normal gait, muscle strength and deep tendon reflexes were normal in all four extremities. Complete blood count showed CD4 count of 19 cells/mcL. Serum Cryptococcal antigen was positive with titer of > 1:512, toxoplasma antibody was negative. CT scan (Computerized Tomography) of the head showed no evidence of raised intra cranial pressure and hydrocephalus or mass lesions. Lumbar Puncture (LP) showed opening pressure of 16cm H20, clear colorless CSF fluid with WBC count of 3/mcL (93% lymphocytes, 7% monocytes, 0% neutrophils), total protein of 45 mg/dl and glucose of 56 mg/dl. Indian ink stain for Cryptococcus species was negative. Cryptococcus Latex Agglutination (LA) tests was negative with pronase pretreated CSF sample. Despite negative CSF agglutination test, patient was initiated on amphotericin B and flucytosine for Cryptococcemia in accordance with current Infectious Diseases Society of America (IDSA) guidelines [4]. SF culture later grew Cryptococcus neoformans. Latex agglutinationtestre performed on the initial CSF sample using dilution techniques was stillnegative for CSF cryptococcal antigen. Patient continued antifungal therapy for culture confirmed Cryptococcal meningitis. Patient completed 2 weeks of induction therapy with Amphotericin and flucytosine followed by oral fluconazole consolidation and maintenance therapy with good response.
Discussion
Cryptococcal meningitis is the most common cause of life threatening meningio encephalitis in HIV infected individuals with estimated 1 million cases worldwide and 650,000 deaths per year [4]. 100% mortality was reported in untreated cases, with morality rates declining to <14% with aggressive therapy with amphotericin B and flucytosine. High index of clinical suspicion for Cryptococcal meningitis is warranted especially in advanced HIV cases (CD4 <100) presenting with isolated fevers and headache. Serum Cryptococcal antigen can be used as a screening tool, however it will be positive in both meningeal and nonmeningieal infections and may present weeks to months even before onset of symptoms [5]. So Lumbar Puncture (LP) preceded by neuro imaging should be performed in clinically suspicious meningo encephalitis cases with positive serum antigen. Though not specific, CSF analysis with elevated protein, mononuclear pleocytosis and low glucose can be available early and may point to cryptococcal meningitis. However, CSF profile can be normal in 25 to 30% of cases with culture positive meningoencephalitis [6-8]. CSF culture considered to be the gold standard for diagnosis, usually takes 3 to7 days, making positive Indian ink stain or positive Cryptococcal CSF antigen tests as alternative diagnostic tools for early diagnosis andinitiation of therapy for meningo encephalitis. Indian ink staining though highly specific has sensitivities ranging between 50% to 80%, especially in cases with low organism burden [9,10].
Singer and Plotz first described latex agglutination test to identify rheumatoid factor in 1956 [11]. Cryptococcal latex agglutination tests were aimed at detecting the presence of Cryptococcus capsular polysaccharide antigen. Antigen detecting latex agglutination tests were performed by mixing individuals specimen containing antigen of interest to reagent fluid containing latex particles coated preformed antibodies. Then antigen of interest if present binds to the preformed antibody in the reagent to form an agglutination complex. The concentrations of antigen in the specimen should be in equivalence with the concentration of antibody in the reagent for optimum precipitation of agglutination complex. If the concentration of antigen in the specimen is relatively higher compared to that of antibody in the reagent, optimum precipitation of agglutination would not occur. This is called postzone effect or zone of relative antigen excess, which can be eliminated by using serially diluted concentrations of the specimen thereby decreasing the concentration on the antigen to a point where it reaches equivalence with the antibody concentration. The original hook effect was described in agglutination tests used detect the antibody of interest and defined as zone of antibody excess which can be eliminated by serial dilution of specimen containing antibody. Also on the other hand if the antigen concentration is relatively less secondary to early infection or infection with acapsular or weakly capsulated organism leading to zone of relative antibody excess or prozone phenomenon, optimum precipitation of agglutination would not occur leading to a negative result [12,13] (Figure 1).
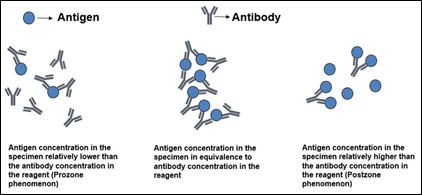
Figure 1: Precipitation reactions.
Sensitivities and specificities of commercially available LA agglutination kits were comparable ranging between 97 to 100% and 93 to 98% respectively especially with pronase treated CSF samples. Though much differences were not noted in sensitivity for CSF samples, significant differences were noted in between the kits for serum samples ranging between 83% to 97% [14,15]. Commercial available kits have high concordance rates of 93 to 96% for qualitative detection of Cryptococcal antigen, but considerable variability was noted with respect to quantitative titres [16]. False negative latex agglutination tests were reported likely secondary to low antigen titers due to early infection or infection with acapsular or weakly capsulated strainsandpostzone like phenomenon [17-22]. False positive Cryptococcal latex agglutination tests attributed to cross reacting antigens in cases of invasive trichosporon asahii (beigelii) infection or the bacteriaial genera stomatococcus or Capnocytophaga canimorsus [23-27]. Decreased sensitivity of latex agglutination kits using monoclonal antibodies in detecting Cryptococcus gatti infections was also reported [28,29]. False positivity secondary to interference of agglutination technique with nonspecific cross reacting antibodies, soap, disinfectants, contamination with agar syneresis fluid, improper transportation of specimen in anaerobic devices and cases with hydroxyethyl starch infusions were also reported [30-34]. Pretreatment of the specimen with pronase a mixture of proteases was shown to increase the sensitivity by preventing false positive test secondary to immunological interferences like rheumatoid factor [32].
Sandwich Enzyme Linked Immunosorbent Assays (ELISA) uses a plate coated with antibody to which antigen of interest is added along with capture antibody and enzyme linked secondary antibody that will be added subsequently. A substrate to the enzyme on the antibody will be added converting the substrate to detectable form. Sensitivity and specificity of sandwich ELISA for detection of cryptococcal antigen were reported as 100% and 98% for CSF samples, comparable with LA tests [15,35]. Advantages include clear demarcation between positive and negative tests and the ability to provide quantitative information without the need for dilution eliminating the false negative results. Lateral Flow Assay (LFA) based on the principle of sandwich immuno chromatographic assay was also developed as a point of care testing to detect cryptococcal antigen. Cryptococcal Antigen (Cr Ag) if present in the test sample, binds to gold conjugated antibodies embedded in the test strip and the antigen antibody complex moves up test strip secondary to capillary action until immobilized by anti Cryptococcal antibodies at the test line thereby producing a visible positive test result [36]. Pooled sensitivity and specificity of LFA in detecting cryptococcal antigen in CSF specimens were reported to be 98.9% (95% CI, 97.9% to 99.5%) and 98.9% (95% CI, 98.0% to 99.5%), respectively [37]. Advantages of LFA over LA would be its faster results, easy to use and higher sensitivities for all 4 Cryptococcal serotypes [38-40]. PCR amplification techniques with 92.9% sensitivity and 100% specificity were also proposed; these are especially useful in cases of low organism load [41,42].
Pretreatment of CSF fluid with pronase and performing dilution techniques ruled out presence of non specific CSF proteins and antigen antibody excess respectively as the reasons for false negativity in our case. Infection with weakly capsulated Cryptococcus is less likely given high serum antigen titers. HIV cases with low organism load secondary to partially preserved immune response and thereby leading to false negative test results were reported, however our patient had very low CD4 count of 19 cells/mcL [43]. CSF agglutination test remained negative in our case despite ruling out conventional reasons for false negativity. Even though techniques like lateral flow assay shown to have increasing sensitivity in detecting Cryptococcal antigen especially with low antigen titers, it would not have improved sensitivity given high serum antigen titers in our case [44,45].
Conclusion
Latex agglutination tests are widely used for early detection of CSF Cryptococcal meningitis given their readily availability, usability and cost effectiveness. Our patient was started on treatment for CNS disease based on high serum antigen titers >1:512, despite negative CSF agglutination test even after correction for conventional reasons for false negativity. The limitations in the currently available CSF antigen detection techniques as encountered in our casemay delay initiating appropriate treatment. The main question that remains to be explored is the therapeutic approach especially in cases with false negative CSF antigen test and who don’t meet the IDSA criteria to be treated as CNS Cryptococcal disease based on high serum titer but still having active CNS disease which can be confirmed later with positive cultures.
References
- Salyer WR, Salyer DC, Baker RD (1974) Primary complex of Cryptococcus and pulmonary lymph J Infect Dis 130: 74-77.
- Jarvis JN, Harrison TS (2007) HIV-associated cryptococcal AIDS 21: 2119-2129.
- van der Horst CM, Saag MS, Cloud GA, Hamill RJ, Graybill JR, et al (1997) Treatment of Cryptococcal meningitis associated with the acquired immuno- deficiency National Institute of Allergy and Infectious Diseases My- coses Study Group and AIDS Clinical Trials Group. N Engl J Med 337: 15-21.
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, et al. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23: 525-530.
- French N, Gray K, Watera C, Nakiyingi J, Lugada E, et (2002) Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS 16: 1031-1038.
- Darras-Joly C, Chevret S, Wolff M, Matheron S, Longuet P, et (1996) Cryp- tococcus neoformans infection in France: epidemiologic features of and early prognostic parameters for 76 patients who were infected with human immu- nodeficiency virus. Clin Infect Dis 23: 369-376.
- Garlipp CR, Rossi CL, Bottini PV (1997) Cerebrospinal fluid profiles in ac- quired immunodeficiency syndrome with and without neurocryptococcosis. Rev Inst Med Trop Sao Paulo 39: 323-325.
- Moosa M, Coovadia Y. Cryptococcal meningitis in Durban, South Africa: a comparison of clinical features, laboratory findings, and outcome for human immunodeficiency virus (HIV)-positive and HIV-negative patients. Clin Infect Dis 24: 131-134.
- Dominic RMS, Prashanth HV, Shenoy S, Baliga S (2009) Diagnostic value of latex agglutination in cryptococcal meningitis. J Lab Physicians 1: 67-68.
- Snow RM, Dismukes WE (1975) Cryptococcal meningitis: diagnostic value of cryptococcal antigen in cerebrospinal Arch Intern Med 135: 1155-1157.
- PLOTZ CM, SINGER JM (1956) The latex fixation test. I. Application to the serologic diagnosis of rheumatoid Am J Med 21: 888-892.
- Levinson W (2016) Antigen–Antibody Reactions in the Review of Medical Microbiology and Immunology, 14e, New York, USA.
- Stewart TW, Parnell D (1982) Postzone v JAMA 248: 646-647.
- Chaurasia S, Waghmare A, Joshi AA (2017) Study the Utility of Cryptococcal Latex Agglutination Test in HIV-Positive and Negative Patients Suspected of having Cryptococcal meningitis and Compare with India Ink Preparation and Fungal Int J Curr Microbiol App Sci 6: 484-491.
- Tanner DC, Weinstein MP, Fedorciw B, Joho KL, Thorpe JJ, et al. (1994) Comparison of commercial kits for detection of cryptococcal antigen. J Clin Microbiol 32: 1680-1684.
- Babady NE, Bestrom JE, Jespersen DJ, Jones MF, Beito EM, et al. (2009) Evaluation of three commercial latex agglutination kits and a commercial en- zyme immunoassay for the detection of cryptococcal antigen. Med mycol 47: 336-338.
- Bottone EJ, Toma M, Johansson BE, Wormser GP (1986) Poorly encapsulat- ed Cryptococcus neoformans from patients with AIDS. I: Preliminary observa- AIDS Res 2: 211-218.
- Bottone EJ, Wormser GP (1986) Poorly encapsulated Cryptococcus neofor- mans from patients with II. Correlation of capsule size observed directly in cerebrospinal fluid with that after animal passage. AIDS Res 2: 219-225.
- Currie BP, Freundlich LF, Soto MA, Casadevall A (1993) False-negative ce- rebrospinal fluid cryptococcal latex agglutination tests for patients with cul- ture-positive cryptococcal J Clin Microbiol 31: 2519-2522.
- Liu LL, Lin LR, Tong ML, Zhang HL, Huang SJ, et al. (2014) Incidence and risk factors for the prozone phenomenon in serologic testing for syphilis in a large cohort. Clin Infect Dis 25: 384-389.
Citation:Annangi S, Nutalapati S, Fransua M (2017) False Negative Cryptococcus Latex Agglutination Test Despite Correcting for Conven- tional Reasons: A Case Report. J Case Repo Imag 1: 002.
Copyright: © 2017 Annangi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


