*Corresponding Author:
Elisabeth Baeza,
INRAE, Université de Tours, BOA, 37380 Nouzilly, France
E-mail: elisabeth.baeza-campone@inrae.fr
Abstract
World poultry production reached 122.5 million tons in 2018. A main factor explaining the continuous increase of poultry production is the development of cut pieces and processed products. The diversity of poultry processed products is very wide. In order to adapt the process, the meat industry needs to characterize the nutritional, technological and sanitary quality of poultry meat. This review focus on the different factors involved in the determinism of technological quality of poultry meat that corresponds to its ability to be processed and preserved. The processing ability of poultry meat depends largely on the meat ultimate pH that affects the conformation of muscle proteins and their functionality. All factors modulating muscle glycolytic reserves and metabolism will have an effect on this parameter such as genotype, slaughter age, feeding programme, pre-slaughter conditions and slaughtering procedure. The preservation ability depends more on the muscle lipid and antioxidant contents, fatty acid composition and meat storage conditions. It is then strongly influenced by feed. In conclusion, many factors can influence the technological quality of raw poultry meat inducing a variability of this quality. Then, the aim of processing technologies is to reduce this variability and to increase technological yields and preservation ability of poultry meat products.
Keywords
Meat quality; Poultry; Preservation; Processing
Introduction
World poultry production reached 122.5 million tons in 2018 [1]. It is now the most produced meat in terms of volume, leads by USA, China and Brazil. Relatively low and competitive prices compared to other meats, the absence of cultural or religious obstacles, and dietary and nutritional (protein) qualities are the main factors explaining poultry meat’s attractiveness [2]. It has become a mass consumer product throughout the world. Another factor explaining the continuous increase of poultry production is the development of cut pieces and processed products. Services embedded in products constitute another recent dynamic factor appealing to consumers. The consumption of poultry meat is developing primarily around two main types of consumer service: an economy of preparation time (the ‘ready-to-eat’) and a diversification of places of consumption. Catering outside the home combines both of these aspects. Whole chickens now account for a very small proportion of overall consumption, as opposed to almost 50% in the late 1980s. For example, in the USA, chickens sold under whole carcasses, cut parts or processed products represented 11,40 and 49%, respectively, in 2015 (source: https://www.nationalchickencouncil.org/about-the-industry/statistics/how-broil ers-are-marketed/). In France, these proportions were 22,48 and 29%, respectively, in 2018 (source: https://www.volaille-francaise.fr/wp-content/uploads/rapport2018chiffres-cles.pdf). The diversity of poultry processed products is very wide. In order to adapt the process, the meat industry needs to characterize the nutritional, technological and sanitary quality of poultry meat. The following review will focus on the different factors involved in the determinism of technological quality of poultry meat that corresponds to its ability to be processed and preserved. The preservation ability depends on the intrinsic properties of meat leading or not to lipid and protein oxidation but also on the possible contamination and development of pathogens and/ or spoilage microbial flora. Only the preservation faced to oxidation mechanisms will be presented.
Processing Ability of Poultry Meat
The processing ability can be assessed by the water holding capacity and by measuring the juice losses during storage at +4°C or after thawing and/or cooking and by determining the technological yield by measuring material loss after processing. The water holding capacity depends largely on the meat ultimate pH (pHu) that affects the conformation of muscle proteins and their functionality. Le Bihan-Duval et al. [3] estimated genetic correlations of -0.89 and -0.80 between pHu on one-hand and exudate and juice loss from chicken fillets on the other. Many factors can affect water holding capacity of meat.
Effect of feeding
Feeding has an indirect effect by first modulating the glycolytic reserves of the muscles before slaughter and thus the meat pHu [4]. For example, Berri et al. [5] tested variable digestible lysine content of the finishing diet distributed to standard chickens. When this content increased from 0.83 to 1.13%, the juice loss after 4 days storage at +4°C decreased from 1.10 to 0.87%. Jlali et al. [6] compared two isoenergetic finishing diets with different protein contents. Fillets of chickens fed diet containing 23% proteins had higher juice loss after 4 days of storage at +4°C than chickens fed diet containing 17% proteins (1.30 vs. 1.08%). However, the feed impact on the fillet characteristics depended on genotype, with animals selected for low peripheral fattening being more sensitive to variations in protein intakes. In order to better define the response governing changes in fillet quality as a function of amino acid intake, several studies [7,8] have shown that beyond the amount of proteins, the amino acid profile of diet could influence meat pHu and some associated traits (colour, exudate). The results showed that an excess intake of amino acids (+10%), combined with a low intake of lysine (0.7-0.8%), favoured the production of acid and exudative meat. The hypothesis would be that a chicken subjected to an amino acid intake exceeding its requirements for protein synthesis and muscle growth would use part of its dietary intake for energy purposes, particularly in the form of muscle glycogen, explaining the observed pH decrease [9]. However, the animal response would depend on the initial intake of lysine (and hence crude protein) and the duration of exposure to these imbalances, which, by modulating the nutrient proportion used for protein synthesis or energy storage purposes, will determine the animal metabolic response thresholds for variations in amino acid intakes. Beyond pHu, the degree of muscle oxidation can also affect the technological properties of meat. Thus, the use of DL-HMTBA (hydroxyl-analogue of methionine) significantly improved the functional properties of chicken fillet (exudate and technological yield) by reducing the index of muscle oxidation [10].
Oxidation modifies the biochemical properties of muscle proteins and particularly their ability to bind to water [11]. Therefore, antioxidants have direct effect on the water holding capacity of meat. For example, by increasing the amount of selenium yeast in feed from 0.15 to 0.60 ppm, Oliveira et al. [12] reduced juice loss after cooking of chicken fillets from 21 to 16%. In this study, the dietary intake of selenium under organic form was more effective in reducing meat juice loss after cooking than the intake of selenium under mineral form. However, in turkey, the feed supplementation with selenium at 0.3 to 0.5 mg/kg had no effect on the juice loss of fillets stored at +4°C for 48 h [13]. The feed supplementation with 40 or 80 ppm zinc also increased the water holding capacity of chicken fillets compared to not supplemented controls (63-66 vs. 56%) [14].
Effect of rearing and pre-slaughter conditions
The water holding capacity can also be influenced by age, genotype, pre-slaughter conditions (mainly heat stress) and post-mortem evolution of meat pH. The effect of age on the water holding capacity is variable according species. For standard heavy chickens slaughtered between 35 and 63 days of age, juice loss after 4 days storage at +4°C and after cooking decreased from 2.02 to 1% and from 18.8 to 17.4%, respectively [15]. Conversely, for fillets of Muscovy ducks slaughtered between 8 and 15 weeks of age, the juice loss after 24 h storage at +4°C increased from 0.68 to 1.62% [16]. For fillets of mule ducks slaughtered between 8 and 13 weeks of age, juice loss after 24 h storage at +4°C was not affected by age [17].
The effect of genotype will depends on the ability of the animal to store energy in muscles. For example, juice loss after 2 days storage at +4°C was higher in fillets of Label Rouge chickens compared to fillets of standard chickens (1.64 vs. 1.24%) [18]. Consistent with this result, the technological yield after curing and cooking fillets was higher in standard chickens compared to Label Rouge chickens (106.8 vs. 100.0%). Sibut et al. [19] compared two chicken lines divergently selected on the proportion of abdominal fat relative to live weight (4.66 vs. 2.09%), and showing differences in pHu fillets (5.79 vs. 5.66 for lean and fat lines, respectively). They showed that fillets of fat chickens had slightly higher juice loss after 4 days storage at +4°C than lean chickens (1.35 vs. 1.12%). A chicken line, selected for a high pHu (average 6.09) in the fillet, had lower juice loss after 5 days storage at +4°C or after cooking than the line selected for a more acid pHu (2.20 vs. 3.80% and 10.2 vs. 11.9%, respectively) [20]. Consistent with these differences, the technological yield after curing and cooking was higher for the high pHu line (86.6 vs. 80.5%). Chicken fillets with a severe “white striping” defect had higher cooking losses than normal fillets (26.7 vs. 21.3%) [21]. Those with “wooden breast” defect had higher juice loss after cold storage but also after cooking than normal fillets (1.19 vs. 0.93% and 28.0 vs. 21.6%, respectively) [22]. Marinade intake and technological yield were also much lower for “wooden breast” fillets compared to normal fillets (6.94 vs. 13.15% and 87.3 vs. 94.5%, respectively).
The pre-slaughter conditions can also affect the glycolytic reserves of muscles and therefore its water holding capacity. For example, comparing exposure of chickens to different temperatures during pre-slaughter transport, Dadgar et al. [23] showed that negative temperatures (-14 to -17°C) negatively impacted muscle glycogen reserves and thereby increased pHu and reduced fillet cooking loss (10.52 vs. 13.45%) compared with positive temperatures (20 to 22°C).Juice loss after 48 h storage at +4°C of fillets from chickens exposed to 33°C for 2 hours before slaughter were higher than those of chickens exposed to 21°C (3.7 vs. 2.0%) [24]. Finally, a too fast post-mortem pH drop negatively affects the technological yield. For example, turkey fillets with pH values measured 20 min after slaughter of 5.90 or 6.55 had technological yields after curing and cooking of 97.4 and 98.3%, respectively [25].
Preservation Ability of Poultry Meat
The preservation ability is estimated by the oxidation susceptibility of meat lipids and proteins. First, the preservation ability depends on the muscle type and poultry species. Thighs having a higher lipid content than fillets (4.5% vs. 1.3% for standard chickens) [26] are more susceptible to oxidation [27,28]. Turkey meat is more susceptible to oxidation than chicken and duck meat (Figure 1) due to its lower ability to bind antioxidant molecules such as vitamin E in muscle tissues [29]. Poultry meat has a high content in unsaturated fatty acids (around 30% of monounsaturated fatty acids, MUFA and 30% of polyunsaturated fatty acids, PUFA) [26] that increases its susceptibility to oxidation.
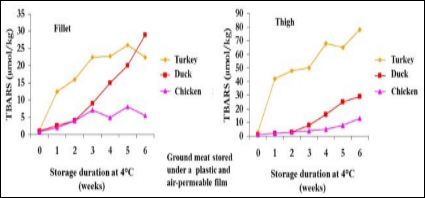
Figure 1: Oxidation susceptibility of chicken, duck and turkey thighs and fillets stored at +4°C (Gong et al., 2010).
The preservation ability is strongly influenced by feed that will affect the lipid, PUFA and antioxidant contents of meat. It also depends on the preservation conditions. For example, Cortinas et al. [30] compared 4 levels of PUFA in feed (15, 34, 45 and 61%) and 4 conditions to preserve chicken thighs (raw meat, raw meat refrigerated 3 days at +4°C, cooked meat and cooked meat stored 2 months at +4°C). The oxidation susceptibility of meat increased with PUFA content (Figure 2). The TBARS (Thio-BArbituric Reactive Substances) index (indicator of lipid peroxidation) increased with storage duration at +4°C. Cooking also promoted oxidation (Figure 2). Vacuum-packed and cooked chicken fillets stored at +4°C were more susceptible to oxidation than raw chicken fillets vacuum-cooked only at the end of the storage period [31]. Jankowski et al. [32] compared three different oils in feed (soybean, rapeseed and linseed). The highest TBARS index in turkey fillets was obtained with the lowest ratio FA n-6/FA n-3 in feed (17.07 vs. 15.64 and 10.91 nmol/g for linseed, rapeseed and soybean based diets, respectively). A storage of turkey fillets for 4 months at -20°C strongly promoted lipid oxidation especially for the linseed oilfed group for which the TBARS index was multiplied by 4.7 when compared to non-frozen meat. The use of linseed oil in the feed also promoted protein oxidation in chicken thighs [28]. The incorporation of 2% micro-algae in feed increased the oxidation susceptibility of chicken fillets stored 6 days at +4°C and then cooked compared with fillets of chickens fed diet containing soybean and palm oils (1.79 vs. 0.49 mg equivalent MDA/kg meat) [33]. Mercier et al. [27] also showed that protein oxidation was higher in the Sartorius thigh muscle of turkeys fed diet containing soybean oil compared with turkeys fed diet containing tallow. The production system can also have an impact. The TBARS index of fillets and thighs from chickens reared under organic conditions was higher than that measured for chickens reared under standard conditions, whereas for the lipid content, it was the reverse [34].
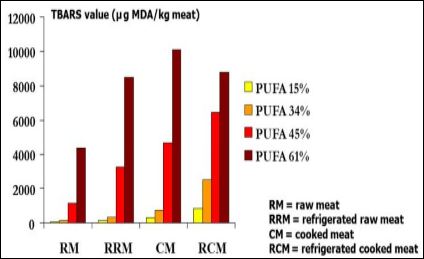
Figure 2: Effect of diet polyunsaturated fatty acid (PUFA) content on the oxidation susceptibility of chicken thighs (Cortinas et al., 2005).
The pH may also affect the oxidation susceptibility of meat. The more acid a meat is, the greater the risk of oxidation. In a chicken line selected for an acid pHu in breast muscle, the TBARS index of fillets preserved at +4°C for 8 days was higher than that measured in chicken fillets of the line selected for a high pHu (0.65 vs. 0.50 mg MDA equivalent/kg meat) [35].
The use of antioxidants in feed limits the oxidation susceptibility of meat. For example, a dietary supplementation with 400 mg vitamin E/ kg feed divided by 2 to 3 the TBARS value measured in the Pectoralis major and Sartorius muscles of turkeys stored at +4°C for 1, 3 or 9 days [25]. In chicken, a supplementation with 200 mg of vitamin E/kg was sufficient. Vitamins A and C did not have the same antioxidant power [36,37]. Vitamin E supplementation also limited the formation of cholesterol oxidation products. For chicken meat cooked, then stored 12 days at +4°C, this reduction was 42% and 75% for fillets and 50% and 72% for thighs when vitamin E supplementation was 200 or 800 mg/kg compared to a control diet supplemented with 20 mg vitamin E/kg [38]. The antioxidant effect of vitamin E can be increased when combined with other compounds such as oregano essential oil [39]. The preservation of chicken meat placed under vacuum in a package composed of biopolymers with antioxidant molecules can also limit the oxidation risk. For example, Sogut and Seydim [40] stored chicken fillets vacuum-packed with a chitosan biopolymer combined with different concentrations of grape seed extracts (5,10 and 15%) at +4°C. The 15% intake inhibited the oxidation of fillets after 15 days storage.
Conclusion
The processing ability of poultry meat depends largely on the meat pHu that affects the conformation of muscle proteins and their functionality. All factors modulating muscle glycolytic reserves and metabolism will have an effect on this parameter such as genotype, slaughter age, feeding programme, pre-slaughter conditions and slaughtering procedure. The preservation ability depends more on the muscle lipid and antioxidant contents, fatty acid composition and meat storage conditions. It is then strongly influenced by feed. In conclusion, many factors can influence the technological quality of poultry meat inducing a variability of this quality. Until now, studies related to the determinism of technological quality have mainly focused on poultry meat. There are few references in the literature concerning poultry processed products. Berri et al. [18] compared the technological quality of curing-cooking meat from fast-, medium- and slow-growing (FG,MG,SG) commercial chickens slaughtered at their usual market ages (6, 8 and 12 weeks, respectively). The highest curing-cooking yields were obtained with the breast and leg meat from 6wk-FG and the lowest with that from 12wk-LG. Because of their reduced water holding ability, the processed meat of 12wk-LG exhibited the lowest moisture and the white cured-cooked meat showed the driest texture and the best slice cohesiveness. This study suggested that processing ability and processed product characteristics of breast and leg meats are greatly related to the chicken type of production with FG chickens being more adapted to further processing than SG ones in terms of profitability. Singh et al. [41] compared the effect of chicken strain (a high growth rate strain, Cobb 400 and three local Indian strains) on nugget quality. The nuggets produced with Cobb 400 strain had the highest water and lipid contents. They also had the most stable emulsion, and the highest cooking yield. Now, there is really a research need to determine if, and how the production system or rearing conditions can modulate the quality of processed poultry products.
References
- Conway A (2018) World poultry production at nearly 123 million tons in Poult Trends 6-14.
- Valceschini E (2006) Poultry Meat Trends and Consumer Attitudes. CABI 1-10.
- Le Bihan-Duval E, Debut M, Berri C, Sellier N, Santé-Lhoutellier V, et al. (2008) Chicken meat quality: Genetic variability and relationship with growth and muscle characteristics. BMC Genetics 9: 53.
- Bendall JR, Swatland H J (1988) Review of the relationships of pH with phys- ical aspects of pork quality. Meat Sci 24: 85-126.
- Berri C, Besnard J, Relandeau C (2008) Increasing dietary lysine increases final pH and decreases drip loss of broiler breast meat. Poult Sci 87: 480-484.
- Jlali M, Gigaud V, Métayer-Coustard S, Sellier N, Tesseraud S, et al. (2012) Modulation of glycogen and breast meat processing ability by nutrition in chickens: effect of crude protein level in two chicken genotypes. J Anim Sci 90: 447-455.
- Lessire M, Primot Y, Corrent E, Fraysse P, Tesseraud S, et (2013) Lysine supply in finishing broilers: Effect on performances and meat quality. Energy and protein metabolism and nutrition in sustainable animal production 209- 210.
- Guardia S, Lessire M, Corniaux A, Metayer-Coustard S, Mercerand F, et al. (2014) Short-term nutritional strategies before slaughter are effective in modulating the final pH and color of broiler breast Poult Sci 93: 1764-1773.
- Tesseraud S, Bouvarel I, Fraysse P, Metayer-Coustard S, Collin A, et al. (2014) Optimiser la composition corporelle et la qualité des viandes de volailles en modulant le métabolisme par les acides aminés alimentaires. INRA Prod Anim 27: 337-346.
- Mercier Y, Berri C, Baéza E, Bordeau T, Chartrin P, et (2009) Improvement of muscle oxidative stability and processing yield in relation with dietary methionine sources. In Proceedings of Poultry Science Association 98th Annual Meeting, 20-23/07/2009, Raleigh, North Carolina (USA): Abstract 117.
- Huff-Lonergan E, Lonergan SM (2005) Mechanisms of water-holding capacity of meat: The role of post mortem biochemical and structural Meat Sci 71: 194-204.
- Oliveira TFB, Rivera DFR, Mesquita FR, Braga H, Ramos EM, et al. (2014) Effect of different sources and levels of selenium on performance, meat quality and tissue characteristics of broilers. J Applied Poult Res 23: 15-22.
- Juniper DT, Phipps RH, Bertin G (2011) Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in commercial-line turkeys. Anim 5: 1751-1760.
- Salim HM, Lee HR, Jo C, Lee SK, Lee BD (2011) Influence of various levels of organic zinc on the live performance meat quality attributes and sensory properties of broiler chickens. Korean J Food Sci Anim Resources 31: 207-
- Baéza E, Arnould C, Jlali M, Chartrin P, Gigaud V, et al. (2012) Influence of increasing slaughter age of chickens on meat quality, welfare, and technical and economic results. J Anim Sci 90: 2003-2013.
- Baéza E, Salichon MR, Marché G, Juin H (1998) Effect of sex on growth, technological and organoleptic characteristics of the Muscovy duck breast Brit Poult Sci 39: 398-403.
- Baéza E, Salichon MR, Marché G, Wacrenier N, Dominguez B, et al. (2000) Effects of age and sex on the structural, chemical and technological characteristics of mule duck meat. Brit Poult Sci 41: 300-307.
- Berri C, Le Bihan-Duval E, Baéza E, Chartrin P, Picgirard L, et al. (2005) Further processing characteristics of breast and leg meat from fast-, medium- and slow-growing commercial chickens. Anim Res: 123-134.
- Sibut V, Le Bihan-Duval E, Tesseraud S, Godet E, Bordeau T, et al. (2008) Adenosine monophosphate-activated protein kinase involved in variations of muscle glycogen and breast meat quality between lean and fat chickens. J Anim Sci 86: 2888-2896.
- Alnahhas N, Berri C, Boulay M, Baéza E, Jégo Y, et (2014) Selecting broiler chickens for ultimate pH of breast muscle: Analysis of divergent selection experiment and phenotypic consequences on meat quality, growth and body composition traits. J Anim Sci 92: 3816-3824.
- Petracci M, Mudalal S, Bonfiglio A, Cavani C (2013) Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poult Sci 92: 1670-1675.
- Mudalal S, Lorenzi M, Soglia F, Cavani C, Petracci M (2015) Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Anim 9: 728-734.
- Dadgar S, Lee ES, Leer TLV, Crowe TG, Classen HL, et al. (2011) Effect of acute cold exposure, age, sex and lairage on broiler breast meat Poult Sci 90: 444-457.
- Sandercock DA, Hunter RR, Nute GR, Hocking PM, Mitchell MA (1999) Physiological responses to acute heat stress in broilers: Implications for meat Proceedings of XIVth European Symposium on the Quality of Poultry Meat, 19-23/09/99, Bologna (Italy): 271-276.
- Fernandez X, Santé V, Baéza E, Le Bihan-Duval E, Berri C, et al. (2002) Effects of the rate of muscle post mortem pH fall on the technological quality of turkey meat. Brit Poult Sci 43: 245-252.
- Rabot C (1998). Vitesse de croissance et caractéristiques lipidiques et sensorielles des muscles de poulet. PhD thesis, INAPG, Paris: 156 p.
- Mercier Y, Gatellier P, Viau M, Rémignon H, Renerre M (1998) Effect of dietary fat and vitamin E on colour stability and on lipid and protein oxidation in turkey meat during storage. Meat Sci 48: 301-318.
- Kralik G, Pavic V, Galovic D, Skrtic Z, Kralik Z, et (2012) Fatty acid composition, and lipid and protein oxidation products in muscles of broilers fed with different plant oils. Arch Geflügelk 76: S259-S269.
- Gong Y, Parker RS, Richards MP (2010) Factors affecting lipid oxidation in breast and thigh muscle from chicken, turkey and J Food Biochem 34: 869-885.
- Cortinas L, Barroeta A, Villaverde C, Galobart J, Guardiola F, et al. (2005) Influence of the dietary polyunsaturation level on chicken meat quality: Lipid Poult Sci 84: 48-55.
- Hong GE, Kim JH, Ahn SJ, Lee CH (2015) Changes in meat quality characteristics of the sous-vide cooked chicken breast during refrigerated storage. Korean J Food Sci Anim Resources 35: 757-764.
- Jankowski J, Zdunczyk Z, Mikulski D, Juskiewicz J, Naczmanski J, et al. (2012) Fatty acid profile, oxidative stability and sensory properties of breast meat from turkeys fed diets with a different n-6/n-3 PUFA European J Lipid Sci Technol 114: 1025-1035.
- Baéza E, Chartrin P, Lessire M, Méteau K, Chesneau G, et al. (2015) Is it possible to increase n-3 fatty acid content of meat without affecting its technological and/or sensorial quality and the growing performance of chickens? Brit Poult Sci 56: 543-550.
- Castellini C, Mugnal C, Dal Bosco A (2002) Effect of organic production system on broiler carcass and meat quality. Meat Sci 60: 219-225.
- Alnahhas N, Le Bihan-Duval E, Baéza E, Chabault M, Chartrin P, et (2015) Impact of divergent selection for ultimate pH of Pectoralis major muscle on biochemical, histological and sensorial attributes of broiler meat. J Anim Sci 93: 4524-4531.
- King AJ, Uijttenboogaart T, De Wries AW (1995) α-tocophérol, β-carotene and ascorbic acid as antioxidants in stored poultry muscle. J Food Sci 60: 1009-1012.
- Bartov I, Sklan D, Friedman A (1997) Effect of vitamin A on the oxidative stability of broiler meat during storage: lack of interactions with vitamin Brit Poult Sci 38: 255-257.
- Galvin K, Morrissey PA, Buckley DJ (1998) Cholesterol oxides in processed chicken muscle as influenced by dietary alpha-tocopherol supplementation. Meat Sci 48: 1-2.
- Botsoglou NA, Grigoropoulou SH, Botsoglou E, Govaris A, Papageorgiou G (2003) The effect of oregano essential oil and α-tocopheryl acetate on lipid oxidation in raw and cooked turkey during refrigerated storage. Meat Sci 65: 1193-1200.
- Sogut E, Seydim AC (2018) The effects of chitosan and grape seed extract-based edible films on the quality of vacuum packaged chicken breast Food Packaging Shelf Life 18: 13-20.
- Singh VP, Pathak V, Bharti SK, Sharma S, Ojha S (2016) Effect of chicken breeds on quality characteristics of meat Nutr Food Sci 46: 432-440.
Citation: Baeza E (2020) Factors Influencing the Processing Ability and Oxidation Susceptibility of Poultry Meat. J Nutr Food Sci 3: 012.
Copyright: © 2020 Baeza E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.


