*Corresponding Author:
Jürg Barandun,
Department of Pulmonology, Lung Center Hirslanden Zurich, Switzerland
Tel: 044 3873000
Fax: 044 3872255
Email: j.barandun@lungenzentrum.ch
Abstract
The fungus of the kind Aspergillus fumigatus is environmentally ubiquitous and can lead to various pulmonary diseases, often depending on the patient’s immune status. Patients with sensitization to the fungus’s antigens can develop a condition called Allergic Bronchopulmonary Aspergillosis (ABPA). In individuals with insufficient immunological response, the fungus can grow invasively. Preformed lung-cavities can be colonized by the fungus, forming fungal masses referred to as aspergillomas. In the following, we describe an unusual and severe case of cavernous destructive ABPA in a young asthmatic without any proven form of immunodeficiency. Cavernous growth of Aspergillus fumigatus in patients with functioning immune system is highly unusual. Despite extensive therapy, the fungus formed repeated cavities, which had to be surgically removed respectively.
The patient is now successfully treated, being free from symptoms and radiological findings, but the process leading up to that point showed to be extraordinarily challenging and the measures taken will be presented and discussed in this report.
Keywords
Allergic bronchopulmonary Aspergillosis; Aspergilloma; Aspergillus fumigates; Cavern; Invasive Aspergillosis
Introduction/Background
The Aspergillus fungus family can cause various pulmonary condi- tions such as allergic bronchopulmonary Aspergillosis (ABPA), inva- sive Aspergillosis and aspergillomas.
The fungi are present worldwide and exposure is common for most individuals.
Human infections are predominantly caused by the species Asper- gillus fumigatus, which is responsible for 95% of incidences [1]. How- ever, most of the population isn’t affected by frequent contact to the fungus due to an adequate immunological response.
ABPA is a disease, which is caused by an allergic reaction (Type I and III) to Aspergillus antigens. Cases are mostly limited to patients with pre-existing conditions such as asthma (1-2% of asthmatics) and cystic fibrosis (2-15% of cystic fibrosis patients) [2].
Fungal balls called aspergillomas can form in preformed cavities of the lung such as ones being present after tuberculosis, sarcoidosis, pneumoconiosis, healed abscesses or cystic fibrosis, due to phagocy- tosis being hindered in such cavities [1,3].
The fungus can be able to form cavities itself by secretion of di- gestive enzymes but mostly under the circumstances of an underlying immunodeficiency [1]. Immunocompetent hosts with expansive, en- capsulating fungal growth are highly unusual and insufficiently de- scribed in scientific literature.
Case Presentation
The patient in question is in his mid-thirties and suffers from anal- lergic asthma, because of which he was treated in our institution start- ing January 2012. He works in an open space office and is of caucasian race. He never smoked.
His asthma is known since early adulthood and presents itself with constant elevation of Fractional expirational Nitrogen monoxide (FeNO) levels, undulating around 30 parts per billion (ppb) despite steroidal therapy. Consistent with the atopic predisposition there were sensitisations towards a variety of pollen and pet hair, specified with a skin prick test. Interestingly, the initial skin prick test in December 2012 didn’t indicate any Aspergillus sensitisation, despite later test- ing and clinical presentation confirming an allergic response to the fungus. This poses the theory, whether there has been a newly occur- ring sensitisation in the period between January 2013 and the start of ABPA symptoms. More likely however is, that the skin prick test showed a false negative result, due to its insufficient positive predictive value [4].
The patient was in good physical condition and responded very well to the anti-asthmatic treatment, consisting of salmeterol and flu- ticasone (50/250 mcg) once per day during pollen season.
Treatment of the patient’s asthma continued for approximately three years without any incidents. The patient has even been able to perform significant physical exercise such as endurance training and ultimately participating in marathon running events.
However, in November 2014, he developed symptoms of fever and coughing that lasted for 5 weeks. Also remarkable was an eosinophilia in the blood count with up to 2 billion eosinophils per liter (G/L) and C-reactive-protein (CRP) values of 173 mg/L.
A Computer Tomography (CT) (Figure 1) showed a huge cavity in the left upper lobe of his lung, measuring 80 times 100 mm with a capsule thickness of 5 to 10 mm and a prominent air-fluid-level. In the right upper lobe, a smaller cavity is also clearly visible.
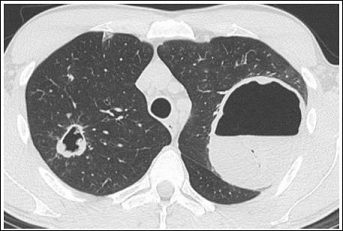
Figure 1: Cavern in the left upper lobe with clearly visible air-fluid-level. Smaller cavern in the right upper lobe.
Suspecting tuberculosis the patient was isolated and bronchosco- pied. The bronchoalveolar lavage showed no signs of mycobacterium tuberculosis but some fungal hyphae were discovered.
Under the assumption of a fungus associated cavern formation, immediate combined treatment with itraconazole, prednisone, tazo- bactam and piperacillin was started after the bronchoalveolar lavage.
This however didn’t affect the cavern formation and necrotic pro- cess, which lead to the decision to resect the cavern seven days after the initial discovery. The resection was performed thoracoscopically in December 2014 and the removed cyst contained massively necrotic and purulent material. Fungal hyphae, consistent with the assumption of a fungus, specifically Aspergillus fumigatus, associated pathology could be detected via the application of Grocott-dye.
Specific Immunoglobulin E (IgE) levels showed sensitivity towards mites, pollen and fungal spores. Specific precipitins for Aspergillus fumigatus were serologically detected and in combination with the pre-existing eosinphilic asthma and high IgE-values (3960 kilo units per liter (kU/L)), the criteria were met for diagnosing ABPA [5].
The necrotizing and cavitary growth however is highly unusual be- cause as far as previous literature goes, cavities formed by the fungus itself are limited to immunodeficient individuals [6]. These would also not be clearly defined by a capsule of this thickness but manifest in invasive, infiltrative destruction of lung parenchyma.
The cavities present in this case are clearly defined by a thick cap- sule. Aspergilloma would fit the radiological description, but would require there to be a pre-existing cavern, which could be colonized by the fungus, forming a fungal ball inside. The observed encapsulation, combined with the destructive and necrotic fungal growth is not doc- umented in scientific literature.
To explain the necrotizing activity of the fungus, testing for HIV as well as for other immunological conditions such as Hyper-IgE-syn- drome (HIES) was commissioned, but turned out negative.
There were no elevated anti-nuclear or anti-neutrophilic antibod- ies and histological biopsies showed no signs of malignant or angioin- flammatory processes.
The therapy that was commenced consisted of voriconazole 200 mg 1-0-1as an antifungal component in combination with omalizum- ab150 mg subcutaneously once a month due to the high IgE values and continuation of the standard asthma therapy with topical corti- costeroids. This showed to be quite successful by reducing the radio- logical findings and also normalising the FeNO over a period of two months. After that, the treatment with voriconazole was stopped but omalizumab and the antiasthmatic medication was continued.
The patient underwent frequent controls by CT-scan and was in good physical condition, not mentioning any discomforts.
Unfortunately, in a control CT-scan in late April 2015, the rapid growth of one of the smaller, previously stagnant infiltrates was seen, forming another cavern in the right upper lobe measuring 33 times 30 mm (Figure 2). In response to this finding, the antifungal therapy with voriconazole (200 mg 1-0-1) was started again. This caused the cavity to shrink to 28 times 25 mm in size and reduce capsule-thickness by a small amount over a period of one month. The lung function wasn’t impaired compared with ones a few years ago. Only the FeNO was elevated to 49 ppb.
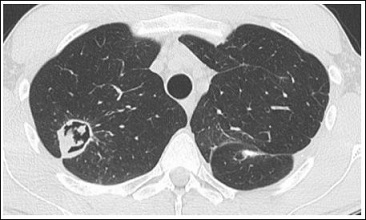
Figure 2: Cavern in the right upper lobe containing necrotic material.
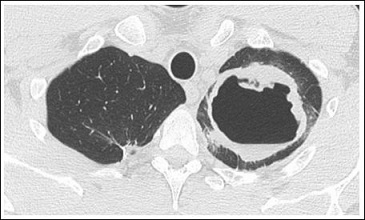
Figure 3: Cavern in the left upper lobe with prominent encapsulation.
A CT-scan of the nose sinuses was performed to dismiss the possi- bility of an aspergilloma-like finding. The examination showed a poly- pous pansinusitis of intermediate severity but no evidence of fungal activity. In response to the fungus still being persistent in the lung, an extensive search for environmental contamination was arranged and ideal growth-circumstances were found at the patient’s workplace. In response to this finding, we contacted the patient’s superiors and tried to achieve relocation to another building. This however wasn’t possi- ble because of various reasons.
A continuous enlargement of the newly discovered cavern was ob- served to a size of 46 times 58 mm by the end of March.
Suspecting that the inhaled glucocorticoids might benefit Aspergil- lus growth by suppressing the immune response needed to combat the fungus, they were stopped in April 2016. This risked increased severity of asthmatic distress but was deemed necessary for best treatment of the main pathology.
Lung function remained satisfactory even without steroidal treat- ment with normal FEV1 and FeNO (21 ppb) values. Eosinophilia however was significant with 1.13 G/L.
The lung function kept being controlled but FeNO rose to 72 ppb at the end of April 2016.
This suggested continuation of treatment with fluticasone once per day (250 mcg), reducing the FeNO to 50 ppb by the end of May.
Unfortunately, an enlargement of the existing cavern with new- ly occurring air-fluid-level was observed at that time. Once again a resection was performed thoracoscopically in early June 2016 under perioperative treatment of caspofungin 70 mg. Additionally a central venous port was implanted to allow the daily continuation with caspo- fungin therapy (50 mg per day) until the 30th of June. Afterwards the intravenous antifungal medication was changed to voriconazole two times 200 mg per day. Due to insufficient systemic concentration, the dose was increased to 400 mg in the morning and 200 mg in the eve- ning after three weeks.
Steroidal therapy was discontinued again after the resection, due to the previous worsening of the Aspergillus infection.
In July 2016 the patient’s lung function showed a light obstruction for the first time. Additionally, pleural effusion and newly occurring various nodule formation in the basal pulmonary segments were ob- served. In early August, the lung function returned to stable condi- tions but FeNO rose to 82 ppb, likely due to the lack of steroidal treat- ment. In September 2016, the previously discovered nodular findings were declining during a three-week holiday abroad but a tree-in-bud sign was observed in the right upper lobe. On the 16th of September, we replaced the monthly omalizumab treatment with mepolizumab. This meant replacing the more general anti-IgE-therapy with anti-in- terleukin-5-treatment, which offers a more specific and slightly more effective treatment of severe allergic asthma [7].
The FeNO improved well despite no steroidal medication, being at 31 ppb a few days after the start of mepolizumab. In October, as an additional line of measures for recovery, the patient was advised to work from home, due to high exposition to Aspergillus spores at his workplace. The home-based work has been continued to this date.
In mid-October, ground-glass-infiltrates were observed and treat- ed with prednisone 40 mg and moxifloxacin 400 mg daily for two weeks. Afterwards, moxifloxacin was stopped and Prednisone reduced to 20 mg daily for an additional week. This was effective, reducing the infiltrates and further lowering the FeNO to 22 ppb.
According to advise from the infectiology department, antifungal medication with voriconazole was stopped in mid-November 2016. This was decided considering the lack of infiltrative Aspergillus activi- ty, shown by the absence of eosinophilia, lack of CRP elevation, FeNO of 20 ppb, normal haematological parameters overall and a moder- ately high but, compared to usual values, reduced IgE of 1467 kU/L (compared to 2965 kU/L in September).
FeNO rose again to 71 ppb in February 2017 and small infiltrates were observed in the radiological controls. This was thought to be due to a conventional ABPA and indicated there continuation of topical corticosteroid therapy with fluticasone and salmeterol 250 mg 1-0-1, which produced the desired results of reducing the FeNO and achiev- ing remission of the infiltrates.
This antiasthmatic treatment is continued to this day alongside with monthly mepolizumab injections. The ABPA is persistent and has to be monitored quite thoroughly, causing fluctuating FeNO and IgE levels. But cavernous necrotizing fungus growth could be suc- cessfully avoided by effective and patient-specific treatment alongside with environmental fungus-prophylaxis.
Discussion
After the successful treatment of this unusual case of ABPA and its complications, the question remains why a physically fit, young and immunologically competent individual showed such a heavy manifestation of this disease. The main point of discussion hereby concerns differentiating between, whether the lung tissue destruction was caused by invasive fungus growth or by chronic inflammatory processes, designed to combat the Aspergillus, which damaged the lung parenchyma.
Due to the persisting pathology, immunological testing for a Signal Transducer and Activator of Transcription 3 (STAT3) gene mutation was commissioned in late 2016, which stands in association with HIES [8,9].
HIES is an immunodeficiency syndrome associated with high IgE levels and a low count of Th17-cells. The patient indeed showed very high levels of IgE with peaks around 5000 G/L in May 2016, but a Th17 cell deficiency is not yet verified.
However, genetic testing for STAT3 mutation produced negative results. The hyper-IgE-score was 25, scores below 20 signify a low probability of HIES, whereas scores over 40 represent a high probability [10]. Further search for alternate mutations causing HIES such as DOCK8 was not pursued due to lack of clinical symptoms such as atopic dermatitis [11].
If there was an immunodeficiency, this would support the assumption of a classical invasive Aspergillosis, allowing for opportunistic invasive fungal growth. Although it is unlikely that the pathology would becaused by this deficiency alone, being quite severe in manners of lung parenchyma destruction that would rather be expected with serious forms of immunosuppression such as individuals suffering from Acquired Immune Deficiency Syndrome (AIDS) or transplant recipients.
As mentioned previously, encapsulation is regularly observed in aspergilloma but not in cases of invasive Aspergillosis, indicating the existence of an additional pathological process, causing the immune system to form capsules with thicknesses up to 1.5 cm. Radiological findings, such as the ones present, showing highly differentiated caverns filled with necrotic material, are more commonly associated with cavitary pulmonary diseases, an example being tuberculosis.
It is known that granulocytes act as the main agonists in the acute stage of Aspergillosis, being essential for pathogen elimination but also may damage lung tissue by excessive release of oxidants and proteases. Contra intuitively, this inordinate immunological answer can then in turn be exploited by the fungus, increasing its pathogenicity [9].
In a patient prone to granulocyte over-excitation upon contact with Aspergillus, also shown by the ABPA, this may promote the invasive characteristics of the fungus and simultaneously cause chronically persisting inflammatory processes, responsible for the capsule formation.
In this respect, the reason for the severity of this Aspergillus fumigates infection and its invasiveness may not solely be an immunodeficiency but also an incorrectly mediated inflammatory response, producing a highly atypical pathological pattern.
The current understanding of Aspergillus infection does not allow for a definite conclusion and the exact mechanisms present in this particular case still pose a challenge yet to be solved completely. However, taking this case as an example, Aspergillus infection should be considered in cases of cavitary pulmonary diseases, even if the patient in question is not immunodeficient.
Acknowledgement
Seife Hailemariam, Pathological Institute for Histological and Cytological Diagnostics; Aarau Antonios Kolios, Immunology Department University Hospital Zurich.
References
- Moodley L, Pillay J, Dheda K (2014) Aspergilloma and the J Thorac Dis 6: 202-209.
- Greenberger PA, Bush RK, Demain JG, Luong A, Slavin RG, et al. (2014) Allergic Bronchopulmonary J Allergy Clin Immunol Pract 2: 703- 708.
- Kolilekas L, Kalomenidis I, Manali E, Liberopoulos P, Papiris S (2008) Re- current Hemoptysis in a Patient with Previous Respiration. 78: 453-454.
- Heinzerling L, Mari A, Bergmann KC, Bresciani M, Burbach G, et (2013) The skin prick test - European standards. Clin Transl Allergy 3: 3.
- Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, et al. (2013) Allergic bronchopulmonary Aspergillosis: review of literature and proposal of new di- agnostic and classification criteria. Clin Exp Allergy 43: 850-873.
- Blanchard E, Gabriel F, Jeanne-Leroyer C, Servant V, Dumas PY (2018) [In- vasive pulmonary Aspergillosis]. Rev Mal Respir.
- Cockle SM, Stynes G, Gunsoy NB, Parks D, Alfonso-Cristancho R, et al. (2017) Comparative effectiveness of mepolizumab and omalizumab in severe asthma: An indirect treatment comparison. Respir Med 123: 140-148.
- Arora M, Bagi P, Strongin A, Heimall J, Zhao X, et (2017) Gastrointestinal Manifestations of STAT3-Deficient Hyper-IgE Syndrome. J Clin Immunol 37: 695-700.
- Sharma S, Saikia B, Goel S, Rawat A, Minz RW, et al. (2016) TH17 Cells in STAT3 Related Hyper-IgE Syndrome. Indian J Pediatr 83: 1104-1108.
- Schimke LF, Sawalle-Belohradsky J, Roesler J, Wollenberg A, Rack A, et al. (2010) Diagnostic approach to the hyper-IgE syndromes: immunologic and clinical key findings to differentiate hyper-IgE syndromes from atopic dermati- J Allergy Clin 126: 611-617.
- Su HC (2010) DOCK8 (Dedicator of cytokinesis 8) deficiency. Curr Opin Aller- gy Clin Immunol. 10: 515-520.
Citation:Gardin F, Roeder M, Mende K, Schöb O, Ruef C, Barandun J (2018) Extraordinary Manifestation of Cavernous Allergic Broncho- pulmonary Aspergillosis in an Immunocompetent Patient. J Case Repo Imag 2: 005.
Copyright: © 2018 Gardin F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


