*Corresponding Author:
Vladimir Zaichick,
Radionuclide Diagnostics Department, Medical Radiological Research Centre, Russia
Tel: +7 (48439) 60289
Fax: +7 (495) 956 1440
E-mail: vzaichick@gmail.com
Abstract
Thyroid Adenomas (TA) are benign tumors, but there is a 20% possibility of malignant transformation. The distinguishing between the TA and Thyroid Cancer (TC) is tricky, therefore new TA biomarkers are needed. Furthermore, the role of Chemical Elements (ChE) in etiology and pathogenesis of TA is unclear. The aim of this exploratory study was to examine the content of Silver (Ag), Bromine (Br), Calcium (Ca), Chlorine (Cl), Cobalt (Co), Chromium (Cr), Cooper (Cu), Iron (Fe), Mercury (Hg), Iodine (I), Potassium (K), Magnesium (Mg), Manganese (Mn), Sodium (Na), Rubidium (Rb), Ammonium (Sb), Scandium (Sc), Selenium (Se), Strontium (Sr), and Zinc (Zn) in the normal and adenomatous thyroid.
Thyroid tissue levels of twenty Chemical Elements (ChE) were prospectively evaluated in 46 patients with TA and 105 healthy inhabitants. Measurements were performed using non-destructive energy-dispersive X-Ray fluorescent analysis combined with instrumental neutron activation analysis with high resolution spectrometry of short-and long-lived radionuclides. Tissue samples were divided into two portions. One was used for morphological study while the other was intended for ChE analysis. It was found that during an adenomatous transformation the mass fraction of Ag, Br, Cl, Cr, Hg, and Na in thyroid tissue significantly increased, whereas the levels of I, Mg, and Sr decrease. It was supposed that the changes in levels Ag, Br, Cl, Cr, Hg, I, Mg, Na, and Sr in thyroid tissue can be used as TA markers.
Keywords
Chemical elements; Energy-dispersive X-Ray fluorescent analysis; Instrumental neutron activation analysis; Intact thyroid; Thyroid adenomas
Introduction
Thyroid Adenomas (TA) are homogenous, solitary, encapsulated benign tumors, more common in females, and have a good prognosis [1]. However, because there is a 20% possibility of malignant transformation, TA should be differentiated from other thyroid nodular diseases such as Nodular Goiter (NG) and Thyroid Cancer (TC). The distinguishing between the TA and TC is tricky, therefore new differential diagnostics and TA biomarkers are needed [2,3].
For over 20th century, there was the dominant opinion that NG, including TA, is the simple consequence of iodine (I) deficiency. However, it was found that NG is a frequent disease even in those countries and regions where the population is never exposed to I shortage [4]. Moreover, it was shown that I excess has severe consequences on human health and associated with the presence of thyroidal disfunctions and autoimmunity, nodular and diffuse goiters, adenomas and malignant tumors of gland [5-8]. It was also demonstrated that besides the I deficiency and excess many other dietary, environmental, and occupational factors are associated with the NG incidence [9-11]. Among them a disturbance of evolutionary stable input of many (ChE) in human body after industrial revolution plays a significant role in etiology of thyroidal disorders [12].
Besides I involved in thyroid function, other ChE have also essential physiological functions such as maintenance and regulation of cell function, gene regulation, activation or inhibition of enzymatic reactions, and regulation of membrane function [13]. Essential or toxic (Goitrogenic, Mutagenic, Carcinogenic) properties of ChE depend on tissue-specific need or tolerance, respectively [13]. Excessive accumulation or an imbalance of the ChE may disturb the cell functions and may result in cellular degeneration, death, benign or malignant transformation [13-15].
In our previous studies the complex of in vivo and in vitro nuclear analytical and related methods was developed and used for the investigation of I and other ChE contents in the normal and pathological thyroid [16-22]. Level of I in the normal thyroid was investigated in relation to age, gender and some non-thyroidal diseases [23,24]. After that, variations of ChE content with age in the thyroid of males and females were studied and ageand gender- dependence of some ChE was observed [25-41]. Furthermore, a significant difference between some ChE contents in normal and cancerous thyroid was demonstrated [42-47].
To date, the etiology and pathogenesis of TA has to be considered as multifactorial. The present study was performed to clarify the role of some ChE in the TA etiology. Having this in mind, our aim was to assess the Silver (Ag), Bromine (Br), Calcium (Ca), Chlorine (Cl), Cobalt (Co), Chromium (Cr), Cooper (Cu), Iron (Fe), Mercury (Hg), I, Potassium (K), Magnesium (Mg), Manganese (Mn), Sodium (Na), Rubidium (Rb), Ammonium (Sb), Scandium (Sc), Selenium (Se), Strontium (Sr), and Zinc (Zn) contents in TA tissue using Energy Dispersive X-ray Fluorescent Analysis (EDXRF) combined with non-destructive Instrumental Neutron Activation Analysis with high resolution spectrometry of Sort-Lived Radionuclides (INAA-SLR) and Long-Lived Radionuclides (INAA-LLR). A further aim was to compare the levels of these twenty ChE in the adenomatous thyroid with those in intact (normal) gland of apparently healthy persons.
Material and Methods
All patients suffered from TA (n=19, 16 females and 3 males, mean age M ± SD was 41 ± 11 years, range 22-55) were hospitalized in the Head and Neck Department of the Medical Radiological Research Centre. Thick-needle puncture biopsy of suspicious nodules of the thyroid was performed for every patient, to permit morphological study of thyroid tissue at these sites and to estimate their TE contents. For all patients the diagnosis has been confirmed by clinical and morphological results obtained during studies of biopsy and resected materials. Histological conclusion for all thyroidal lesions was the TA.
Normal thyroids for the control group samples were removed at necropsy from 105 deceased (mean age 44 ± 21 years, range 2-87), who had died suddenly. The majority of deaths were due to trauma. A histological examination in the control group was used to control the age norm conformity, as well as to confirm the absence of micronodules and latent cancer.
All tissue samples were divided into two portions using a titanium scalpel [48]. One was used for morphological study while the other was intended for chemical element analysis. After the samples intended for ChE analysis were weighed, they were freeze-dried and homogenized [49].
The content of Br, Cu, Fe, Rb, Sr, and Zn were determined by EDXRF. Details of the relevant facility for this method, source with 109Cd radionuclide, methods of analysis and the results of quality control were presented in our earlier publications concerning the EDXRF of ChE contents in human thyroid and prostate tissue [25,26,50].
The content of Br, Ca, Cl, I, K, Mg, Mn, and Na were determined by INAA-SLR using a horizontal channel equipped with the pneumatic rabbit system of the WWR-c research nuclear reactor (Branch of Karpov Institute, Obninsk). Details of used neutron flux, nuclear reactions, radionuclides, gamma-energies, spectrometric unit, sample preparation and measurement were presented in our earlier publications concerning the INAA-SLR of ChE contents in human thyroid, scalp hair, and prostate [27,28,51-53].
In a few days after non-destructive INAA-SLR all thyroid samples were repacked and used for INAA-LLR. A vertical channel of the WWR-c research nuclear reactor (Branch of Karpov Institute, Obninsk).was applied to determine the content of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn by INAA-LLR. Details of used neutron flux, nuclear reactions, radionuclides, gamma-energies, spectrometric unit, sample preparation and measurement were presented in our earlier publications concerning the INAA-LLR of ChE contents in human thyroid, scalp hair, and prostate [29,30,51,54].
To determine contents of the ChE by comparison with a known standard, Biological Synthetic Standards (BSS) prepared from phenol-formaldehyde resins were used [55]. In addition to BSS, aliquots of commercial, chemically pure compounds were also used as standards. For each method ten certified reference material IAEA H-4 (animal muscle) and IAEA HH-1 (human hair) sub-samples were treated and analyzed in the same conditions that thyroid samples to estimate the precision and accuracy of results.
A dedicated computer program for INAA mode optimization was used [56]. All thyroid samples were prepared in duplicate, and mean values of ChE contents were used. Mean values of ChE contents were used in final calculation for the Br, Fe, Rb, and Zn mass fractions measured by two methods. Using Microsoft Office Excel, a summary of the statistics, including, arithmetic mean, standard deviation, standard error of mean, minimum and maximum values, median, percentiles with 0.025 and 0.975 levels was calculated for ChE contents. The difference in the results between two groups (normal thyroid and TA) was evaluated by the parametric Student’s t-test and non-parametric Wilcoxon-Mann-Whitney U-test.
All studies were approved by the Ethical Committees of the Medical Radiological Research Centre, Obninsk. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
Depicts our data for Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn mass fraction mass fractions in ten sub-samples of IAEA H-4 (Animal Muscle) and IAEA HH-1 (Human Hair) certified reference material and the certified values of this material.
The comparison of our results for the Br, Fe, Rb, and Zn mass fractions (mg/kg, dry mass basis) in the normal human thyroid obtained by both EDXRF and INAA methods is shown in (Tables 1-3).
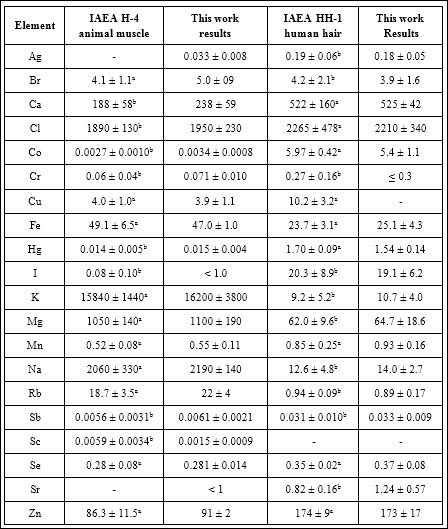
Table 1: EDXRF, INAA-SLR and INAA-LLR data of chemical element contents in certified reference material IAEA H-4 (animal muscle) and IAEA HH-1 (human hair) compared to certified values ((mg/kg, dry mass basis).
M – arithmetical mean, SD – standard deviation, a – certified values, b – information values.

Table 2: Comparison of the mean values (M ± SD) of the chemical element mass fractions (mg/kg, dry mass basis) in the normal human thyroid obtained by both EDXRF and INAA methods.
M – arithmetic mean, SEM – standard error of mean.
Presents certain statistical parameters (Arithmetic Mean, Standard Deviation, Standard Error of Mean, Minimal and Maximal Values, Median, Percentiles with 0.025 and 0.975 Levels) of the Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn mass fraction mass fraction in normal and adenomatous thyroid.
The comparison of our results with published data for Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn mass fraction in normal and adenomatous thyroid [57-82]. Is shown in (Table 4).
The ratios of means and the difference between mean values of Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn mass fractions in normal and adenomatous thyroid are presented in (Table 5).

Table 3: Some statistical parameters of Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn mass fraction (mg/kg, dry mass basis) in normal and adeno- matous thyroid.
M – arithmetic mean, SD – standard deviation, SEM – standard error of mean, Min – minimum value, Max – maximum value, P 0.025 – percentile with 0.025 level, P 0.975 – per- centile with 0.975 level.
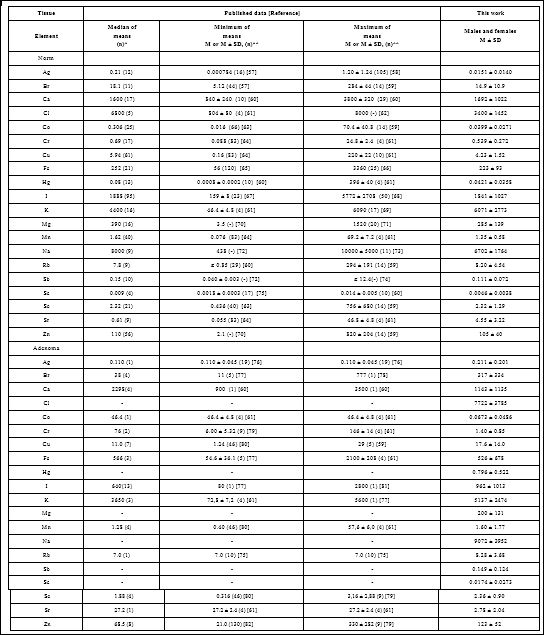
Table 4: Median, minimum and maximum value of means of twenty chemical element contents in the normal and adenomatous thyroid according to data from the literature in comparison with our results (mg/kg, dry mass basis).
M – arithmetic mean, SD – standard deviation, (n)* – number of all references, (n)** – number of samples.
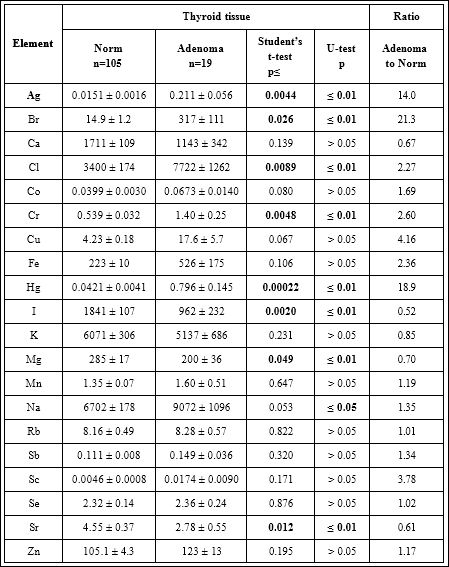
Table 5: Differences between mean values (M ± SEM) of Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn mass fraction (mg/kg, dry mass basis) in normal and adenomatous thyroid.
M – arithmetic mean, SEM – standard error of mean, Statistically significant values are in bold.
Discussion
Precision and accuracy of results
A good agreement of our results for the Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn mass fractions with the certified values of CRM IAEA H-4 and CRM IAEA HH-1 (Table 1) as well as the similarity of the means of the Br, Fe, Rb, and Zn mass fractions in the normal human thyroid determined by both EDXRF and INAA methods (Table 2) demonstrates an acceptable precision and accuracy of the results obtained in the study and presented in (Tables 3-5).
The mean values and all selected statistical parameters were calculated for twenty ChE (Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn) mass fractions (Table 3). The mass fraction of Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn were measured in all, or a major portion of normal and adenomatous tissue samples.
Comparison with published data
Values obtained for Br, Ca, Cl, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, and Zn contents in the normal human thyroid (Table 4) agree well with median of mean values reported by other researches [57-92]. The obtained means for Ag and Co were almost one order of magnitude lower whereas mean for Sr was 7.46 times higher than median of previously reported means, but, nevertheless, inside the range of means (Table 4). A number of values for ChE mass fractions were not expressed on a dry mass basis by the authors of the cited references. However, we calculated these values using published data for water (75%) [83] and ash (4.16% on dry mass basis) [84] contents in thyroid of adults.
Data cited in (Table 4) for normal thyroid also includes samples obtained from patients who died from different non-endocrine diseases. In our previous study it was shown that some non-endocrine diseases can effect on ChE contents in thyroid [24]. Moreover, in many studies the “Normal” thyroid means a visually non-affected tissue adjacent to benign or malignant thyroidal nodules. However, there are no data on a comparison between the ChE contents in such kind of samples and those in thyroid of healthy persons, which permits to confirm their identity.
In adenomatous thyroid (Table 4) our results were comparable with published data for Ag, Ca, Cu, Fe, I, K, Mn, Rb, Se, and Zn contents. The obtained means for Br, Co, Cr, and Sr were approximately one order of magnitude lower median of previously reported means. The obtained mean for Br was inside the range of reported means, whereas the obtained mean for Cr was lower the minimal published mean for this element (Table 4). The data on Ag, Co, Rb, and Sr content in TA were found in one paper. No published data referring Cl, Hg, Mg, Na, Sb, and Sc contents of adenomatous thyroid were found.
The range of means of Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn level reported in the literature for normal and for adenomatous thyroid vary widely (Table 4). This can be explained by a dependence of ChE content on many factors, including “Normality” of thyroid samples (see above), the region of the thyroid, from which the sample was taken, age, gender, ethnicity, mass of the gland, and the adenoma stage, histology and functional activity. Not all these factors were strictly controlled in cited studies. However, in our opinion, the leading causes of inter- observer variability can be attributed to the accuracy of the analytical techniques, sample preparation methods, and inability of taking uniform samples from the affected tissues.
It was insufficient quality control of results in these studies. In many scientific reports, tissue samples were ashed or dried at high temperature for many hours. In other cases, thyroid samples were treated with solvents (Distilled Water, Ethanol, Formalin etc). There is evidence that during ashing, drying and digestion at high temperature some quantities of certain ChE are lost as a result of this treatment. That concerns not only such volatile halogen as Br, but also other ChE investigated in the study [49,85,86].
Effect of adenomatous transformation on ChE contents
From (Table 5), it is observed that in adenomatous tissue the mass fraction of Ag, Br, Cl, Cr, Hg, and Na are approximately 14.0, 21.3, 2.27, 2.60, 18.9, and 1.35 times, respectively, significantly higher than in normal tissues of the thyroid. In contrast, the mass fractions of I, Mg, and Sr are 48%, 30%, and 39%, respectively, lower. Thus, if we accept the ChE contents in thyroid glands in the control group as a norm, we have to conclude that with an adenomatous transformation the levels of Ag, Br, Cl, Cr, Hg, I, Mg, Na, and Sr in thyroid tissue significantly changed.
Role of ChE in adenomatous transformation of the thyroid
Characteristically, elevated or reduced levels of ChE observed in adenomatous thyroid are discussed in terms of their potential role in the initiation and promotion of thyroid adenoma. In other words, using the low or high levels of the ChE in adenomatous tissues researchers try to determine the role of the deficiency or excess of each ChE in TA etiology. In our opinion, abnormal levels of many ChE in TA could be and cause, and also effect of adenomatous transformation. From the results of such kind studies, it is not always possible to decide whether the measured decrease or increase in ChE level in pathologically altered tissue is the reason for alterations or vice versa.
Silver: Ag is a ChE with no recognized trace metal value in the human body [87]. Ag in metal form and inorganic Ag compounds ionize in the presence of water, body fluids or tissue exudates. The silver ion Ag+ is biologically active and readily interacts with proteins, amino acid residues, free anions and receptors on mammalian and eukaryotic cell membranes [88]. Besides such the adverse effects of chronic exposure to Ag as a permanent bluish-gray discoloration of the skin (Argyria) or eyes (Argyrosis), exposure to soluble Ag compounds may produce other toxic effects, including liver and kidney damage, irritation of the eyes, skin, respiratory, and intestinal tract, and changes in blood cells [89]. More detailed knowledge of the Ag toxicity can lead to a better understanding of the impact on human health, including thyroid function.
Bromine: This is one of the most abundant and ubiquitous of the recognized ChE in the biosphere. Inorganic bromide is the ionic form of bromine which exerts therapeutic as well as toxic effects. An enhanced intake of bromide could interfere with the metabolism of iodine at the whole-body level. In the thyroid gland the biological behavior of bromide is more similar to the biological behavior of iodide [90].
In our previous studies, we found a significant age-related increase of Br content in human thyroid [25-28,31,32]. Therefore, a goitrogenic and, probably, carcinogenic effect of excessive Br levels in the thyroid of old females was assumed. On the one hand, elevated levels of Br in TA tissues, observed in the present study, supports this conclusion. But, on the other hand, bromide compounds, especially Potassium Bromide (KBr), Sodium Bromide (NaBr), and ammonium bromide (NH4Br), are frequently used as sedatives in Russia [91]. It may be the reason for elevated levels of Br in specimens of patients with TA.
Chlorine: Cl is a ubiquitous, extracellular electrolyte essential to more than one metabolic pathway. Cl exists in the form of chloride in the human body. In the body, it is mostly present as sodium chloride. Therefore, as usual, there is a correlation between Na and Cl contents in tissues and fluids of human body. It is well known that Cl mass fractions in samples depend mainly on the extracellular water volume, including the blood volumes, in tissues [92]. Colloid is the extracellular liquid. Thus, it is possible to speculate that TA are characterized by an increase of the mean value of the Cl mass fraction because the level of colloid is higher than that in normal thyroid tissue.
Chromium: The Cr-compounds are cytotoxic, genotoxic, and carcinogenic in nature. Some Cr forms, including hexavalent chromium (Cr6+), are toxicants known for their carcinogenic effect in humans. They have been classified as certain or probable carcinogens by the International Agency for Research on Cancer (IARC) [93]. Furthermore, it was found that an elevated intake of Cr may induce functional and cellular damage in animal and human thyroid [94,95]. Besides Reactive Oxygen Species (ROS) generation, oxidative stress, and cytotoxic effects of Cr exposure, a variety of other changes like DNA damage, increased formation of DNA adducts and DNA- protein cross-links, DNA strand breaks, chromosomal aberrations and instability, disruption of mitotic cell division, chromosomal aberration, premature cell division, S or G2/M cell cycle phase arrest, and carcinogenesis also occur in humans or experimental test systems [96]. In this connection our finding of elevated Cr content in the adenomatous thyroid confirms the role of this ChE in the TA etiology.
Mercury: Hg is one of the most dangerous environmental pollutants [97]. The growing use of this metal in diverse areas of industry has resulted in a significant increase of environment contamination and episodes of human intoxication. Hg has been classified as certain or probable carcinogen by the IARC [98]. For example, in Hg polluted area thyroid cancer incidence was almost 2 times higher than in in adjacent control areas [99].
Negative effects of Hg are due to the interference of this metal in cellular signaling pathways and protein synthesis during the period of development. Since it bonds chemically with the sulfur hydride groups of proteins, it causes damage to the cell membrane and decreases the amount of RNA [100]. Moreover, it was shown that Hg may be involved in four main processes that lead to genotoxicity: generation of free radicals and oxidative stress, action on microtubules, influence on DNA repair mechanisms and direct interaction with DNA molecules [101]. Thus, the present study suggests that an elevated level of Hg in thyroid may be involved in TA etiology.
Iodine: Compared to other soft tissues, the human thyroid gland has higher levels of I, because this element plays an important role in its normal functions, through the production of thyroid hormones (Thyroxin and Triiodothyronine) which are essential for cellular oxidation, growth, reproduction, and the activity of the central and autonomic nervous system. The I deficiency is one of the main (but not only) cause of adenomatous transformation, which leads to a significant reduction in I content associated with functional characteristics of the human thyroid tissue.
Magnesium: Mg is abundant in the human body. This ChE is essential for the functions of more than 300 enzymes (e.g. Alkaline Phosphatases, ATP-Ases, Phosphokinases, The Oxidative Phosphorylation Pathway). It plays a crucial role in many cell functions such as energy metabolism, protein and DNA syntheses, and cytoskeleton activation. Moreover, Mg plays a central role in determining the clinical picture associated with thyroid disease [102].
Sodium: Na is mainly an extracellular electrolyte and its elevated level in TA might link with a high content of colloid in adenomatous tissue (see Chlorine).
Strontium: A reduced Sr content in TA was also indicated by Reddy et al. [61]. However, the role of Sr in the thyroid function is unknown and we can’t explain why the Sr level in adenomatous tissues is 39% lower than in normal thyroid.
Our findings show that mass fraction of Ag, Br, Cl, Cr, Hg, I, Mg, Na, and Sr are significantly different in TA as compared to normal thyroid tissues (Table 5). Thus, it is plausible to assume that levels of these ChE in thyroid tissue can be used as TA markers. However, this subjects needs in additional studies.
Limitations
This study has several limitations. Firstly, analytical techniques employed in this study measure only twenty ChE (Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn) mass fractions. Future studies should be directed toward using other analytical methods which will extend the list of ChE investigated in normal and adenomatous thyroid. Secondly, the sample size of TA group was relatively small. It was not allow us to carry out the investigations of ChE contents in TA group using differentials like gender, histological types of adenoma, functional status of benign neoplasm, stage of disease, and dietary habits of healthy persons and patients with TA. Lastly, generalization of our results may be limited to Russian population. Despite these limitations, this study provides evidence on adenoma-specific tissue Ag, Br, Cl, Cr, Hg, I, Mg, Na, and Sr level alteration and shows the necessity to continue ChE research of TA.
Conclusion
In this work, ChE measurements were carried out in the tissue samples of normal thyroid and TA using three non-destructive instrumental analytical methods: EDXRF, INAA-SLR, and INAA- LLR. It was shown that the combination of these methods is an adequate analytical tool for the non-destructive determination of Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn content in the tissue samples of human thyroid, including needle-biopsy cores. It was observed that in adenomatous tissues content of Ag, Br, Cl, Cr, Hg, and Na significantly increased whereas the levels of I, Mg, and Sr decreased in a comparison with the normal thyroid tissues. In our opinion, the increase in levels of Ag, Br, Cl, Cr, Hg, and Na, as well as the decrease in levels of I, Mg, and Sr in adenomatous tissue might demonstrate an involvement of these ChE in etiology and pathogenesis of TA. It was supposed that the changes in levels Ag, Br, Cl, Cr, Hg, I, Mg, Na, and Sr in thyroid tissue can be used as TA markers.
Acknowledgements
The author is extremely grateful to Profs. B.M. Vtyurin and V.S. Medvedev, Medical Radiological Research Center, Obninsk, as well as to Dr. Yu. Choporov, Head of the Forensic Medicine Department of City Hospital, Obninsk, for supplying thyroid samples.
References
- Welker MJ, Orlov D (2003) Thyroid Nodules. Am Fam Physician 67: 559-567.
- Kant R, Davis A, Verma V (2020) Thyroid nodules: Advances in evaluation and management. Am Fam Physician 102: 298-304.
- Sato A, Matsuda K, Motoyama T, Mussazhanova Z, Otsubo R, et al. (2021) 53BP1 expression as a biomarker to differentiate thyroid follicular Endocr Connect 10: 309-315.
- Derwahl M, Studer H (2000) Multinodular goitre: ‘much more to it than simply iodine deficiency’. Baillieres Best Pract Res Clin Endocrinol Metab 14: 577-600.
- Zaichick V (1998) Iodine excess and thyroid cancer. J Trace Elem Exp Med 11: 508-509.
- Zaichick V, Iljina T (1998) Dietary iodine supplementation effect on the rat thyroid 131I blastomogenic In: Die Bedentung der Mengen- und Spurenelemente. 18. Arbeitstangung. Friedrich-Schiller-Universität, Jena p 294-306.
- Kim S, Kwon YS, Kim JY, Hong KH, Park YK (2019) Association between Iodine Nutrition Status and Thyroid Disease-Related Hormone in Korean Adults: Korean National Health and Nutrition Examination Survey VI (2013-2015). Nutrients 11: 27-57.
- Vargas-Uricoechea Р, Pinzón-Fernández MV, Bastidas-Sánchez BE, Jojoa-Tobar E, Ramírez-Bejarano LE, et al. (2019) Iodine status in the colombian population and the impact of universal salt iodization: A double-edged sword? J Nutr Metab : 6239243.
- Stojsavljević A, Rovčanin B (2021) Impact of essential and toxic trace metals on thyroid health and cancer: A review. Expo Health.
- Fahim YA, Sharaf NE, Hasani IW, Ragab EA, Abdelhakim HK (2020) Assessment of thyroid function and oxidative stress state in foundry workers exposed to lead. J Health Pollut 10: 200903.
- Liu M, Song J, Jiang Y, Lin Y, Peng J, et (2021) A case-control study on the association of mineral elements exposure and thyroid tumor and goiter. Ecotoxicol Environ Saf 208: 111615.
- Zaichick V (2006) Medical elementology as a new scientific J Radioanal Nucl Chem 269: 303-309.
- Moncayo R, Moncayo H (2017) A post-publication analysis of the idealized upper reference value of 2.5 mIU/L for TSH: Time to support the thyroid axis with magnesium and iron especially in the setting of reproduction medicine. BBA Clin 7: 115-119.
- Beyersmann D, Hartwig A (2008) Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol 82: 493-512.
- Martinez-Zamudio R, Ha HC (2011) Environmental epigenetics in metal Epigenetics 6: 820-827.
- Zaĭchik VE, Raibukhin YuS, Melnik AD, Cherkashin VI (1970) Neutron- activation analysis in the study of the behavior of iodine in the Med Radiol (Mosk) 15: 33-36.
- Zaĭchik VE, Matveenko EG, Vtiurin BM, Medvedev VS (1982) Intrathyroid iodine in the diagnosis of thyroid cancer. Vopr Onkol 28: 18-24.
- Zaichick V, Tsyb AF, Vtyurin BM (1995) Trace elements and thyroid Analyst 120: 817-821.
- Zaichick VYe, Choporov YuYa (1996) Determination of the natural level of human intra-thyroid iodine by instrumental neutron activation J Radioanal Nucl Chem 207: 153-161.
- Zaichick V (1998) In vivo and in vitro application of energy-dispersive XRF in clinical investigations: Experience and the future. J Trace Elem Exp Med 11: 509-510.
- Zaichick V, Zaichick S (1999) Energy-dispersive X-ray fluorescence of iodine in thyroid puncture biopsy specimens. J Trace Microprobe Tech 17: 219-232.
- Zaichick V (2000) Relevance of, and potentiality for in vivo intrathyroidal iodine determination. Ann N Y Acad Sci 904: 630-632.
- Zaichick V, Zaichick S (1997) Normal human intrathyroidal iodine. Sci Total Environ 206: 39-56.
- Zaichick V (1999) Human intrathyroidal iodine in health and non- thyroidal disease. In: New aspects of trace element research (Eds: Abdulla, M.Bost, S.Gamon, P.Arnaud, G.Chazot). Smith-Gordon, London, and Nishimura,Tokyo p. 114-119.
- Zaichick V, Zaichick S (2017) Age-related changes of some trace element contents in intact thyroid of females investigated by energy dispersive X-ray fluorescent analysis. Trends Geriatr Healthc 1: 31-38.
- Zaichick V, Zaichick S (2017) Age-related changes of some trace element contents in intact thyroid of males investigated by energy dispersive X-ray fluorescent analysis. MOJ Gerontol Ger 1: 00028.
- Zaichick V, Zaichick S (2017) Age-related changes of Br, Ca, Cl, I, K, Mg, Mn, and Na contents in intact thyroid of females investigated by neutron activation analysis. Curr Updates Aging 1: 51.
- Zaichick V, Zaichick S (2017) Age-related changes of Br, Ca, Cl, I, K, Mg, Mn, and Na contents in intact thyroid of males investigated by neutron activation analysis. J Aging Age Relat Dis 1: 1002.
- Zaichick V, Zaichick S (2017) Age-related changes of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn contents in intact thyroid of females investigated by neutron activation analysis. J Gerontol Geriatr Med 3: 015.
- Zaichick V, Zaichick S (2017) Age-related changes of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn contents in intact thyroid of males investigated by neutron activation analysis. Curr Trends Biomedical Eng Biosci 4555644..
- Zaichick V, Zaichick S (2018) Effect of age on chemical element contents in female thyroid investigated by some nuclear analytical methods. MicroMedicine 6: 47-61.
- Zaichick V, Zaichick S (2018) Neutron activation and X-ray fluorescent analysis in study of association between age and chemical element contents in thyroid of males. Op Acc J Bio Eng Bio Sci 2: 202-212.
- Zaichick V, Zaichick S (2018) Variation with age of chemical element contents in females’ thyroids investigated by neutron activation analysis and inductively coupled plasma atomic emission spectrometry. J Biochem Analyt Stud 3: 1-10.
- Zaichick V, Zaichick S (2018) Association between age and twenty chemical element contents in intact thyroid of males. SM Gerontol Geriatr Res 2: 1014.
- Zaichick V, Zaichick S (2018) Associations between age and 50 trace element contents and relationships in intact thyroid of Aging Clin Exp Res 30: 1059-1070.
- Zaichick V, Zaichick S (2018) Possible role of inadequate quantities of intra-thyroidal bromine, rubidium and zinc in the etiology of female subclinical hypothyroidism. EC Gynaecology 7: 107-115.
- Zaichick V, Zaichick S (2018) Possible role of inadequate quantities of intra-thyroidal bromine, calcium and magnesium in the etiology of female subclinical hypothyroidism. Int Gyn and Women’s Health 1: IGWHC. ID.000113.
- Zaichick V, Zaichick S (2018) Possible role of inadequate quantities of intra-thyroidal cobalt, rubidium and zinc in the etiology of female subclinical hypothyroidism. Womens Health Sci J 2: 000108.
- Zaichick V, Zaichick S (2018) Association between female subclinical hypothyroidism and inadequate quantities of some intra-thyroidal chemical elements investigated by X-ray fluorescence and neutron activation analysis. Gynaecology and Perinatology 2: 340-355.
- Zaichick V, Zaichick S (2018) Investigation of association between the high risk of female subclinical hypothyroidism and inadequate quantities of twenty intra-thyroidal chemical elements. Clin Res: Gynecol Obstet 1: 1-18.
- Zaichick V, Zaichick S (2018) Investigation of association between the high risk of female subclinical hypothyroidism and inadequate quantities of intra-thyroidal trace elements using neutron activation and inductively coupled plasma mass spectrometry. Acta Scientific Medical Sciences 2: 23-37.
- Zaichick V. Zaichick S (2018) Trace element contents in thyroid cancer investigated by energy dispersive X-ray fluorescent analysis. American Journal of Cancer Research and Reviews 2: 5.
- Zaichick V, Zaichick S (2018) Trace element contents in thyroid cancer investigated by instrumental neutron activation analysis. J Oncol Res 2: 1-13.
- Zaichick V, Zaichick S (2018) Variation in selected chemical element contents associated with malignant tumors of human thyroid gland. Cancer Studies 2: 2.
- Zaichick V, Zaichick S (2018) Twenty chemical element contents in normal and cancerous thyroid. Int J Hematol Blo Dis 3: 1-13.
- Zaichick V, Zaichick S (2018) Levels of chemical element contents in thyroid as potential biomarkers for cancer diagnosis (a preliminary study). J Cancer Metastasis Treat 4: 60.
- Zaichick V, Zaichick S (2018) Fifty trace element contents in normal and cancerous thyroid. Acta Scientific Cancer Biology 2: 21-38.
- Zaichick V, Zaichick S (1996) Instrumental effect on the contamination of biomedical samples in the course of sampling. The Journal of Analytical Chemistry 51: 1200-1205.
- Zaichick V, Zaichick S (1997) A search for losses of chemical elements during freeze-drying of biological J Radioanal Nucl Chem 218: 249-253.
- Zaichick S, Zaichick V (2011) The Br, Fe, Rb, Sr, and Zn contents and interrelation in intact and morphologic normal prostate tissue of adult men investigated by energy-dispersive X-ray fluorescent X-Ray Spectrom 40: 464-469.
- Zaichick S, Zaichick V (2010) The effect of age and gender on 37 chemical element contents in scalp hair of healthy humans. Biol Trace Elem Res 134: 41-54.
- Zaichick S, Zaichick V (2011) INAA application in the age dynamics assessment of Br, Ca, Cl, K, Mg, Mn, and Na content in the normal human J Radioanal Nucl Chem 288: 197-202.
- Zaichick V, Zaichick S (2013) The effect of age on Br, Ca, Cl, K, Mg, Mn, and Na mass fraction in pediatric and young adult prostate glands investigated by neutron activation analysis. J Appl Radiat Isot 82: 145-151.
- Zaichick S, Zaichick V (2011) The effect of age on Ag, Co, Cr, Fe, Hg, Sb, Sc, Se, and Zn contents in intact human prostate investigated by neutron activation analysis. J Appl Radiat Isot 69: 827-833.
- Zaichick V (1995) Applications of synthetic reference materials in the medical Radiological Research Fresenius J Anal Chem 352: 219- 223.
- Korelo AM, Zaichick V (1993) Software to optimize the multielement INAA of medical and environmental In: Activation Analysis in Environment Protection. Joint Institute for Nuclear Research, Dubna, Russia 326-332.
- Zhu H, Wang N, Zhang Y, Wu Q, Chen R, et al. (2010) Element contents in organs and tissues of Chinese adult men. Health Phys 98: 61-73.
- Vlasova ZA (1969) Dynamics of trace element contents in thyroid gland in connection with age and Proceedings of the Leningrad Institute of Doctor Advanced Training 80: 135-144.
- Salimi J, Moosavi K, Vatankhah S, Yaghoobi A (2004) Investigation of heavy trace elements in neoplastic and non-neoplastic human thyroid tissue: A study by proton- induced X-ray emissions. Int J Radiat Res 1: 211-216.
- Boulyga SF, Zhuk IV, Lomonosova EM, Kanash NV, Bazhanova NN (1997) Determination of microelements in thyroids of the inhabitants of Belarus by neutron activation analysis using the k0-method. J Radioanal Nucl Chem 222: 11-14.
- Reddy SB, Charles MJ, Kumar MR, Reddy BS, Anjaneyulu CH, et al. (2002) Trace elemental analysis of adenoma and carcinoma thyroid by PIXE method. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 196: 333-339.
- Woodard HQ, White DR (1986) The composition of body tissues. Brit J Radiol 708: 1209-1218.
- Stojsavljević A, Rovčanin B, Krstić D, Borković-Mitić S, Paunović I, et al. (2019) Risk assessment of toxic and essential trace metals on the thyroid health at the tissue level: The significance of lead and selenium for colloid goiter disease. Expo Health 12: 255-264.
- Reitblat MA, Kropachyev AM (1967) Some trace elements in thyroid of the Perm Pricam’ya Proceedings of Perm Medical Institute 78: 157-164.
- Ataullachanov IA (1969) Age changes in the content of manganese, cobalt, copper, zinc and iron in the endocrine glands of women. Probl Endocrinol (Mosk) 15: 98-102.
- Kamenev VF (1963) About trace element contents in thyroid of In: Trace Elements in Agriculture and Medicine. Buryatia publishing-house, Ulan-Ude, Russia12-16.
- Neimark II, Timoschnikov VM (1978) Development of carcinoma of the thyroid gland in person residing in the focus of goiter Problemy Endocrinilogii 24: 28-32.
- Zabala J, Carrion N, Murillo M, Quintana M, Chirinos J, et al. (2009) Determination of normal human intrathyroidal iodine in Caracas J Trace Elem Med Biol 23: 9-14.
- Forssen A (1972) Inorganic elements in the human body. Ann Med Exp Biol Fenn 50: 99-162.
- Kortev AI, Dontsov GI, Lyascheva AP (1972) Bioelements and a human Middle-Ural publishing-house, Sverdlovsk, Russia.
- Li AA (1973) Level of some macro- and trace element contents in blood and thyroid of patients with endemic goiter in Kalinin PhD thesis. Kalinin medical institute, Kalinin.
- Boulyga SF, Becker JS, Malenchenko AF, Dietze HJ (2000) Application of ICP-MS for multielement analysis in small sample amounts of pathological thyroid tissue. Microchimica Acta 134: 215-222.
- Soman SD Joseph KT, Raut SJ, Mulay CD, Parameshwaran M, et al. (1970) Studies of major and trace element content in human tissues. Health Phys 19: 641-656.
- Zakutinsky DK, Parfyenov YuD, Selivanova LN (1962) Handbook of the toxicology of radioactive isotopes. State Publishing House of Medical Literature, Moscow.
- Kvicala J, Havelka J, Nemec J, Zeman V (1992) Selenium and rubidium changes in subjects with pathologically altered thyroid. Biol Trace Elem Res 32: 253-258.
- Predtechenskaya VC (1975) Nucleic acids and trace elements in thyroid Proceedings of the Voronezh Medical Faculty 94: 85-87.
- Maeda K, Yokode Y, Sasa Y, Kusuyama H, Uda M (1987) Multielemental analysis of human thyroid glands using particle induced X-ray emission (PIXE). Nucl Instrum Methods Phys Res B 22: 188-190.
- Turetskaia ES (1961) Studies on goitrous thyroid glands for iodine and bromine content. Probl Endokrinol Gormonoter 7: 75-80.
- Zagrodzki P, Nicol F, Arthur JR, Słowiaczek M, Walas S, et al. (2010) Selenoenzymes, laboratory parameters, and trace elements in different types of thyroid tumor. Biol Trace Elem Res 134: 25-40.
- Stojsavljević A, Rovčanin B, Krstić D, Borković-Mitić S, Paunović I, et al. (2019) Evaluation of trace metals in thyroid tissues: Comparative analysis with benign and malignant thyroid Ecotoxicol Environ Saf 183: 109479.
- Tadros TG, Maisey MN, Ng Tang Fui SC, Turner P (1981) The iodine concentration in benign and malignant thyroid nodules measured by X-ray fluorescence. Br J Radiol 54: 626-629.
- Koch HJ, Smith ER (1956) The determination of copper and zinc in normal and pathologic thyroid tissue. J Clin Endocrinol 16: 123-129.
- Katoh Y, Sato T, Yamamoto Y (2002) Determination of multielement concentrations in normal human organs from the Japanese. Biol Trace Elem Res 90: 57-70.
- Schroeder HA, Tipton IH, Nason AP (1972) Trace metals in man: Strontium and barium. J Chron Dis 25: 491-517.
- Zaichick V (1997) Sampling, samplestorage and preparation of biomaterials for INAA in clinical medicine, occupational and environmental In: Harmonization of Health-Related Environmental Measurements Using Nuclear and Isotopic Techniques. IAEA Vienna 123-133.
- Zaichick V (2004) Losses of chemical elements in biological samples under the dry aching process. Trace Elements in Medicine 5:17-22.
- Lansdown AB (2007) Critical observations on the neurotoxicity of Crit Rev Toxicol 37: 237-250.
- Lansdown AB (2006) Silver in health care: Antimicrobial effects and safety in use. Curr Probl Dermatol 33: 17-34.
- Drake PL, Hazelwood KJ (2005) Exposure-related health effects of silver and silver compounds: A review. Ann Occup Hyg 49: 575-585.
- Pavelka S (2016) Radiometric determination of thyrotoxic effects of some xenobiotics. Rad Applic 1: 155-158.
- Maschkovsky MD (2005) The sedatives. In: The Medicaments. 15th ed. Novaya Volna, Moscow 72-86.
- Zaichick V (1998) X-ray fluorescence analysis of bromine for the estimation of extracellular water. J Appl Radiat Isot 49: 1165-1169.
- Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68: 167-182.
- Qureshi IZ, Mahmood T (2010) Prospective role of ascorbic acid (vitamin C) in attenuating hexavalent chromium-induced functional and cellular damage in rat thyroid. Toxicol and Health 26: 349-359.
- Petrosino V, Motta G, Tenore G, Coletta M, Guariglia A, et al. (2018) The role of heavy metals and polychlorinated biphenyls (PCBs) in the oncogenesis of head and neck tumors and thyroid diseases: A pilot Biometals 31: 285-295.
- Nigam A, Priya S, Bajpai P, Kumar S (2014) Cytogenomics of hexavalent chromium (Cr 6+) exposed cells: A comprehensive Indian J Med Res 139: 349-370.
- Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36: 609-662.
- Hazelhoff MH, Bulacio RP, Torres AM (2012) Gender related differences in kidney injury induced by mercury. Int J Mol Sci 13: 10523-10536.
- Malandrino P, Russo M, Ronchi A, Cataldo D, Regalbuto C, et (2016) Increased thyroid cancer incidence in a basaltic volcanic area is associated with non-anthropogenic pollution and biocontamination. Endocrine 53: 471-479.
- Abnoos H, Fereidoni M, Mahdavi-Shahri N, Haddad F, Jalal R (2013) Developmental study of mercury effects on the fruit fly (Drosophila melanogaster). Interdiscip Toxicol 6: 34-40.
- Crespo-López ME, Macêdo GL, Pereira SI, Arrifano GP, Picanço-Diniz DL, et al. (2009) Mercury and human genotoxicity: Critical considerations and possible molecular mechanisms. Pharmacol Res 60: 212-220.
- Moncayo R, Moncayo H (2017) Applying a systems approach to thyroid physiology: Looking at the whole with a mitochondrial perspective instead of judging single TSH values or why we should know more about mitochondria to understand metabolism. BBA Clin 7: 127-140.
Citation: Zaichick V (2021) Evaluation of Twenty Chemical Element Contents in Thyroid Adenomas using X-Ray Fluorescent and Neutron Activation Analysis. J Cell Mol Onco 3: 007.
Copyright: © 2021 Zaichick V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.