*Corresponding Author:
Ravi Jadeja,
Associate Professor and Food Safety Specialist,106 Robert M Kerr Food and Agriculture Products Center Oklahoma State University, Stillwater, OK, 74078, USA
E-mail: ravi.jadeja@ okstate.edu
Abstract
In this study the suitability of Sodium Acid Sulfate (SAS) as an antimicrobial intervention to reduce Escherichia coli O157:H7, Salmonella Typhimurium DT 104 and Listeria monocytogenes from cantaloupe and bell pepper was investigated. The produce were spot inoculated by placing 20μl inoculum of approximately (high) 9 or (low) 6 logs CFU/ml target pathogens on marked spots on produce. The inoculated produced were treated by submerging in 1,2, or 3% SAS, peroxyacetic acid (85 ppm, PAA), sodium hypchlorite (200 ppm), and deionized (DI) water control for one minute. After treatment, inoculated portions of the produce were excised and placed in 10% sodium metabiosulfate neutralizing solution. Microbial enumeration of target pathogens from the produce were carried our using appropriate selective media. For all produce inoculated with low levels of pathogens, all treatments reduced the number of target pathogens below plating detection limit. When produce items were inoculated with a higher level of pathogens, all treatments were more effective in remove pathogens form bell pepper in comparison of cantaloupe. Among all treatments applied to produce inoculated with the high levels of pathogens, DI control was the least effective in removing pathogens form produce while, 3% SAS treatment was found to be the most effective treatment except for L. monocytogenes removal from bell pepper. The finding of the study suggests that antimicrobial SAS treatment could be a suitable antimicrobial intervention for bell pepper and cantaloupes.
Keywords
Bell Pepper; Cantaloupe, Sodium acid sulfate, Antimi- crobials
Introduction
The food industry relies on Good Agricultural Practices to minimize the amount of contamination found on fresh produce; however it is difficult to eliminate every food safety risk in the field [1]. Therefore, the steps taken by both producers and processors are likely the only protection the consumer has from consuming foodborne pathogens such as L. monocytogenes, E. coli 0157:H7 and Salmonella Typhimurium DT104 [2]. When produce is processed, it is typically handled in a recirculated wash water system.
Therefore, the water within the dump tank becomes high in organic matter and is a potential point of cross contamination for bacterial pathogens. It is vital to employ an effective antimicrobial within the wash water of the dump tank to control and reduce the likelihood of bacterial pathogen contamination on produce [3].
The two types of fresh produce that this research will be focusing on are bell pepper, and cantaloupe. These produce types are at risk of being contaminated because they are typically consumed raw [4].
Being consumed raw means that pathogens which may be present are not likely to be killed prior to their consumption. The cantaloupe specifically has an additional risk because its rough surface allows the bacteria to adhere itself to the surface and does not come off with a simple tap water rinse [5].
One of the most prominent foodborne outbreaks related to fresh produce to date in the USA was the Jensen Farms outbreak in which cantaloupe contaminated with L. monocytogenes was determined to be the cause [6]. According to the CDC, this outbreak was responsible for 147 illnesses, 143 hospitalizations, 33 deaths and one miscarriage. An appropriate, efficient antimicrobial wash step could potentially have prevented this outbreak as well as the consequences.
There are multiple chemical antimicrobial washes in place to minimize contamination, the most common being the use of chlorinated water (50-200 ppm) [7]. However, this step is not always effective as chlorine has limited effectiveness when it is applied to fresh produce for a number of reasons, such as sensitivity to organic load and temperature [1,8]. Additionally, there is a concern with the use of chlorine as it can cause adverse health effects [9]. Chlorine has the potential to produce carcinogenic halogenated by-products and chlorates resulting from breakdown in storage and disinfection reactions that form chlorinated organic compounds. Other antimicrobials such as peroxyacetic acid has been and is currently used in the produce industry, however, it has not been proven to provide a desirable protection against foodborne illnesses associated with the consumption of raw produce [10]. In addition, there have been a rising number of consumer demands for natural antimicrobials, and so alternatives to chlorine and other commonly used sanitizers have been, and continue to be investigated.
In recent years, both the antimicrobial and ant browning properties of SAS have been explored [11], but there is still research needed to look into the efficacy of SAS on various types of produce [12], found that 3% SAS treatment was able to achieve a lower APC as well as more effectively inhibit browning of fresh-cut potatoes over a 14 day period when compared to the results achieved by citric acid in the same study. SAS is an affordable natural food acid which was listed as a Safer Choice Antimicrobial by the Environmental Protection Agency and it gained Generally Recognized as Safe (GRAS) status in 1998. Although the use of SAS as an antibrowning and microbial reduction agent has been explored briefly, further research into its efficacy is required. Understanding the effects, benefits and limitations of this antimicrobial is vital to the future implementation as a produce wash water sanitizer. An effective antimicrobial wash solution that prevents cross-contamination is the key in reducing the number of foodborne outbreaks caused by fresh produce every year [13]. The aim of this research is to explore the use of SAS as an antimicrobial wash step for use during produce processing.
Materials and Methods
Preparation of inoculum
For this study, four strains of L. monocytogenes, five strains of E. coli O157:H7 and five strains of S. Typhimurium DT 104 were used for a total of 14 strains. The four strains of monocytogenes used were monocytogenes Scott A-2, V7-2, PMM39-2 and PMM383- 2.Scott A and V7 are well-known strains, PMM383 was isolated from raw meat products and PMM39 was isolated from RTE meat products. These strains have been adapted to streptomycin (100µg/ ml) for ease of isolation. The E. coli strains used included 1 (Beef isolate), 5 (human isolate), 932 (human isolate), E009 (Beef isolate) and E0122 (cattle isolate); and five strains of S. Typhimurium DT104 used were H2662 (cattle isolate), 11942A (cattle isolate), 13068A (cattle isolate), 152N17-1 (dairy isolate) and H3279 (human isolate). For ease of isolation the E. coli strains were adapted to 50mg/L nalidixic acid and S. Typhimurium strains were adapted to 32 mg/l ampicillin, 16 mg/l tetracycline, and 64 mg/l streptomycin. These strains were individually grown in tryptic soy broth (TSB; Difco, Becton Dickinson, Sparks, MD) at 37°C for S. Typhimurium DT 104 and E. coli O157:H7 and 30°C for L. Monocytogenes. After the strains grew overnight, they were washed by centrifugation (3,000 × g for 15 min), then the excess TSB was disposed of and the pellets were suspended in phosphate buffered saline. A five strain Cocktail was made for both E. coli and S. Typhimurium DT 104 and a 4 strain cocktail was prepared for L. monocytogenes by combining 2ml of each strain. Dilutions were made from the cocktail to reach final concentrations of approximately 9 logs CFU/ml or 6 logs CFU/ml for all target pathogens.
Antimicrobial treatment solution preparation
The antimicrobial efficacy of SAS (Jones-Hamilton Co. Walbridge, OH), Peroxyacetic Acid (PAA) (Jet Harvest Solutions), sodium hypochlorite (C), and deionized water (DI) wash solutions were evaluated to reduce S. Typhimurium DT 104, E. coli O157:H7 and L.monocytogenes from cantaloupes and bell peppers. The treatment solutions were prepared as followed.
SAS: Appropriate SAS samples were weighed and dissolved in deionized water to provide 1,2 and 3% solutions. For each experiment, a fresh solution was prepared and used on the same day.
PAA: PAA was prepared as per the manufacturer’s instruction (Jet Harvest Solutions, Jet-Oxide 15). Briefly3.75 ml of PAA concentrated solution was mixed with 7.57L deionized water to produce a wash solution containing 85 PPM PAA solution.
Sodium Hypochlorite: A 200 PPM sodium hypochlorite solution was prepared from 12.5% sodium hypochlorite solution (Hydrite chemical co., MD) by mixing the appropriate amount of chemical with deionized water. The final concentration of chlorine concentration was confirmed with chlorine test strip (Catalog#2745050, Hach, CO).
Deionized water: DI water was collected from Robert. M. Kerr Food & Ag Products Center, Oklahoma State University, DI distribution system. Fresh DI water was collected before each experiment.
Inoculation procedures
Samples of bell pepper, and cantaloupe were obtained from a local farmer’s market or retailers (Stillwater, OK) and immediately brought back to the lab and stored in the refrigerator at approximately 4 ± 2°C. All samples were used within 48h of refrigeration. All bell pepper and cantaloupe were of a uniform size and weight to ensure consistent samples. Before inoculation, all produce was washed for three minutes with tap water [4].
Three 2.5 cm2 squares were marked using a permanent marker on each sample of produce [14]. The bell pepper and cantaloupe was spot inoculated with 20µl of appropriate inoculum within the marked squares [14]. The samples were then allowed one hour for drying within a laminar airflow hood to allow the pathogens to attach [15]. Then the produce was refrigerated overnight.
Antimicrobial treatment
After the one hour of allotted drying time and overnight refrigeration, the bell pepper and cantaloupe were washed in 6L of respective treatment solution for one minute. In order to mimic industry washing practices, the produce was agitated within the wash water. Five-gallon food grade buckets were used to wash the produce. After the one-minute washing period, the samples were immediately removed from the antimicrobial treatment and the squares were excised immediately using a sterile scalpel. All three squares were then placed in 27 ml of 10% sodium metabiosulfate (Sigma-aldrich, MO) neutralizing solution in filter bag (WhirlPak 24oz; Serial#851). Samples were stomached at the normal setting for two minutes (AES Laboratoire EasyMIX™). All treatment results were compared with DI treatment control.
Microbial enumeration
One ml from the stomached sample was taken and appropriate serial dilutions were made. Then, 0.1 ml samples were plated on sorbitol MacConkey agar (SMA; Oxoid, Basingstoke, UK) with 50 mg/L nalidixic acid added for E. coli 0157:H7, xylose lysine deoxycholate agar (XLD; Becton Dickinson, Sparks, MD) supplemented with 32 mg/L ampicillin, 16 mg/L tetracycline, and 64 mg/L streptomycin for S.Typhimurium DT104 or Tryptic Soy Agar (TSB; Difco, Becton Dickinson, Sparks, MD) supplemented with 100µg/ml streptomycin for L. monocytogenes.
Finished plates were stored in an incubator at 37°C or for E. coli 0157:H7 and S. Typhimurium DT104 and 30°C for L. monocytogenes for up to 48h prior to counting. Plates were observed for typical E. coli 0157:H7 which are colorless, S. Typhimurium DT104 which produce black colonies and colorless opaque colonies for L. monocytogenes. Recovered bacteria were confirmed using biochemical (API, Biomerieux, NC) and serological testing.
Statistical analysis
All of the results presented are the result of three independent repeated experimental trials. The statistical analysis was performed using JMP PRO 13 (SAS Institute, Inc., Cary, NC). The Tukey- Kramer test at the probability level of P ≤ 0.05 was used for the pairwise comparisons of means.
Results and Discussion
Recovery of S. Typhimurium and E. coli 0157:H7 from cantaloupe and bell pepper inoculated with high levels of pathogens
Cantaloupe and bell pepper were washed with antimicrobial solutions to evaluate the effectiveness of each antimicrobial. Spot inoculation was chosen as a way of mimicking a single contamination point, as would likely occur with contaminated soil, water, feces, human contact, or other potential sources of contamination. A DI water wash was used as a control to identify the wash off effect of water. After the DI treatment, recoveries of S. Typhimurium and E.coli were observed to be 4.68 and 4.62 log CFU/in2 respectively.
After antimicrobial treatment, bacterial recovery of S. Typhimurium DT 104 from cantaloupes was observed to be 4.25, 4.45, 4.49, 4.21, and 3.74 for C, PAA, 1% SAS, 2% SAS, and 3% SAS log CFU/ in , respectively. The recoveries of the target pathogen were not significantly different (P ≤ 0.05) for all antimicrobial treatments, except for 3% SAS (Figure 1). In the case of bell pepper, a similar trend of bacterial recoveries was observed (Figure 2). The recovery of S. Typhimurium DT 104 was observed to be 01, 3.40, 3.01, 2.91 and <1.4 log CFU/in2 respectively for C, PAA, 1% SAS, 2% SAS, and 3% SAS. The highest concentration of SAS solution was able to reduce S. Typhimurium DT 104 to non-detectable levels by direct plating but, after enrichment, samples were found positive for S. Typhimurium DT 104. To the best of our knowledge this is the first study which employed SAS as a produce wash treatment therefore; direct comparison of the data with previous work is not possible. A study [12] found that the reduction of Salmonella on cantaloupe achieved by 200ppm total chlorine was 0.7 log10 CFU/in in comparison to a DI water wash after a 60s soaking time, which is slightly more compared to our study (0.31 log log10 CFU/ in ) with chlorine. This difference in recoveries could be explained by the difference in inoculation methods. For our study, the produce was dried overnight whereas in the study conducted by Parnell et al., 2005, the produce was only dried for approximately one hour. Our longer incubation time could allow time for more pathogens to adhere to the surface of the produce. Even with a longer incubation period, 3% SAS solution was found to be more effective (0.94 log10 CFU/ in2 reduction) in comparison to chlorine treatment.
The recoveries of E. coli 0157:H7 from cantaloupe are presented in Figure 1. It was observed that 3% SAS treatment was the most effective in reducing E. coli O157:H7 but, all other wash treatments including deionized water treatments reduced targeted pathogen at a similar rate (P ≤ 0.05). Deionized water treatment was the least effective treatment to reduce E. coli O157:H7 from bell peppers (Figure 3) and 3% SAS was again found to be most effective (P ≤ 0.05). A previous study conducted by [16], found that E. coli 0157:H7 was reduced by 1.5 log CFU/cm2 from cantaloupe compared to cantaloupe, which did not undergo any treatment. This increase in reduction compared to our results can be explained by the no- treatment control compared to our DI water wash. An approximate 1 log CFU/g is typically expected when a water wash is employed, as water is thought to rinse off debris and other contaminants [17].
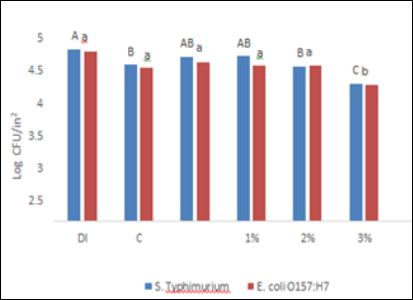
Figure 1: Efficacy of SAS and other antimicrobials to reduce E. coli O157: H7 and Salmonella inoculated at high levels. Typhimurium DT 104 from cantaloupe. DI: De- ionized water, C: Chlorine, PAA: peracetic acid, 1%: 1% SAS solution in water, 2%: 2% SAS solution in water and 3%: 3% SAS solution in water. A-C, means bearing with no common letter are significantly different (P ≤ 0.05) a-c, means bearing with no com- mon letter are significantly different (P ≤ 0.05).
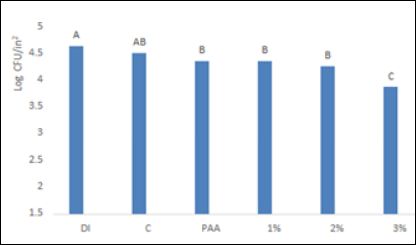
Figure 2: Efficacy of SAS and other antimicrobials to reduce L. monocytogenes from cantaloupe inoculated at high levels. DI: Deionized water, C: Chlorine, PAA: peracetic acid, 1%: 1% SAS solution in water, 2%: 2% SAS solution in water and 3%: 3% SAS solution in water. A-C, means bearing with no common letter are significantly different (P ≤ 0.05)
It was observed that washing treatments were more effective in reducing pathogens from bell pepper than cantaloupe. The increase in reduction on bell pepper compared to cantaloupe can be explained by the difference in surface structure properties. The smooth surface of bell peppers allows for more effective produce decontamination, consistent with results found by researchers conducting similar studies [16,18,19].
Recovery of L. monocytogenes from cantaloupe and bell pepper inoculated with high levels of pathogens
The DI water wash for L. monocytogenes yielded bacterial recoveries of 4.45 and 4.31 log CFU/ in2 for cantaloupe and bell pepper respectively.
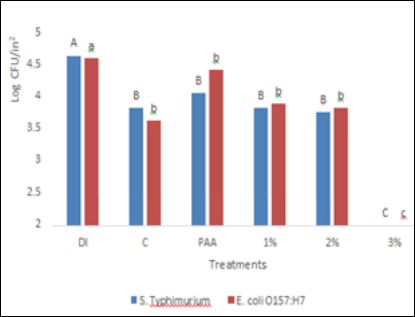
Figure 3: Efficacy of SAS and other antimicrobials to reduce E. coli O157: H7 and Salmonella inoculated at high levels. Typhimurium DT 104 from bell pepper inoculat- ed at high levels. DI: Deionized water, C: Chlorine, PAA: peracetic acid, 1%: 1% SAS solution in water, 2%: 2% SAS solution in water and 3%: 3% SAS solution in water A-C, means bearing with no common letter are significantly different (P ≤ 0.05) a-c, means bearing with no common letter are significantly different (P ≤ 0.05).
The recovery of L. monocytogenes from cantaloupe for C, PAA, 1% SAS, 2% SAS and 3% SAS was 4.27, 4.05, 4.05, 3.92,and 3.37 log CFU/in2 respectively (Figure 2). A similar trend of bacterial recoveries was observed with bell pepper. Bell pepper, after treatment yielded a recovery of 3.81, 3.60, 3.64, 3.54 and 3.12 log CFU/in2 for C, PAA, 1% SAS, 2% SAS and 3% SAS respectively (Figure 4). DI water treatment and chlorine treatments were similarly effective in reducing L. monocytogenes from both produce items. Our results are in agreement with a previous study by [20], where they found that 200 ppm chlorine treatment did not significantly reduce L. monocytogenes from cantaloupe in comparison to a water wash. The lack of effectiveness of chlorine was attributed to the presence of organic matter present in the wash solution. Our study identified that 3% SAS solution was significantly more effective in reducing L. monocytogenes from cantaloupe and bell pepper in comparison to all other treatments. In a study by [21], the authors successfully utilized different combinations of SAS and PAA in reducing Listeria innocua from apples. A 3% SAS solution in combination with 60 ppm PAA provided a 2.57 log CFU/g reduction of L. innocua from apple surfaces. In comparison to the study by [21], we had a modest reduction of targeted pathogens which could be the function of differences in bacterial type, surface of produce, or the combination of PAA with SAS [22,23].
Recovery of S. Typhimurium DT 104, E. coli 0157:H7 and L. monocytogenes from cantaloupe and bell pepper inoculated with low levels of pathogens
Samples from the low inoculum level did not yield countable colonies for all treatments (data not shown). However, the results of enrichment were positive for the targeted pathogenic organisms. This could be an indication that the amount of bacteria was reduced below detectable limits (1.4 log CFU/g), or that the cells were damaged and would require a longer time to recover [24].
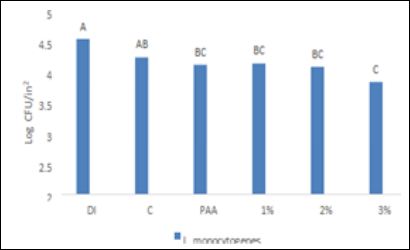
Figure 4: Efficacy of SAS and other antimicrobials to reduce L. monocytogenes from bell pepper inoculated at high levels. DI: Deionized water, C: Chlorine, PAA: peracetic acid, 1%: 1% SAS solution in water, 2%: 2% SAS solution in water and 3%: 3% SAS solution in water. A-C, means bearing with no common letter are significantly differ- ent (P ≤ 0.05)
.Conclusion
The study’s findings suggest that SAS antimicrobial treatment could be an effective antimicrobial intervention for the produce industry. But, the impact of SAS treatment on the quality of treated produce should be investigated before use.
Acknowledgements
The authors appreciate Jones-Hamilton Co. for providing antimicrobial agents and partial financial support for this research. Partial financial support for this research was provided by the Virgil & Marge Jurgensmeyer Endowed Professorship.
References
- https://producesafetyalliance.cornell.edu/curriculum/download-V1-2/
- Chen X, Hung YC (2017) Effects of organic load, sanitizer pH and initial chlorine concentration of chlorine-based sanitizers on chlorine demand of fresh produce wash waters. Food Control 77: 96-101.
- Starobin A, Foong-Cunningham S (2017) Fruit and Vegetable Washing in Food Retail Environments. Food Protection Trends 37: 70-73.
- Amrutha B, Sundar K, Shetty PH (2017) Spice oil nanoemulsions: Potential natural inhibitors against pathogenic coli and Salmonella spp. from fresh fruits and vegetables. LWT-Food Science and Technology 79: 152-159.
- Guzel M, Moreira RG, Omac B, Castell-Perez ME (2017) Quantifying the effectiveness of washing treatments on the microbial quality of fresh-cut romaine lettuce and cantaloupe. LWT 86: 270-276.
- Cosgrove S, Cronquist A, Wright G, Ghosh T, Vogt R, et al. (2011) Multistate Outbreak of Listeriosis Associated With Jensen Farms Cantaloupe-United States, August-September 2011. JAMA 11: 2768-2769.
- Weissinger WR, Chantarapanont W, Beuchat LR (2000) Survival and growth of Salmonella baildon in shredded lettuce and diced tomatoes, and effectiveness of chlorinated water as a sanitizer. International Journal of Food Microbiology 62: 123-131.
- Goodburn C, Wallace CA (2012) The microbiological efficacy of decontamination methodologies for fresh produce: A Food Control 32: 418-427.
- Oliveira M, Abadias M, Colás-Medà P, Usall J, Viñas I (2015) Biopreservative methods to control the growth of foodborne pathogens on fresh-cut lettuce. International Journal of Food Microbiology 214: 4-11.
- Rodgers S, Cash J, Siddiq M, Ryser E (2004) A comparison of different chemical sanitizers for inactivating Escherichia coli O157: H7 and Listeria monocytogenes in solution and on apples, lettuce, strawberries, and cantaloupe. Journal of Food Protection 67: 721-731.
- Fan X, Sokorai KJB, Liao CH, Cooke P, Zhang HQ (2009) Antibrowning and Antimicrobial Properties of Sodium Acid Sulfate in Apple Slices. Journal of Food Science 74: M485-M492.
- Calder BL, Kash EA, Davis-Dentici K, Bushway AA (2011) Comparison of sodium acid sulfate to citric acid to inhibit browning of fresh-cut Journal of food science 76: S164.
- Gil M, Gómez-López V, Hung YC, Allende A (2015) Potential of Electrolyzed Water as an Alternative Disinfectant Agent in the Fresh-Cut An International Journal 8: 1336-1348.
- Parnell TL, Harris LJ, Suslow TV (2005) Reducing Salmonella on cantaloupes and honeydew melons using wash practices applicable to postharvest handling, foodservice, and consumer preparation. International Journal of Food Microbiology 99: 59-70.
- Beuchat L, Nail B, Adler B, Clavero L (1998) Efficacy of spray application of chlorinated water in killing pathogenic bacteria on raw apples, tomatoes, and lettuce. Journal of Food Protection 61: 1305-1311.
- Alvarado-Casillas S, Ibarra-Sánchez S, Rodríguez-García O, Martínez- Gonzáles N, Castillo A (2007) Comparison of rinsing and sanitizing procedures for reducing bacterial pathogens on fresh cantaloupes and bell Journal of food protection 70: 655.
- https://apps.who.int/iris/handle/10665/64435
- Annous BA, Burke A, Sites JE (2004) Surface pasteurization of whole fresh cantaloupes inoculated with Salmonella Poona or Escherichia Journal of Food Protecction 67: 1876-1885.
- Ukuku DO, Fett WF (2002) Relationship of cell surface charge and hydrophobicity to strength of attachment of bacteria to cantaloupe rind. Journal of Food Protection 65: 1093-1099.
- Upadhyay A, Chen CH, Yin H, Upadhyaya I, Fancher S, et al. (2016) Inactivation of Listeria monocytogenes, Salmonella and Escherichia coli O157:H7 on cantaloupes by octenidine dihydrochloride. Food Microbiology 58: 121-127.
- Kim SA, Park SH, Knueven C, Basel R, Ricke SC (2018) A decontamination approach using a combination of bisulfate of soda and peracetic acid against Listeria innocua inoculated on whole apples. Food Control 84: 106-110.
- De J, Li Y, Sreedharan A, Schneider GR, Gutierrez A, et (2018) A three- year survey of Florida packinghouses to determine microbial loads on pre- and post-processed tomatoes. Food Control 86: 383-388.
- Muriana P, Quimby W, Davidson A, Grooms J (2002) Postpackage Pasteurization of Ready-to-Eat Deli Meats by Submersion Heating for Reduction of Listeria monocytogenes Journal of Food Protection 65: 963-969.
- Zhang S, Farber JM (1996) The effects of various disinfectants against Listeria monocytogenes on fresh-cut vegetables. Food Microbiology 13: 311-321.
Citation: McDaniel C, Jadeja R (2021) Evaluating the Efficacy of Sodium Acid Sulfate to Reduce Escherichia Coli O157:H7, Salmonella Typhimurium and Listeria Monocytogenes from Cantaloupe and Bell Pepper. J Nutr Food Sci 4: 034.
Copyright: © 2021 McDaniel C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.


