*Corresponding Author:
María del Carmen Beltrán-Orozco,
Laboratorio de Nutrición Maestría, Departamento de Ingeniería Bioquímica, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, México
Tel: +52 1 (56)11908888
E-mail: mariaencb@gmail.com
Abstract
It was studied the enzymatic activity of the crude extract obtained from the pulp of each of the varieties of pitayas included in the present investigation. The enzymatic extracts of catalase (CAT), peroxidase (POD), polyphenoloxidase (PPO), glucose oxidase (GOx), and protease (PRO), from the pulp of, red, purple, orange, and yellow varieties of pitaya Stenocereus griseous H., were obtained using 5% NaCl extracting solution. All the pitaya pulps tested showed enzymatic activity of the five enzymes proved. The orange variety had the maximum activity of CAT. The POD activity was higher in the red variety. The purple variety presented high activity in all the enzymes tested but the enzyme which had the highest activity in this variety of fruit was PPO. The highest activity of the GOx was founded in the purple variety of pitaya all the varieties tested showed a considerable proteolytic activity (PRO). The presence of enzymes such as PRO, CAT, POD, and GOx place these fruits as important functional foods given the protection offered by these enzymes as antioxidants and/or antimicrobial agents. Proteolytic enzymes help digestive problems, as well as can act as anti-inflammatory compounds. On the other hand, these fruits can be a source for the extraction of enzymes for industrial uses.
Keywords
Catalase; Enzymatic activity; Glucose oxidase; Peroxidase; Polyphenoloxidase; Protease; Pulp of pitaya fruit; Stenocereus griseus H
Introduction
The Stenocereus griseus species complex (CESG) [1] has historically been a complicated group of taxonomic understanding [2-4], includes 6 recognized species: S. griseus, S. heptagonus, S. huastecorum, S. laevigathus, S. stellatus, and S. pruinosos. These species are geographically distributed in certain regions ranging from central Mexico to Central America [5,6]. These delicious and juicy exotic fruits tend to grow in soils that are limited to other species [7]. Stenocereus griseus H., known as “May Pitaya” for the time of its harvest and marketing [8], presents characteristics, like the rest of the fruits of the Cactaceae, which do not occur in any other order of angiosperms such as the presence of betaines (Esquivel, 2004), given the structural diversity of betacyanin and betaxanthins, the genus Cactaceae represent a promising source of natural dyes [6,8].
The oxidative enzymes present in these fruits are related to their deterioration, as catalysts for browning reactions, as well as to their antioxidant properties in their role as a functional food [9].
The production of Reactive Oxygen Species (ROS) is a normal process, it can be increased not only during the maturation and senescence of the tissues, but also by stressors such as heavy metals, radiation, modification of the atmosphere, extreme temperatures, fiscal damage, and by the attack of microorganisms.
CAT and POD protect cells from the toxic effect produced by H2O2 , which is released during the redox reactions of metabolism. CAT degrades H2O2 in water and oxygen. POD catalyzes the oxidation of certain proton donor compounds, such as phenols (guaiac, pyrogallol) and aromatic amines (o-phenylenediamine) when they react with peroxides H2O2 [10,11].
In addition to its role within the antioxidant system of tissues POD has been related to PPO as a cause of enzymatic browning of fruits and vegetables. PPO catalyzes two different reactions in the presence of O2: the o-hydroxylation of phenolic substrates to o-diphenols, and the oxidation of o-diphenols to quinones. These quinones can spontaneously polymerize through non-enzymatic pathways generating brown pigments known as melanins [12].
GOx (β-D-glucose: oxygen oxidoreductase) is a flavoprotein that catalyzes the oxidation of β-D-glucose in gluconic acid using oxygen as an acceptor of electrons with the consequent formation of H2O2. It is also used as a food preservative to help remove oxygen and glucose from food when packaged such as dry egg powder to prevent unwanted browning and undesired taste [13]. GOx is also found in honey, GOx on the surface of honey reduces atmospheric oxygen to hydrogen peroxide, which acts as an antimicrobial barrier. In the manufacturing industry, GOx is used as an additive due to its oxidizing effects. In a bakery it is used to obtain firmer doughs, replacing oxidants such as bromate. Enzyme electrode biosensors detect glucose levels by keeping track of the electrons that pass through the enzyme. The enzyme is deposited on an electrode and what is measured is the electrical potential generated by the reaction. This has made possible the manufacture of microsensors that are true nanotechnological feats and that are used, for example, in-home sensors that measure the level of blood glucose used by many diabetics [14].
The proteases present in the fruits help the digestion process of food. In addition, they are the most widely used enzymatic groups in the food industry, for example, in cheese making, brewing beer, digestive drugs, as anti-inflammatories. In the chemical industry in the production of detergents.
The aim of this research was identifying the enzymatic activity of protease, polyphenoloxidase, catalase, peroxidase, and glucose oxidase in the pulp of four varieties of red, purple, orange, and yellow pitaya pulp.
Materials and Methods
Raw Materials
Four varieties of May pitaya (S. griseus H.) were studied: red, purple, orange, and yellow, (Figure 1) which were acquired, during May, at the “Central de Abates” in Mexico City, and kept frozen (at -20°C) until use.

Figure 1: May pitaya fruit (Stenocereus griseus H).
Methods
Enzyme extraction
The pulp of the fruits subject to the study was macerated with a 5% NaCl extracting solution at a ratio of 1:1, until it had a homogeneous mixture, which was left at rest for 48 h in refrigeration (3°C), after that time it was filtered and kept frozen until use. Each of the extracts obtained was identified its enzymatic activity, the tests were carried out six times.
The activity of the oxidative enzyme was expressed in Activity Units (AU), where one activity unit is equal to the quantity of the enzyme that decomposes 1 μg of substrate per min under specific conditions.
CAT activity [7,8]
The disappearance of peroxide by the action of CAT is followed spectrophotometrically (Jenway UV-vis spectrophotometer model 7305) at A240, at 25°C. They were pipetted into each cuvette 2.0 mL of phosphate regulator at pH 7.0, 3 mL of the substrate (H202 0.059 M, in phosphate regulator), and 0.3 mL of enzyme extract, and the decrease in A240 absorbance was recorded every min for 5 min.
PPO activity [9,10]
It is evaluated by the rate of the catechol oxidating: 0.2 mL of the enzymatic extract was placed in a cuvette, 2.4 mL of phosphate buffer (10 mM, pH 6.5), and 0.4 mL of 0.5 M catechol was added. The increase in absorbance was measured against a blank at A460 with intervals of 30 s at a temperature of 2°C.
POD activity [11]
The measurement of POD activity is based on the rate of breakdown of H O by the enzyme peroxidase, in the presence of o-dianisidine as a proton donor. The speed of color development is measured. At 6 mL of the substrate were added 0.05 mL of the dye (o-dianisidine), 2.9 mL of the mixture was transferred to each of the two cuvettes of the spectrophotometer. In the first cuvette (blank) were added 0.1 mL of phosphate regulator, in the second (problem) were added 0.1 mL of the enzyme extract, it was stirred and recorded the increase in A460 every minute for a period of 10 min.
GOx activity [12]
This procedure, taking advantage of the specificity of glucose oxidase, is based upon the conversion of glucose to gluconic acid and hydrogen peroxide by glucose oxidase and the subsequent oxidation of o-dianisidine to its oxidized form, measurable at A420, by hydrogen peroxide with peroxidase. A sample solution (0.5 mL), containing 0-50 μg of glucose, was mixed with glucose oxidase reagent (3.0 mL) in a test tube and incubated in a water bath at 37 °C for 60 min. The glucose oxidase reagent was prepared by creating a mixture of glucose oxidase (125,000 units), horse-radish peroxidase (0.5 mg), and 1% o-dianisidine in 95% ethanol (0.5 mL) to 100 mL with 0.5 M sodium phosphate buffer, pH 7.0, and, if necessary, filtering the solution. After incubation, the A420 was measured against a blank. The sample content is then calculated from a standard curve.
PRO activity [13,14]
The Kunitz method modified by [15], was used. It is based on quantifying the production of peptides and amino acids released during the enzymatic hydrolysis of a protein leveraging the ability of reaction products to selectively A280. A tyrosine-type curve vs. A280 was developed.
The substrate was prepared by suspending 2 g of Hammarstein type casein in phosphate regulator at pH 7.6, 0.05 M. The substrate was denatured by heating into a water bath for 20 min, then cooled and calibrated to 190 mL with the regulator. It was pipetted 1.9 mL of the substrate into a test tube and 0.1 mL of the enzyme extract, the mixture was allowed to react in a bath at 35°C for 24 h, the reaction was stopped by adding 5% Trichloroacetic Acid (TCA), was filtered, and A280 was recorded. The absorbance of the extracts was interpolated in the type curve of tyrosine (y=0.0019x + 0.0093, R²=0.9959, where y=A280, and x=µmol tyrosine). The results were expressed as µg tirosyne released/100 g of pulp/min.
Results and Discussion
In the pulp of the four varieties of pitaya investigated, namely: red, purple, orange and yellow, oxidative enzymatic activity was found, as well as proteolytic enzymatic activity, although in different proportions.
CAT activity
The orange pitaya presented a very high CAT activity, this would indicate that this variety is unstable towards environmental oxygen; therefore CAT is present to protect the antioxidants in the pulp. This agrees with the important contents of phenolic compounds, as well as ascorbic acid, found in four varieties of the pitaya fruit Stenocereus stellatus Riccobono, which belongs to the same genus as the fruit object of the present investigation [11].The other varieties showed a lower activity of CAT enzyme, as can be seen in Figure 2.

Figure 2: CAT activity (AU CAT/100 g of pulp/min) present at red, purple, orange, and yellow varieties of pitaya fruit.
CAT is one of the most efficient known enzymes, so much so that it cannot be saturated by H2O2 at any concentration, catalyzing its conversion into H2O and O2, to protect the cells from the H2O2 that is generated inside. With H donors (methanol, ethanol, formic acid, phenols ...) it shows peroxidase activity.
Therefore, H2O2 is enzymatically catabolized in aerobic organisms by catalase and other peroxidases. CAT has an important role in the acquisition of tolerance to oxidative stress in the adaptive response of cells. It captures H2O2 before it can escape the cell and converts it to molecular oxygen [15].
The knowledge of the enzymatic activity in the fruits is of great relevance, since it allows to design methods of conservation of the same, as well as to know their role as functional foods. For instance: Wang, et al., 2005, have studied the effect of different controlled atmospheres on the activities of lipoxygenase (LOX), peroxidase (POD), superoxide dismutase (SOD) and catalase (CAT), as well as malondialdehyde (MDA) content and membrane integrity of the Peach fruits (Amygdalus persica cv. Okubao) during storage periods of post-storage ripening at 20°C. The results indicated that the decrease of SOD and CAT might contribute to the development of chilling injury in peach fruits due to the protective effect of these enzymes as antioxidant agents that protect cells from the spread of ROS.
Evidence is presented that the high levels of two enzymes implicated in antioxidative defense, superoxide dismutase (SOD) and catalase (CAT), are involved in delaying the senescence process in nonnetted muskmelon (Cucumis melo L.) variety Clipper and this could explain, at least, to some extent, the long storage life of Clipper, longer than 14 days [16].
POD activity
All the varieties studied had POD activity, the highest activity was founded in the red variety (Figure 3). The POD enzyme serves to protect the substances that give the fruit color, and not undergo oxidation by exposing the pulp to oxygen, as well as to protect other antioxidant compounds present in the fruit, as phenolic compounds, and ascorbic acid presents in the fruits of genus Stenocereus [11].
POD is found in all plants and animals and is essential for living systems. This is in turn helps to prevent lipid peroxidation and maintain intracellular homeostasis as well as redox balance. They catalyze the abstraction of one or two electrons (usually two) via a single-electron transfer from hydrogen peroxide or an organic substrate, being used as an electron acceptor [17].
The role of PPO enzyme in the browning phenomenon in fruits and vegetables is linked also with the action of POD enzyme. It has been suggested that PPO works as a promoter for POD activity because hydrogen peroxide which is a product of PPO reaction with phenolic compounds is essential for POD action [18]. POD enzymes catalyze oxidation of phenolic compounds in the presence of hydrogen peroxide to form brown compounds. Besides changing the color, action of PPO and POD enzymes has significant impact on the flavor and aroma of horticultural products, since phenolic compounds play a role in giving bitter, sweet, pungent, or astringent tastes in fruits, vegetables, and spices [19,20].

Figure 3: POD activity (AU POD/100 g of pulp/min) present at red, purple, orange, and yellow varieties of pitaya fruit.
Peroxidases and their mimetic systems have several technological and biomedical applications such as environment protection, energy production, bioremediation, sensors and immunoassays design, and drug delivery devices [21].
PPO activity
All the varieties studied had important PPO activity, the differences found between the activities of the said enzyme, in the varieties of the pulps studied, were not as great, as in the case of CAT (Figure 4).
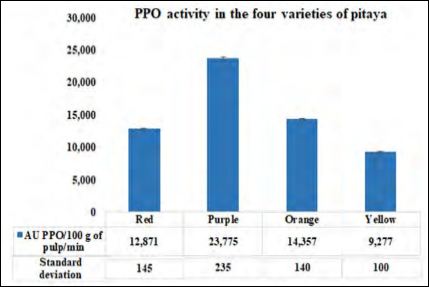
Figure 4: PPO activity (AU PPO/100 g of pulp/min) present at red, purple, orange, and yellow varieties of pitaya fruit.
Regarding PPO and GOx, the purple pitaya is the one with the highest activity and therefore the one with the highest amount of phenols and therefore protects them from oxidation, which is beneficial for humans (Figures 4 and 5). The oxidases present in the different pitayas are beneficial for the fruit and the consumer by keeping our body protected due from the spread of ROS. Vegetables and fruits antioxidants work as singlet and triplet oxygen quenchers, free radical scavengers, peroxide decomposers, and enzyme inhibitors [22]. Many of their protective biological effects are derived from their antioxidant’s functions [23].
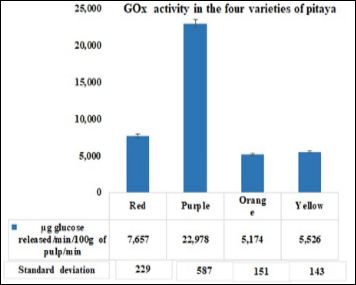
Figure 5: GOx activity (µg glucose released/100 g of pulp/min) present at red, purple, orange, and yellow varieties of pitaya fruit.
PPO is a class of naturally occurring enzymes that occur commonly in plants and animals. Their role in natural systems is typically as a form of resistance to microbial infection, and their effect on our food is more of a secondary one. The polyphenol structures often act as antioxidants in plants, and often are responsible for their pigmentation. In the presence of oxygen and PPO the aromatic rings of the polyphenols become progressively oxidized, resulting in melanin, as well as other flavor compounds. Melanin is a product that gives this reaction its characteristic brown color. Enzymatic browning is often detrimental to a large portion of fruits, vegetables, and seafood. But this process is also known to produce a variety of important components that give tea its distinctive flavor and aroma. In some darker teas, such as black tea, the leaves are crushed up upon picking, thereby increasing the rate of oxidation and the rate of color/ flavor formation [24].
As it was said, PPO and POD are the main enzymes responsible for quality loss due to phenolic degradation. The different factors affecting phenolic‐related food quality include internal (genetic) and environmental (agronomic) factors, technological treatments applied during postharvest storage of fruits and vegetables, as well as processing and storage of the processed products. The different strategies that are required to either maintain or enhance the phenolic‐ related quality of foods are critically reviewed. Genetic modification designed to decrease polyphenol oxidases or peroxidases is not always a feasible method, owing to side problems related to the growth and defense of the plant [23].
GOx activity
The highest activity of the enzyme GOx which plays an important role as oxidoreductase was found in the pulp of the purple variety, followed by that of the red, yellow, and orange varieties (Figure 5).
Plants are the basis of all traditional medicinal therapy [25] and in 1992 the positive effect of antioxidants was found in fruit and vegetables [26-29]. Free radicals are the leading cause of degenerative diseases such as several forms of cancer, cardiovascular disease, and neurological diseases [30], hence the importance of consuming these foods to help prevent contracting degenerative diseases.
GOx is an oxidoreductase that catalyzes the oxidation of β-D- glucopyranose to D-glucono-1, 5-lactone with the formation of H2O2. Among this group of sugars, the oxidation of the glucose is the most rapid. Therefore, it is mostly used for measuring glucose concentration in different samples [31].
The antimicrobial activity of the GOx system is based on the cytotoxicity of H2O2. The associated inhibitory effects depend on the concentration of enzyme, and on the concentration of glucose; higher inhibitory activity will be observed at higher enzyme and substrate concentrations. The antimicrobial activity of GOx, has been associated with a significant reduction in growth of various food- borne pathogens, namely Salmonella infantis, S. aureus, Clostridium perfringens, B. cereus, Campylobacter jejuni, and L. monocytogens. GOx has found several commercial applications, including removal of glucose and other fermentable sugars from egg albumin and whole eggs, prior to drying, to prevent browning during storage afterward; improvement of color and flavor, and extension of shelf life of food products; oxygen removal from fruit juices and canned beverages; and prevention of oxidative deterioration of mayonnaise and salad dressings [32].
The presence of the oxidative enzymes CAT, PPO, POD, and GOx position these fruits as an important source of these enzymes, as well as give us useful information for their preservation. These enzymes can play a double role in fruits, as oxidizing agents that cause deterioration in them, or as antioxidant agents that protect cells from the spread of ROS.
[11], partially characterized the CAT, POD, and PPO enzymes extracted from the skin of a cactus fruit: yellow pitahaya (Acanthocereus pitajaya). They found one inhibitory effect of H2O2 on the activity of POD has been and may be related to the oxidative effect of this compound on some amino acids of the active site, these results agree with those obtained by [33,34].
It has been reported that, in general, the kinetic parameters against H2O2 found for CAT and POD indicate that these enzymes have a complementary role in terms of the degradation of this compound, potentially toxic to cells [35].
Features found in POD and PFO indicate that these enzymes can actively participate in the enzymatic browning process, under various stress conditions [36].
PRO activity
Proteolytic activity was found in all the varieties of pitaya studied (Figure 6). Proteolytic activity is important because it demonstrates the presence in the fruits of digestive enzymes when said fruits are consumed help the proper functioning of the digestive system. If these enzymes are isolated, products such as cheese, protein hydrolysates, and medicines among others could be prepared.

Figure 6: PRO activity (µg tirosyne released/100 g of pulp/min) present at red, purple, orange, and yellow varieties of pitaya fruit.
The pitaya (S. griseus H.) joins the plants that contain proteases, by virtue of the important proteolytic activity found in them. The pulp of these fruits’ places them as nutraceutical foods, due to the role that these enzymes play in digestive processes, and another health benefits.
As is well known, fruits rich in proteolytic enzymes, such as papaya and pineapple are a digestive aid and a natural anti-inflammatory fruit [37]. Besides their role in digestion, they have other health benefits. Bromelain (from pineapple) supplements are particularly popular among athletes for treating all sorts of physical aches and injuries [38].
Proteases of plant origin, such as papain, chymopapain, ficin, bromelain, asclepain, mexicain, euphorbain, solanin, flicin and white gourd protease, have been reported by many workers. Recently, it has also been reported that some proteases are present in ginger and princemelon [39]. Found that green asparagus, kiwi fruit and miut have high proteolytic activities.
Proteases in addition have been used in the pharmaceutical, and food industries. Papain, bromelain or asclepain have already been used in the fields of food and medicine, as meat tenderizers, digestive and anti-inflammatory agents, and preventers of turbidity in beer, etc.
[40-49], investigated the presence of new proteases in plants: green asparagus, kiwi and miut, where they found high proteolytic activity, applicable in many fields.
Conclusions
The findings of this work show that in the pulp of the pitaya fruit (Stenocereus griseus H.) there is an important enzymatic activity, of oxidative enzymes such as CAT, PPO, POD, oxidoreductases as GOx, as well as proteolytic enzymes (PRO), consequently.
Pitayas are fruits of great nutraceutical and industrial value; they have important bioactive components, which open an important field for scientific research.
Acknowledgement
The authors thanks for the support of the Instituto Politécnico Nacional in carrying out this work through Project SIP # 20201469.
We appreciate the correct observations on the manuscript by Dr. Laura Almazán Rodrígez, professor at the Department of Food Engineering, ENCB, Instituto Politécnico Nacional.
References
- Gibson AC (1991) The systematics and evolution of subtribe Stenocereinae The species group of Stenocereus griseus. Cactus and Succulent Journal 63: 92-99.
- Alvarado-Sizzo H, Casas A, González-Rodríguez A, Arreola-Nava HJ, Terraza T (2019) Clave dicotómica y distribución del complejo de especies de Stenocereus griseus (Cactaceae). Revista Mexicana de Biodiversidad 90: e902675.
- Alvarado-Sizzo H, Casas A, Parra F, Arreola-Nava HJ, Terrazas T, et al. (2018) Species delimitation in the Stenocereus griseus (Cactaceae) species complex reveals a new species, huastecorum. PLOS ONE 13: 1-25.
- INEGI (Instituto Nacional de Estadística Geografía e Informática). (2001). Conjunto de datos vectoriales fisiográficos. Continuo Nacional escala 1:1 000 000 serie I (Subprovincias fisiográficas) - 2001 escala: 1:1 000 000. Recuperado el 01 febrero, 2016
- Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by J Biol Chem 195: 133-140.
- Worthington Enzyme Worthington, K. and Worthington, V. (2011). Worthington Biochemical Corporation. Date of access Oc- tober 20, 2020.
- Bravo-Hollis H (1978) Las Cactáceas de México. México: UNAM.
- Bravo-Hollis H Scheinvar L (1995) El Interesante Mundo de las Cactáceas: Fondo de Cultura Económica.
- Beltrán-Orozco MC, Oliva-Coba TG, Gallardo-Velázquez T, Oso- rio-Revilla G (2009) Ascorbic acid, phenolic content, and antioxidant capacity of red, cherry, yellow and white types of pitaya cactus fruit (Stenocereus stellatus Riccobono). Agrociencia 41: 53-161.
- Ayala-Camarillo K (2008) Caracterización del fruto y pulpa de la pitaya Stenocereus griseus , de su capacidad antioxidante y ex- tracción de pigmentos y compuestos fenólicos. (Tesis de Maestría). Instituto Politécnico Nacional. México.
- Whitaker JR, Dekker M (1994) Principles of enzymology for the food sciences, 2nd edition. John R. Whitaker, Marcel Dekker, Inc., 270 Madison Ave., New York, NY 10016.
- Castro Rivera JA, Baquero Duarte LE, Narváez Cuenca CE (2006) Catalasa, peroxidasa y polifenoloxidasa de pitahaya amarilla (Ac- anthocereus pitajaya). Rev Colomb Quím (Bogotá) 35: 91-100.
- Wong CM, Wong KH, Chen XD (2008) Glucose oxidase: natural occurrence, function, properties, and industrial Applied Microbiology Biotechnology 78:927-938.
- https://web.archive.org/
- Kimberly W, Wissermann W, Lee CY (1981) Characterization of polyphenoloxidase from ravat 51 and Niagara grape. J Fd Sci 46: 506-508.
- Flukery WH, Jen JJ (1978) Peroxidase and polyphenoloxidase ac- tives in peaches. J Food Sci Chicago 43: 1826-1828.
- Ortega ML, Castillo LM (1966) Actividad de la mexicaína en pres- encia de altas concentraciones de urea. Ciencia México 24: 5-6.
- Bender M, Kezdy F (1965) Mechanisms of action of proteolytic en- Annual Review of Biochemistry 34: 49-76.
- https://www.monografias.com/trabajos24/enzimas-antioxidantes/ enzimas-antioxidantes.shtml
- Lacan D, Baccou JC (1998) High levels of antioxidant enzymes correlate with delayed senescence in nonnetted muskmelon Planta 204: 377-382.
- Meunier B (2013) Bio-coordination Chemistry, in Comprehensive Coordination Chemistry II. 2nd Edition. From Biology to Editors: J. A. McCleverty T.J. Meyer. Editor in Chief: Edwin Constable. Hardcover.
- Tomás-Barberán FA, and Espin JC (2001) Phenolic compounds and related enzymes as determinants of quality in fruits and vege- Journal of the Science of Food and Agriculture 81: 853-876.
- Al-Amrani M, Al-Alawi A, Al-Marhob I (2020) Enzymatic Browning and Evaluation of Antibrowning Methods on Dates. Hindawi Inter- national Journal of Food Science 6: 1-9.
- Carmona Ribeiro A, Prieto T, Nantes IL (2015) Nanostructures for Front Mol Biosci 2:50.
- Wang SY, Lim HS (2000) Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and devel- opment stage. J Agric Food Chem 48: 140-146.
- Halliwell B (1994) Free radicals and Nutrition Rev 52: 253-265.
- Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 46: 413-417.
- https://chemistryoffood.weebly.com/
- Zheng W, Wang SY (2001) Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 49: 5165- 5170.
- Ames BM, Shigenaga MK, Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of Proc of the National Academy of Sci U S A 90: 7915-7922.
- Hertog MGL, Hollman PCH, Katan MB (1992) Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in The J Agric Food Chem 40: 2379-2383.
- Hertog MGL, Feskens EJ, Hollman PCH (1993) Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen eldery Lancet 342: 1007-1011.
- Hertog MGL, Kromhout D, Aravanis C (1995) Flavonoid intake and long-term risk of coronary-heart-disease and cancer in the 7 countries study. Arch Internal Medicine 155: 381-386.
- Tsutomu Y, Yukiko Y, Imao T, Hisashi K (1982) Proteolytic Enzymes in Green Asparagus, Kiwi Fruit and Miut: Occurrence and Partial Characterization. Agricultural and Biological Chemistry 46: 1983-1986.
- Podgornik A, Vodopivecb M, Podgornikc H, Baruta M, Štrancara Stability A (1998) Stabilization of Biocatalysts in Progress in Bio- technology, in Carbohydrate Bioengineering, Volume 10. 1st Edi- tion. Editors: S.B. Petersen, B. Svensson, and S. Pedersen.
- Ramos OS, Malcata FX (2011) Industrial Biotechnology and Commodity Products in Comprehensive Biotechnology (Second Edition). Editor in Chief: Murray Moo-Young. Hardcover ISBN: eBook ISBN: 9780080885049.
- Halpin B, Pressey R, Jen J, Mondy N (1989) Purification and characterization of peroxidase isoenzymes from green peas (Pisum sa- tivum). J Food Sci 54: 644-649.
- Rivera CAP, Restrepo P, Narváez CC. (2004) Polyphenoloxidase and peroxidase from caimarona grape pulp (Pourouma cecropiifo- lia). Rev Col Quim 33: 57-66.
- Hossain F, Akhtar S, Anwar M (2015) Nutritional Value and Medic- inal Benefits of Pineapple. International Journal of Nutrition and Food Sciences 4: 84-88.
- Yu BP (1994) Cellular defenses against damage from reactive oxy- gen species. Physiol Rev 76: 139-162.
- Stryer L (1995) Biochemistry. NH New York. Freeman and Company.
- Sánchez-Ferrer A, Rodríguez-López JN, García-Cánovas F, García-Carmona F (1995) Tyrosinase: A comprehensive review of its mechanism. Biochem Biophys Minutes 1247: 1-11.
- Henzler T, Steudle E (2000) Transport and metabolic degradation of hydrogen peroxide in chara corallina: Model calculations and measurements with the pressure probe suggest transport of h202 across water channels. Journal of Experimental Botany 51: 2053-2066.
- Arreola-Nava HJ (2006) Sistemática filogenética del género Stenocereus (Cactaceae). (Tesis doctoral). Colegio de Posgradu- México.
- Chiba T (2003) Encyclopedia of Food Sciences and In P Finglas, F Toldra, and B Caballero (Eds.), Visible Spectroscopy and Colorimetry. Glucose (glucose oxidase method) (Second, Issue 1996).
- Hansberg TW (2002) Biology of reactive oxygen Biochem- ical Message 26: 19-54.
- Hayyan M, Hashim MA, Alnashef IM (2016) Superoxide Ion: Gen- eration and Chemical Implications. Chemical Reviews 116: 3029-3085.
- Yu BP Raskin I (1992) Role of salicylic acid in Annual Review of Plant Physiology Mol Biol 43: 439-463.
- Wang Y, Tian S, XuY (2005) Effects of high oxygen concentration on pro- and anti-oxidant enzymes in peach fruits during postharvest periods. Food Chemistry 91: 99-104.
Citation: Beltrán-Orozco MC, Ayala-Camarillo KC, Cruz y Victoria MT (2020) Enzymatic Activity Present In The Pulp of Four Varieties of Pitaya Fruit (Stenocereus Griseus H.), Red, Purple, Orange, and Yellow.J Nutr Food Sci 3: 023.
Copyright: © 2020 Beltrán-Orozco MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.


