*Corresponding Author:
Israel Alfonso-Trujillo,
Dermatology Department, Clinical Surgical Hospital: Hermanos Ameijeiras, Havana, HAV 10200, Cuba
E-mail: isralfonso@infomed.sld.cu
Abstract
Introduction: The search for painless and low-invasive alternatives to correct the signs of infraorbital aging is a challenge in the field of aesthetic medicine.
Objective: To evaluate the efficacy and safety of intradermal microinjection of Autologous Platelet Concentrate (APC) in the treatment of infraorbital signs of aging.
Method: An observational, analytical and longitudinal study was carried out in 60 patients from the Hospital Clínico Quirúrgico: “Hermanos Ameijeiras”, in the period between March 1, 2017 and March 31, 2020. The treatment was applied monthly for 1 year. The final evaluation was carried out 3 months after the end of the treatment.
Results: 60 women with an average age of 45 ± 4.3 years were treated. After treatment, there were significant changes in the Glogau Photodamage Scale (P=0.019), in the Global Aesthetic Improvement Scale (P<0.021) and in the Allergan Infraorbital Gaps Scale (P=0.011). The adverse events found were pain, inflammation and ecchymosis. The degree of satisfaction reported by the patients was good (6.6%) and very good (93.4%) (P<0.0033).
Conclusions: Autologous platelet concentrate proved to be effective and safe in reducing infraorbital signs of aging, associated with a high degree of patient satisfaction.
Keywords
Autologous platelet concentrate; Infraorbital aging; Infraorbital hollows; Platelet rich plasma; Rejuvenation of the infraorbital hollows; Skin photoaging
Introduction
The aging process causes the loss of volume in the infraorbital area, causing the appearance of wrinkles and dark circles (sagging or marked furrow around the eyes associated or not with a coloration or increased pigmentation of the lower eyelids). Several methods have been used for its correction (lower eyelid blepharoplasty, chemical peel, subdermal injections of fillers, radiosurgery, laser, and intense pulsed light and autologous fat transfer), however, they can be associated with significant risks for the patient. At present, there is a great international boom in the use of less invasive aesthetic medical procedures based on the application of Platelet-Rich Plasma (PRP) and its growth factors (FC) [1,2], but few studies objectively evaluate the efficacy of the same, which motivated the realization of the present investigation.
Goals
The primary objective was: to determine the effectiveness and safety of the microinjection of Autologous Platelet Concentrate (APC) in the treatment of infraorbital aging signs and the secondary objectives were: 1) to evaluate the clinical response to treatment, 2) to evaluate type and intensity of adverse events that occur and 3) describe the degree of patient satisfaction.
Methods
An observational, analytical, longitudinal study was carried out in 60 patients at the Hospital Clínico Quirúrgico: “Hermanos Ameijeiras”, in the period between March 1, 2017 and March 31, 2020.
Treatment with CPA was applied monthly for 12 months. Three months after the end of the treatment, the response to it was evaluated (final evaluation), comparing the current state of the lesions (infraorbital subsidence, loss of infraorbital volume, wrinkles, pigmentation) with the initial state; for this, and the patient had to attend the scheduled consultation. Throughout the study there was a rigorous control of adverse reactions. Before and after the procedure, the platelets were quantified to determine the quality of the applied product (the average degree of concentration of the platelets after the procedure increased 10.8 times its initial value). Microbiological culture of the extracted plasma was performed to guarantee that a sterile germ product was administered.
Inclusion criteria
Patients between 20 and 60 years old, of any sex and skin phototype, skin photoaging grade II, III (Glogau classification) [3], grades 1 to 4 of the Allergan Infraorbital Gaps Scale [4], normal complementary tests (hemogram with differential, coagulogram, blood chemistry and serology for HIV, hepatitis B and C), with signed informed consent (Table 1).
Elimination criteria
Patients who wish to abandon the study, presence of an adverse event and / or complication that prevents continuing with the treatment or patients who have missed a treatment session.
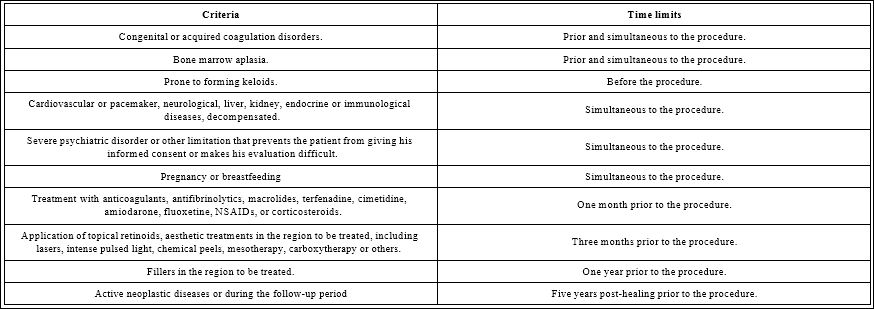
Table 1: Exclusion criteria and their relationship with the time limits to perform the procedure.
Treatment
Once the patients gave informed consent, the included subject’s registry template and the investigator’s internal registry were filled out. All information on the included patients was compiled in the data collection notebook (CRD). The blood was extracted (500 milliliters), then the CPA was obtained with the Rotixa centrifuge (221 mm radius) according to international standards [5]. To obtain the CPA, a first light centrifugation of the whole blood was carried out in the plastic bag for 3 minutes at 2800 rpm at 22 oC, with a centrifugation force of 2000 g, in this way 250 ml of red blood cells and 250 ml were obtained. of PRP; then a second weighted centrifugation was performed with PRP in the plastic bag for 5 minutes at 4500 rpm at 22 oC, with a centrifugation force of 5000 g. Once the heavy centrifugation had been carried out, the supernatant plasma was transferred through the tubes that have the plastic bags for blood collection and only 10 ml were left and it is in said volume that by shaking the platelets that were deposited in the cell were resuspended. Bottom of the bag as results of the centrifugation procedure. Subsequently, the red blood cells were returned to the patients and finally a microinjection of 10 milliliters of the CPA was performed, distributed among the infraorbital hollows, the facial area, the back of the hands, the V of the décolleté and the neck.
Variables Related to the Response to Treatment
The response to treatment was evaluated taking into account the clinical examination of the patient, using the Glogau photodamage classification scale) (Table 2) [3], the Allergan infraorbital void scale (Table 3) [3] and the Global Aesthetic Improvement Scale (GAIS) (Table 4) [6].
Adverse Events
Adverse events reported in the reviewed literature are pain, edema, and ecchymosis at the microinjection site [1,2] (Table 5) [7].
Degree of Satisfaction of Patients to Treatment
The degree of satisfaction (PSSS) of the patients with the treatment was evaluated taking into account what was reported by the patient according to the scale (Table 6) [8].
Bioethical considerations
The protocol was submitted to the consideration and approval of a Review and Ethics Committee (PRE) for Clinical Research created for this purpose, which evaluated it from an ethical point of view. Additionally, this protocol was submitted to scientific and methodological review and approval by the Institutional Scientific Council (CCI) of the Hospital Clínico Quirúrgico “Hermanos Ameijeiras”.
Statistical Methods Used
The medical records of the patients included in the study were stored in the Department’s file. With the information gathered, a Microsoft Office version XP database in Excel format was created, which was exported to the SPSS version 21.0 system for analysis. To summarize the information of the study sample, the arithmetic mean, standard deviation and minimum and maximum values were used. For all quantitative variables, the student’s t test was used. For all qualitative variables (degree of aesthetic improvement, degree of infraorbital subsidence, and degree of satisfaction), absolute numbers and percentages were calculated before and after treatment, which were compared using Pearson’s Chi-square test. In all hypothesis tests carried out, a significance level α = 0.05 was used.
Sample’s size calculation
The sample size was calculated using the C4-Study Design Pack computerized program. (C4SDP) for sample size calculation (CTM). Version 1.1 ® Glaxo Wellcome. SA [9] considering the following values: percentage of success reported in the literature 70%, percentage of success in the current study of 80%. With an alpha error of 0.05, a power of 80% and covering a loss of 5% of the patients, it was necessary to have 60 subjects in total.
Results
The study sample consisted of 60 women with skin phototypes between II and IV. The average age ranged around 45 ± 4.3 years (Table 7).
Regarding the Glogau PhotoDamage Scale, 51 patients were classified as grade III, and 9 as grade II before the start of the study. After treatment, 38/51 (63.3%) patients who were classified as grade III were reclassified as grade II and 6/9 (66.6%) patients who were classified as grade II were reclassified as grade I (p=0.019); the rest of the patients remained in the same grade assigned before treatment.

Table 2: Classification of photoaging according to Glogau [3].
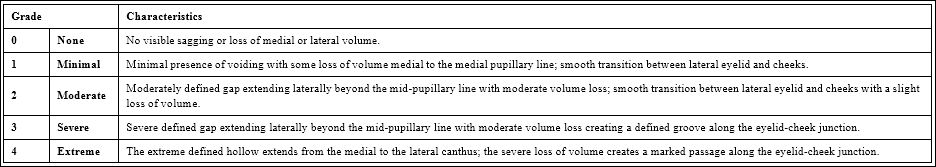
Table 3: The Allergan infraorbital void scale [4].

Table 4: Global aesthetic improvement scale (GAIS) [6].

Table 5: Intensity scale of adverse events [7].

Table 6: Scale of the degree of patient satisfaction [8].
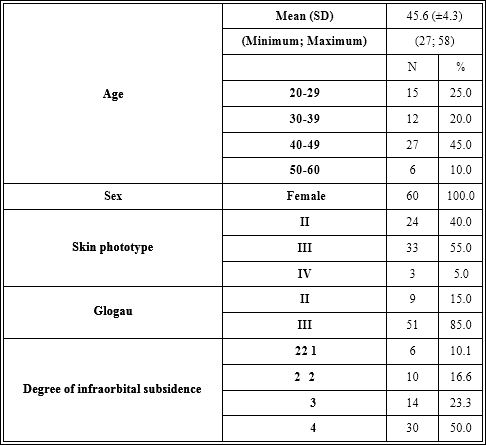
Table 7: Epidemiological and clinical characteristics of the subjects.

Table 8: Adverse events.

Table 9: Degree of satisfaction, according to the patients’ own satisfaction scale (PSSS).

Figure 1: Images showing the modifications in the Allergan Infraorbital Gap Scale (1A) before and after (1B) treatment with CPA.
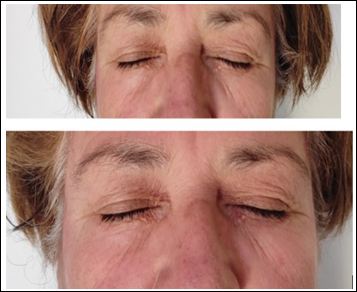
Figure 2: Images showing the modifications in the Allergan infraorbital gap scale (2A) before and after (2B) treatment with CPA.
According to the Global Aesthetic Improvement Scale, there were significant changes after treatment (p <0.021); 10/60 (16.6%) patients achieved a total response, 34/60 (56.6%) patients achieved a marked partial response, and 16/60 (26.6%) patients achieved a slight partial response.
Regarding the Allergan infraorbital gap scale, 30 patients were classified as grade 4, 14 as grade 3, 10 as grade 2 and 6 as grade 1, before the start of the study. After treatment, 22/30 (73.3%) patients who were classified as grade 4 were reclassified as grade 3, 10/14 (71.4%) patients who were classified as grade 3 were reclassified as grade 2, 6/10 (60.0%) patients who were classified as grade 2 were reclassified as grade 1 and 4/6 (66.6%) patients who were classified as grade 1 were reclassified as grade 0 (p=0.011); the rest of the patients remained in the same grade assigned before treatment (Figures 1 and 2).
All the patients reported some adverse event (pain, inflammation and ecchymosis), which were of slight intensity, did not imply changes before the intervention and were completely resolved. The pain occurred during the procedure and disappeared immediately after completion of the procedure (100%), the inflammation (90%) lasted 2 to 3 days and the ecchymoses at the puncture sites (85%) they were of short duration (five to seven days in duration) (Table 8).
Discussion
The skin of the eyelids is extremely thin and decreases in thickness with the aging process, which gives it translucency, allows us to see the superficial vascular network and gives it a reddish-bluish hue. The loss of infraorbital fat, structural in extremely thin people or with wasting disease, contributes to this effect. Sun exposure dries out the skin and destroys collagen and elastin fibers, making it even thinner. There are also structural conditions that cause changes in light reflection and create infraorbital “purple shadows”, such as deep orbits, enophthalmos, and prominence of the superciliary arch and prominence of the nasal bridge. The loss of volume and tension of the orbital malar ligament associated with age reduces the support of the midface structures and favors the formation of an infraorbital sulcus. As the region plays an important role in facial appearance, rejuvenation of the area has immense cosmetic benefit, and various treatment modalities have been used to achieve this [10].
The first randomized controlled clinical trial for PRP skin rejuvenation was conducted in 2018 by Alam M and colleagues who injected 3 milliliters of PRP and saline (monotherapy) into the same subjects on one cheek and the contralateral cheek, respectively. At the six-month follow-up, two masked dermatologists performed the subject evaluation and found that the PRP-treated cheek compared to the saline-treated cheek showed a significant improvement in skin texture (P=0.02) and in wrinkles (P=0.03) [11].
Mehryan P et al. Treated 10 patients with a single session of intradermal injections of 1.5 ml of PRP in the area of the lacrimal canal and crow’s feet wrinkles on each side. The improvement in infraorbital color homogeneity was statistically significant (P=0.010), but no statistically significant changes were observed in melanin content, stratum corneum hydration, wrinkle volume, and visibility index. The participants’ satisfaction score and the physician’s global assessment score were 2.2 and 1.7, respectively, on a scale of 0-3 [12].
Ozer K et al. Subjected 9 patients complaining of infraorbital darkness to 3 sessions with monthly PRP injection frequency. Patient-reported results for FACE-Q satisfaction and quality of life for FACE-Q before and after the procedure showed a statistically significant improvement (P<0.05). Overall satisfaction with the result was 83.33±16.25 (range 63-100). Only transient ecchymoses and edema were observed and improved during follow-up [13].
Aust M et al. Published 20 patients treated three times at monthly intervals with 2 ml of PRP for each infraorbital region, administered laterally using 27 G 38 mm cannulas. The patients were evaluated on the days of treatment and one month after the third injection by means of photographic images and measurements of the firmness and elasticity of the skin using a cutometer to objectify the subjective evaluations of the questionnaires of the patient and the doctor. A progressive improvement of the aesthetic result and a high level of patient satisfaction were determined. Cutometer measurements showed a statistically significant higher level of skin firmness (P=0.0005) (due to increased collagen production) and a statistically significant increase in skin elasticity (P=0.0021) (thanks to increased elastin production). In addition to the swelling visible immediately after injection, there were no other undesirable side effects or complications. The typical burning sensation during injection was not reported [14].
Evans AG et al. conducted a systematic review and meta-analysis on rejuvenation of the periorbital area with PRP. They found 19 studies where 455 patients were treated (95% female, age range 2860). The patients were treated a mean of 3 times (range 1-8) at mean intervals of 23 days (range 14-56 days). Follow-up averaged 3 months (range 1 to 6 months). Meta-analysis of 3 randomized controlled clinical trials showed that PRP-treated patients had higher satisfaction over controls (saline, platelet-poor plasma, mesotherapy, and as an adjunct to laser therapy) (P=0.001) [15].
In our study, 60 women with an average age of 45 ± 4.3 years were treated. After treatment, there were significant changes in the Glogau Photo Damage Scale (P=0.019), in the Global Aesthetic Improvement Scale (P<0.021) and in the Allergan Infraorbital Gaps Scale (P=0.011). The adverse events found were pain, inflammation and ecchymosis. The degree of satisfaction reported by the patients was good (6.6%) and very good (93.4%) (P<0.0033).
Despite the heterogeneity in the form of preparation, the number of doses, the interval between sessions and in the evaluation methods, all the studies reviewed and also ours demonstrated that therapy with injectable PRP induces an improvement in appearance, texture , pigmentation and fine lines of the periorbital skin, so we can conclude that autologous platelet concentrate proved to be effective and safe in reducing infraorbital signs of aging, associated with a high degree of patient satisfaction.
References
- Du R, Lei T (2020) Effects of autologous platelet-rich plasma injections on facial skin rejuvenation. Experimental and Therapeutic Medicine 19: 3024-3030.
- Draelos ZD, Rheins LA, Wootten S, Kellar RS, Diller R (2019) Pilot study: Autologous platelet‐rich plasma used in a topical cream for facial rejuve J Cosmet Dermatol 18: 1348-1352.
- Glogau RG (1996) Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg 15: 134-138.
- Donofrio L, Carruthers J, Hardas B, Murphy DK, Jones D, et al. (2016) Development and Validation of a Photonumeric Scale for Evaluation of Infraorbital Dermatol Surg 42: S251-S258.
- https://booksmedicos.org/manual-tecnico-aabb-17a-edicion/
- Savoia A, Accardo C, Vannini F, Pascale B, Baldi A (2014) Outcomes in thread lift for facial rejuvenation: A study performed with happy lift revi Dermatol Ther (Heidelb) 4: 103-114.
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, et al. (2010) CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 340: c869.
- Larson L, Rovers J, Mackeigan L (2002) Patient satisfaction with pharmaceutical care: Update of a validated instrument. J Am Pharm Assoc 42: 44-50.
- https://www.scielo.br/pdf/rbf/v32n4/aop13810.pdf
- https://piel-l.org/blog/wp-content/uploads/2016/10/Manejos-de-las-pdf
- Alam M, Hughart R, Champlain A, Geisler A, Paghdal K, et al. (2018) Effect of platelet-rich plasma injection for rejuvenation of photoaged facial skin: A randomized clinical trial. JAMA Dermatol 154: 1447-1452.
- Mehryan P, Zartab H, Rajabi A, Pazhoohi N, Firooz A (2014) Assessment of efficacy of platelet‐rich plasma (PRP) on infraorbital dark circles and crow’s feet J Cosmet Dermatol 13: 72-78.
- Ozer K, Ozlem (2019) Evaluation of platelet-rich plasma on infraorbital dark circles by using the FACE-Q Int J Res Dermatol 5: 224-230.
- Aust M, Pototschnig H, Jamchi S, Busch KH (2018) Platelet-rich Plasma for Skin Rejuvenation and Treatment of Actinic Elastosis in the Lower Eyelid Cureus 10: e2999.
- Evans AG, Ivanic MG, Botros MA, Pope RW, Halle BR, et (2021) Rejuvenating the periorbital area using platelet-rich plasma: A systematic review and meta-analysis. Arch Dermatol Res.
Citation: Alfonso-Trujillo I, Cruz-León Y, Vázquez AAM, Núñez-Jordán EJ, Morales-Novo YL (2021) Efficacy and Safety of Autologous Platelet Concentrate in the Treatment of Infraorbital Aging. J Clinic Exper Cosme Derma 4: 015.
Copyright: © 2021 Alfonso-Trujillo I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


