*Corresponding Author:
Harikrishnan Ramasamy,
Department of Zoology, Pachaiyappa’s College for Men, Tamil Nadu, India
Tel: +91-4362227937
E-mail: rhari123@ yahoo.com
Abstract
Differential tissue expression of tumor necrosis factor α (TNFα) gene and their mediated antimictobial immune response were investigate in challenged and immunized goldfish (Carassius auratus) both in vitro and in vivo against Aeromonas hydrophila (strain KCTC 2358). A correlation between the proliferation of immune head kidney (HK) leucocytes and the production of macrophage activating factor (MAF) were also studied in the present experiment. The proliferative immune response of HK leucocytes of infected goldfish immunized with formalin killed (FK) A. hydrophila vaccine was significantly higher compared with that of non-immunized goldfish. The production of reactive oxygen and nitrite intermediates from the resident goldfish phagocytes of control was enhanced after incubating with the MAF-containing supernatants for 48 h. Both cytokine-containing supernatants and the incubated phagocytes of the immune HK leucocytes also exhibited a significant increase of bactericidal activity. The study also showed that nitric oxide (NO) production and microbicidal activity of the antigen-activated phagocytes were partially blocked with NG-monomethyl-L-arginine (NG-MMLA), suggesting that NO might be an important anti-microbial effector in goldfish phagocytes.
Keywords
Aeromonas hydrophila, Bactericidal activity, Carassius auratus, Macrophage-activating factor (MAF), Tumor necrosis factor α (TNFα), Vaccination
Introduction
Bony fish and teleost fish possess both humoral-mediated immunity (HMI) and cell-mediated immunity (CMI) [1], however, yet most of studies on protective immunity of fish towards pathogenic agents have focused on the role of antibodies directed against whole bacterial cells as well as their toxic products [2]. The production of macrophage-activating factor (MAF) by leucocytes under the stimulation of mitogens or antigens threw some light on the mechanisms involved in CMI was only recent research. Tumor necrosis factor α (TNFα) is a powerful pleiotropic pro-inflammatory cytokine secreted by several cells including monocytes (MCs), macrophages (MΦ), neutrophils (ANCs), polymorphonuclear leucocytes (PMNL), mast cells (MCs), smooth muscle cells (SMCs), natural-killer cells (NKCs), and T-cells following their stimulation by bacterial lipopolysaccharide (LPS) or during acute inflammation or infection. It was responsible for a different range of signaling events within cells including inflammatory, infectious, and malignant conditions [3]. TNF is well-defined role as an important mediator in resistance against parasitic, bacterial, and viral diseases [4-6]. The TNF belong to a large family of structurally related proteins called ‘TNF Ligand Superfamily’ (TNFSF) which diverse and profound involving inflammation, apoptosis, cell proliferation, and the stimulation of various aspects of the immune system [7]. It is unclear, what may have diverged in the evolution of vertebrates, the receptors and the cellular mechanism by which the ligands function [8,9].
TNFα and β has been discovered and characterized by a large number of mammalian taxa which are recently cloned in various bony fish and teleost’s fish species [10-17]. These studies have revealed the existence of some obvious differences from their mammalian counterparts and the multiple isoforms of TNFα present in various teleost fishes [10-12,14-16]. It was contains four exons and three introns, and the deduced protein sequences that possess in the TNF family signature and it conserve by two cysteine residues. Recently, there are two non-mammalian TNFα mRNAs isoforms have been discorded, such as TNF1 and TNF2 in different fishes [10-19], which the TNF2 gene exhibits high constitutive expression in different tissues of healthy fish, but it relatively poor expression (up-regulation) when fish immune challenge in vitro and in vivo [11,13,14].
A number of studies have provided an indirect evidence for the biological role of TNFα suggested that an important MAF produced by leukocytes, which stimulates macrophage function in vitro. Expression analysis of the TNFα isoforms has been demonstrated in several fish species that can be up-regulated in macrophages by stimulated with bacterial LPS and TNF2 being expressed at a higher level than TNF1 [7,14,17 and 20]. In Japanese flounder, TNFα was first identified as a single copy gene, which inducible mRNA expression by LPS, and phorbol myristate acetate (PMA) stimulated the leukocytes [18]. In rainbow trout, seabream, goldfish, and catfish, homologous MAF-containing supernatants have been shown to induce a typical activated-macrophage response by LPS, evidenced by increases in respiratory burst activity (RBA), phagocytosis, and nitric oxide (NO) production [21-27]. Similarly, carp and turbot the head kidney (HK) leukocytes induce by LPS that results in proliferation, NO production, inhibited with pentoxifylline (PTX), and a known blocker of TNFα production [17,28]. Interestingly, in salmonids have been reported to be constitutively expressed in HK and gill tissues and it is LPS-inducible in isolated HK leukocytes [14], while in trout TNFα induced leukocyte migration and phagocytosis [15]. On the other hand, the recombinant TNFα (rTNFα) in gilthead sea bream increased phagocyte mobilization and primed the RBA [29]. Thus the above studies are strongly indicates that CMI may play an important role in the immune protection of fishes against pathogens.
The most unpredicted and exciting difference between fish and mammal TNFα alarms the weak in vitro effects on phagocytosis. Consequently, high concentration of rTNFα is weakly induced chemotaxis, RBA, phagocytosis, and NO response of macrophages in goldfish [29] and in channel catfish [11]. However, TNFα alone or combined with LPS failed to trigger the RBA of phagocytes in turbot [30] and in gilthead seabream [31], but it induce significant NO production of phagocytes in turbot [30]. Perhaps one of the importance roles of TNFα is macrophage-mediated cytotoxicity due to the proapoptotic effects. Nevertheless, TNFα is increasingly recognized as a key regulator of lipid metabolism in adipose tissue and protein catabolism in muscle [7]. Therefore, this weak in vitro activity of fish TNFα sharply contrasts with the powerful actions exerted by injection of rTNFα in gilthead sea bream, which includes the recruitment of phagocytes to the injection site with a concomitant strong increase in their RBA [31]. These information’s were raise questions regarding the evolution of TNFα functions and, particularly, the roles played by this cytokine in the regulation of the inflammatory response in different vertebrate groups.
Goldfish are popular, small, inexpensive, colorful, and economic important ornamental fish species. It can be kept in pond or aquarium throughout year in temperate and subtropical climates. This fancy goldfish are more susceptible to different pathogens, including bacteria, virus, and fungus. A Gram-negative Aeromonas hydrophila bacterium is major problem in goldfish [32]. Currently no information is available concerning the expression of the TNFα cytokine gene, which does not strongly respond to the majority of known cytokine expression inducers in goldfish against A. hydrophila. In our laboratory for the first time preliminary results show that A. hydrophila inhibits TNFα mRNA expression in goldfish macrophages both when pre-treated and when added simultaneously with LPS (Harikrishnan; unpublished results). Therefore, the present study investigated first time release of cytokines from HK leucocytes susceptibility to A. hydrophila infected and after immunized goldfish with heat-killed (HK) A. hydrophila vaccine and their possible immune protection like proliferation of HK leucocytes, RBA, NO production, phagocytosis, intracellular killing activity, and differential tissue expression.
Material and Methods
Fish
Healthy goldfish, Carassius auratus weighing approximately 38 g were purchased from a local fish farm at Tiruchirapalli, Tamil Nadu, India and transported to the laboratory in plastic bags filled with oxygenated water. The fishes were stocked randomly into 150-L aquaria. All fish were acclimated for 2 week under natural laboratory conditions (14/10 h light/dark cycle) prior to challenge or immunization. The use of goldfish for this study was approved by an official Ethics Committee of Bharathidasan University, Tamil Nadu, India.
The aquaria water quality parameters were monitored during the experimental period as dissolved oxygen concentration 5.5 7.4 mg l-1 (Winkler’s method), pH 5.6 7.3, and temperature at 18 21 ºC. The fish were fed a commercial pellet diet ad libitum twice a day at 5% of their body weight throughout the experiment. The 50% of water of the aquaria was renewed five days once to remove the unfed and fecal materials.
Preparation of A. hydrophila culture and vaccine
A. hydrophila (KCTC 2358) was obtained from Korean Collection for Type Cultures (KCTC) in Daejeon, South Korea and maintained in the laboratory. Subcultures were maintained on tryptic soy agar (TSA, Sigma) in slopes at 5 ºC and routinely tested for pathogenesis [33], by inoculation into goldfish [34]. Stock culture in tryptic soy broth (TSB, Sigma) was stored at -70 ºC in 0.85% NaCl with 20% glycerol (v/v) to provide stable inoculate throughout the experiment [32]. Subculture of A. hydrophila was taken on TSA slope and harvested by TSB. The inoculated TSB was incubated for 24 h in a shaker at 30 ºC, and then centrifuged at 12000 g for 10 min at 4 ºC [32]. The supernatant was discarded and the bacterial pellet was washed three times with phosphate-buffered saline (PBS) at pH 7.2. The number of A. hydrophila cells ml-1 in one day culture was enumerated using standard plate count methods on TSA plates supplemented with 5% sheep’s blood [32].
For the preparation of vaccine, an aliquot of 25 µl of culture used on BHI agar plates and incubated for 48 h at 26 ºC. Pure culture of A. hydrophila was taken and inoculated in TSB for 24 h at 28 °C, and then washed with PBS for three times. Cells were then suspended in PBS containing 0.4% formalin to a final concentration of 1.0 x 105 cells ml-1. The formalin killed cells (FKC) thereafter diluted with an equal volume of Freund’s complete adjuvant (FCA; ICN Biomedicals) and stored at 4 °C until use. Before use, the vaccines were kept at room temperature (RT) and check the viability of bacteria on BHI agar plates.
Experimental design, immunization and cumulative mortality
The goldfish were divided into three groups of 50 fish (3x50=150 fish) each in triplicate groups (3x150= 450 fish) namely: (i) control group (C) received with 100 µl PBS or FCA; (ii) fish challenged intra-peritoneally with 100 μl of phosphate-buffered saline (PBS, pH 7.2) containing 1.0 x 105 cells ml-1 of A. hydrophila (I); infected fish immunized intra-peritoneally with 100 µl of formalin-killed (FK) A. hydrophila (1.0 x 105 cells ml-1) in FCA (T). The same was booster after 2nd week. After booster at weeks 1, 2, and 4, the HK leucocytes were collected all groups for in vitro mediated antimicrobial immune response and differential tissue expression by RT-PCR namely: (i) incubate only supplemental L15 medium (C); (ii) incubate supernatant of control HK leucocytes without bacterial cells (CHK); (iii) incubate supernatant of control HK leucocytes stimulated by bacterial cells (CHKB); (iv) incubate supernatant of immunized HK leucocytes without bacterial cells (IHK); (v) incubate supernatant of immunized HK leucocytes stimulated by bacterial cells (IHKB); (vi) incubate supernatant of immunized HK leucocytes stimulated by bacterial cells in the presence of 1000 ìM NG-MMLA (IHKB-NG-MMLA).
Sample collection
The antiserum sample (500 µl) were collected via the caudal vein puncture of six fish each experiment (control or exposed) from one of the triplicate aquaria at 1, 2, and 4 weeks of post-challenged with bacteria or vaccination after fish anesthetized in a 100 mg l-1 solution of tricaine methanesulfonate (MS-222). Immediately after blood sampling tissues sample of muscle, gills, liver, anterior kidney/head kidney (HK), heart, spleen, and intestine were rinsed in cold PBS (Gibco) at pH 7.2 and stored in 1-ml Trizol® (Invitrogen) frozen at -80 ºC in liquid nitrogen until DNA or RNA extraction. Individual fish was sampled only once to avoid the influence on the assays due to multiple bleeding and handling stress on the fish.
Proliferation of head kidney (HK) leucocytes
The HK leucocytes were obtained from goldfih following Marsden et al. [37]. Goldfish HK cell suspensions were diluted in L-15 medium (Leibovitz, Sigma) containing 10 iu ml-1 heparin, 100 µg ml-1 penicillin/100 iu ml-1 streptomycin (P/S) and 10% foetal calf serum (FCS) and layered over 51% Percoll density gradients (All are Sigma). It was centrifuged at 400 g for 45 min at 4 ºC. After leucocytes were carefully removed from the Percoll medium interface and counted by a Coulter counter (Coulter Electronic Ltd.). The reliability of the HK leucocytes cells were checked by trypan blue exclusion (TBE) assay. The HK leucocytes cell suspension was then adjusted to 5 x 106 cells ml-1. Each well of a 96-well tissue culture plate (Falcon Laboratories) was seeded with 100 µl of HK leucocytes in L-15 medium containing 100 µg or 100 iu ml-1 P/S, and 10-5 M 2-mercaptoethanol (Sigma). A volume of 5 µl medium containing 1 x 105 FK A. hydrophila cells was added and incubated for 3 h at 25 ºC before the cultures were supplemented with 10% heat-inactivated FCS and 5% heat-inactivated homologous fish plasma. The propagation of HK leucocytes were determined by colorimetric 3-[4,5-dimethylthiazol-2-yl.-2,5-diphenyltetrazolium bromide (MTT) assay [38]. After 48 h culture, 20 µl of MTT (5 mg ml-1 PBS) was added to each well of the leucocyte cultures and incubated further 25 ºC for 4 h, then centrifuged at 500 g for 10 min. After centrifugation, the supernatant fluid was carefully discarded without disturbing the cell pellet or precipitate. A 200 µl of DMSO (Sigma) and 25 µl of glycine buffer (0.1 M glycine, 0.1 M NaCl, pH 10.5) was added and mixed thoroughly in each well then incubated at RT for 10 min. The formazan development was determined at 570 nm using Bio-kinetics reader (EL 312e, BIO-TEK Instruments). The HK leucocytes obtained from bacteria-immunized goldfish 4 weeks post-immunization and the control fish were referred to as ‘immune HK leucocytes’ and ‘control HK leucocytes’, respectively.
Determination of specific antibody
Antibody (Ab) titres against the A. hydrophila strain were determined by an agglutination test in 96-well microtitre plates (Sigma) following Robertson [36]. A volume of 50 µl each serum samples were serially diluted with PBS and 50 µl of formalin-killed A. hydrophila (1 x 105 cells ml-1) was added to each well and mixed thoroughly then incubated at RT overnight prior to examination for agglutination. The Ab titres were expressed as the reciprocal of the highest serum dilution giving positive agglutination.
Production of cytokine-containing supernatants
Cytokine (CK)-containing supernatants were prepared following Marsden et al. [39]. HK cell suspensions from immunized and control goldfish were prepared as above and then adjusted to 5 x 106 cells ml-1. All the well (24-well tissue culture plate, Falcon Laboratories) was seeded with 1 ml-1 of leucocytes in supplemented L-15 medium mixed with 0.5-ml medium containing 1 x 105 FK A. hydrophila cells (KCTC 2358) and incubated at 25ºC for 3h before the cultures were supplemented with 10% heat-inactivated FCS and 5% heat-inactivated homologous fish plasma. Further 48 h incubation, the supernatants were harvested by centrifugation at 10000 g to remove bacteria and cell debris. The supernatants were then sterilized by passing through a 0.22-µm sterile filter (Millipore), aliquoted, and stored at -70 ºC prior to use.
Detection of Macrophage Activating Factor (MAF) activity
The goldfish HK phagocytes were incubated with supernatant samples prepared before the assay of activation [40]. The HK leucocyte suspensions preparation as described above except that cells were layered over a 34/51% Percoll density gradient. The cell density was adjusted to 5 x 106 ml-1 in L-15 medium containing 1% P/S and 0.1% FCS. A volume 100 µl sample of the suspension was added in each well (96-well tissue culture plates) and incubated at 25 ºC overnight before gentle washing to remove non-adherent cells. Further 100 µl fresh supplemented L-15 medium was added to each well. Then 25 µl of leucocyte culture supernatant prepared from immunized or control goldfish as above was added to each of 8 replicate wells. The plates were incubated 48 h, the target phagocytes were assayed for respiratory burst (RB) activity [37] and for the production of reactive nitrogen intermediates following method of Wang et al. [41]. The optical density (O.D.) was measured by Bio-kinetics reader EL 312e at 630 and 570 nm.
Phagocytosis and bactericidal activity of phagocytes
The phagocytosis assays in replication of bacteria of cultured goldfish phagocytes were determined by Yin et al. [42] with a slight modification. HK phagocytes from six of control or immunized goldfish at 1, 2, and 4 weeks post-immunization were collected separately and adjusted to 5 x 106 ml-1 in L-15 medium containing 0.1% FCS. Then 1 ml-1 was added to each well and incubated at 25 ºC overnight. Phagocyte monolayers were prepared by the removal of non-adherent cells through gentle washing. Each of three replicate wells was added 1 µl of L-15 medium containing 0.1% FCS and 250 µl of the CK-supernatant samples. The bactericidal assays were determined after incubation with 48 h. Live A. hydrophila was opsonized with goldfish specific antiserum. They were then added to the phagocyte monolayers and incubated for 30 min before gentamycin was added to kill extracellular bacteria. Antibiotic was removed after 1 h incubation and viable bacteria in each well were counted immediately (initial counts) and then after 6 h incubation with antibiotic-free medium used for final counts. The intracellular bacterial population at 0 h (initial count) represented the effects on phagocytosis. The replication index was calculated by dividing the population of A. hydrophila at 6 h by the intracellular population at 0 h. Values recorded were arithmetic means ± S.D. of counts from six monolayers.
Direct killing effect of the CK-supernatants
A volume of 100 µl of live bacterial suspension (about 105 cells ml1) was added to 900 µl of various culture supernatants, mixed thoroughly and incubated at 25 ºC for 4 h. The bactericidal activities of the supernatants were expressed as percentages of the mean number of bacteria surviving in various culture supernatants as compared to those in tissue culture medium.
Isolation of total RNA and 1st strand synthesis
Total RNA was isolated using Trizol® (Invitrogen) following the manufacturer’s instructions and stored at -80 ºC for later use. The concentration of total RNA was quantified by UV spectrophotometry (Ultrospec 6300 pro, Amersham Biosciences) at 260 nm. To remove any genomic DNA, the RNA was treated with DNase I (Solgent) according to the manufacturer’s instructions. For reverse transcription, 2 µg of total RNA from the goldfish tissues with an oligo (dT20) primer and Superscript™ III reverse transcriptase (Solgent), and DEPC (Solgent) treated dH2O was added to a final volume of 14 µl. This mixture was incubated at 70 ºC for 10 min and chilled on ice for at least 3 min. The specific primers for goldfish TNFα gene, Gen Bank accession no. ABU50128 and goldfish β-actin, GenBank accession no. BAA92339 was utilized as the internal control (Table 1). Reverse transcription to cDNA was carried out. Three microgram RNA in 14 µl DEPC-water was incubated with 1 µl oligo (dT) 15-20 (500 µg ml-1, Invitrogen) for 10 min at 70 ºC and chilled on ice for at least 3 min. One microlitre Moloney Murine Leukaemia Virus (MMLV) reverse transcriptase (Solgent), 4 µl 5x first strand buffer (Solgent), 2 µl 10 mM dNTPs (Solgent), 1 µl 0.1M DTT (Solgent) 0.5 µl RNase inhibitor (Solgent) and 1 µl Diastar RNase were added, and the reaction was incubated at 50 ºC for 50 min followed by 5 min at 95 ºC. The resultant cDNA was dissolved in water and stored at -20 ºC.
cDNA synthesis and reverse transcriptase (RT) PCR of TLR and β-actin genes
PCR was carried out using different primer sets and conditions (Table 1). cDNA (1 µl) was added to an RNAse free tube, followed by 25 µl of a premix (Solgent) containing 12.5 µl 2x Taq premix, 1 µl forward primer, 1 µl reverse primer (Genotech), 1 µl cDNA sample and 11.5 µl DEPC-water. The solution was mixed and briefly centrifuged and then subjected to a gene-specific heat-protocol (Table 1) in a Astec (PC-707) thermal cycler (Japan). A β-actin PCR was initially performed, at 26 cycles, and the amount of template added adjusted to give approximately equal PCR products between samples. During this procedure the reproducibility of the products obtained from individual fish was confirmed. The same amount of cDNA was then used for TNFα gene PCRs as a way of normalizing the data in order to give a more quantitative result. Optimal cycling number of the other genes was determined to generate a product that was visible but not saturated, and found to be between 32 and 37 cycles, depending on the specific gene. All of the data were gathered from triplicate experiments and were expressed as fluorescence relative to β-actin.
Analysis of expression profile and the relative expression ratio
The resultant PCR products were separated on 2% agarose gel containing 100 ng ml-1 ethidium bromide. The products were run on the gel for 1 h at 100 V, using a 100 bp DNA ladder (Bioneer) as a size marker. All the gel was then visualized using a Gel Doc image analysis system (Bio-Rad). The relative folds (ratio) of TNFα gene expression relative to β-actin expression using the pixel density for each product were determined by Lab Works image acquisition and analysis software. This enabled the evaluation of differential expression of TLRα gene between different sample groups.
Statistical analysis
Statistical analysis of mediated antimicrobial immune response and TNFα gene expression results (mean ± SE) was performed using the Tukey’s test compared the means between groups in SPSS at P < 0.05 levels.
Results
Antibody production
The serum agglutinating Ab titres of control goldfish was less than 1:32. In compare to control, the immunized group was prominent humoral response on week 2. After 4 weeks of post-immunization with FK A. hydrophila vaccine was significantly high level compared to control. All these immune sera had Ab titers of lower than 1:32 against live A. hydrophila.
Proliferation of HK leucocytes
The proliferative response after weeks 1, 2, and 4 of post-challenge without live A. hydrophila (CHK) of goldfish HK leucocytes supernatant were incubated in vitro did not significantly (P > 0·05) enhanced compared with the control. On the other hand, HK leucocytes incubated with live A. hydrophila (CHKB) was significantly enhanced the proliferation of HK leucocytes. Immunized goldfish (FK A. hydrophila vaccine) HK leucocytes supernatant and incubated with or without live A. hydrophila (IHK and IHKB) or immunized goldfish HK leucocytes supernatant and incubated with A. hydrophila in the presence of presence of 1000 ìM NG-MMLA (IHKB-NG-MMLA) were higher proliferative response than those of control (Figure 1).
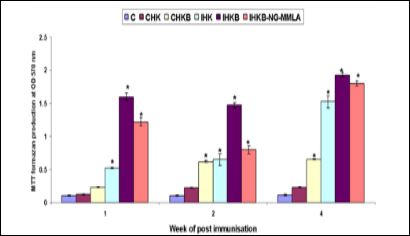
Figure 1: Proliferation of HK leucocytes isolated from control and immunised goldfish (means ± SE, n = 6) on 1, 2, and 4 weeks of post-immunisation with formalin-killed A. hydrophila vaccine, and cultured for 48 h in the absence or presence of live A. hydrophila in vitro at OD 570 nm. *Indicates statistically (P < 0.05) significant difference compared to control group at the same sampling week. C: incubate only supplemental L15 medium, CHK: incubate supernatant of control HK leucocytes without bacterial cells, CHKB: incubate supernatant of control HK leucocytes stimulated by bacterial cells, IHK: incubate supernatant of immunized HK leucocytes without bacterial cells, IHKB: incubate supernatant of immunized HK leucocytes stimulated by bacterial cells, IHKB-NG-MMLA: incubate supernatant of immune leucocytes stimulated by bacterial cells in the presence of 1000 ìM NG-MMLA.
MAF in supernatants
Production of reactive oxygen intermediates
The production of reactive oxygen intermediates (ROI/O free radical) was measured by detecting the reduction of the redox dye nitroblue tetrazolium (NBT) caused by target phagocytes. The resident HK phagocytes of goldfish was incubated with the supernatants of control HK leucocytes with or without live A. hydrophila (KCTC 2358) showed a high level of RB activity than those of supernatants from control HK leucocytes in vitro (CHK and CHKB). On the other hand, the supernatants from immune phagocytes incubated in vitro with the homologous bacterial antigen exhibited the highest stimulation on fish phagocytes (IHKB) then incubated without bacterial antigen (IHK). The production of ROI of immunized goldfish (FK A. hydrophila vaccine) HK leucocytes supernatant and incubated with live A. hydrophila in the presence of presence of 1000 ìM NG-MMLA (IHKB-NG-MMLA) also high level of RB activity compared to control. These results suggested that the secretion of MAF from immunized goldfish HK phagocytes resulted from the activity of the specific immune reaction (Figure 2).
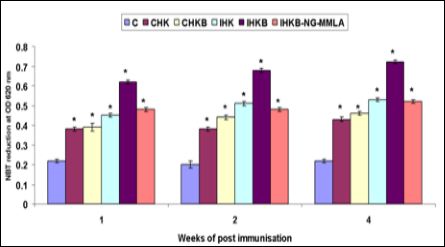
Figure 2: Reduction of NBT by resident HK phagocytes from control goldfish (means ± SE, n = 6) on 1, 2, and 4 weeks of post-immunisation with formalin-killed A. hydrophila vaccine, and incubated for 48 h in the absence or presence of live A. hydrophila in vitro OD at 620 nm. Abbreviations and statistical information as in Figure 1.
Production of reactive nitrogen intermediates (RNI)
The production of RNI (release of NO -) was often used as a good marker of the NO pathway in macrophages. The present results demonstrated that the supernatants of goldfish HK leucocytes and incubated with or without homologous bacterial antigens in vitro were able to stimulating the resident phagocytes and produced low levels of NO (CHK and CHKB). The supernatant from the immunized goldfish HK leucocytes treated with or without homologous bacterial antigen showed high levels of NO production (IHK and IHKB). However, supernatant from the immunized goldfish HK leucocytes treated with homologous bacterial antigen in the presence of 1000 ìM NG-MMLA (IHKB-NG-MMLA) showed low level of NO production. Therefore, the present results comparing the effects of various supernatants suggest that the release of MAF was related to the proliferation of the HK leucocytes. In addition, the production of NO was indicated that inhibited by addition of 1000 ìM NG-MMLA, an effective inhibitor of NO synthase enzyme (Figure 3).

Figure 3: Nitrite production of resident HK phagocytes from control goldfish (means ± SE, n = 6) on 1, 2, and 4 weeks of post-immunisation with formalin-killed A. hydrophila vaccine, and incubated for 48 h in the absence or presence of live A. hydrophila in vitro OD at 570 nm. Abbreviations and statistical information as in (Figure 1).
Phagocytosis and bactericidal activity of the activated phagocytes
Phagocytic activity of phagocytes treated with various HK leucocyte supernatants was summarized by showing the intracellular cfu numbers at initial counts at 0 h (Figure 4).
The activation effects of the supernatants prepared from control HK leucocytes incubated without live A. hydrophila did not enhance the phagocytic activity (CHK). However, control HK leucocytes incubated with live A. hydrophila significantly enhance the phagocytic activity (CHKB). On the other hand, An inhibition effect on phagocytosis by activated phagocytes was also observed in the supernatant, that from immune leucocyte culture treated with or without homologous bacterial antigen (IHK and IHKB) and in the presence of 1000 ìM NG-MMLA (IHKB-NG-MMLA) showed highest phagocytic activity compared to control. The resident phagocytes treated with supernatants from immune fish leucocytes also showed a significant increase of killing compared with those of the phagocytes treated with supernatants from control leucocytes cultures. The present results suggested that supernatants from immune HK leucocytes exposed in vitro to homologous bacterial antigen exhibited the highest stimulation effect on the bactericidal activity of phagocytes. However, this killing effect of the activated phagocytes was partially inhibited by 1000 ìM NGMMLA (Figure 5).
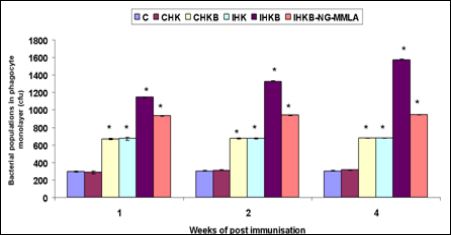
Figure 4: Phagocytosis of resident phagocytes from control goldfish (means ± SE, n = 6) on 1, 2, and 4 weeks of post-immunisation with formalin-killed A. hydrophila vaccine, and incubated for 48 h in the absence or presence of live A. hydrophila in vitro. Abbreviations and statistical information as in (Figure 1).

Figure 5: Intracellular killing activity of resident phagocytes from control goldfish (means ± SE, n = 6) on 1, 2, and 4 weeks of post-immunisation with formalin-killed A.hydrophila vaccine, and incubated for 48 h in the absence or presence of live A. hydrophila in vitro. Abbreviations and statistical information as in (Figure 1).
Killing effect of the supernatants
The supernatants obtained from the immunized goldfish HK leucocytes that had been incubated for 48 h with FK A. hydrophila vaccine then exposed to live bacteria were able to kill almost 98% of the bacteria within 4 h, compared with the number of viable bacteria in the supplemented L15 medium alone. On the contrary, supernatants from the control leucocytes pre-treated with bacterial antigen could only kill 10% of the viable bacteria compared with those grown in medium alone. The results also showed that significant bactericidal activity was present in supernatants from immune leucocytes cultured in the absence of specific antigen (Figure 6).

Figure 6: Ability of supernatants from various cultures of control goldfish HK leucocytes (means ± SE, n = 6) on 1, 2, and 4 weeks of post-immunisation with formalin-killed A. hydrophila vaccine, and incubated for 48 h in the absence or presence of live A. hydrophila in vitro. Abbreviations and statistical information as in (Figure 1).
Disease resistance
The cumulative mortality of A. hydrophila infected goldfish was 40% for 15 days post-challenge. However, the mortality of infected goldfish after immunized with FK A. hydrophila was 25%. There was no mortality in the control group without challenge with A. hydrophila.
TNFα expression after post-challenge goldfish tissues
We used semi quantitative real-time PCR to measure the tissue-specific TNFα gene expression in control, challenged and immunized with FK A. hydrophila vaccine in goldfish. We compared the relative mRNA copy numbers in RNA preparations extracted from muscle, gills, liver, anterior kidney, heart, spleen, and intestine tissues (Figure 7).

Figure 7: Differential tissues expression profile of TNFα gene obtained from goldfish control and after 1, 2, and 4 weeks of post-challenged or immunized with formalin-killed A. hydrophila vaccine. M: muscle, G: gills, I: intestine, S: spleen, K: kidney, H: heart, L: liver.
The TNFα gene mRNA expression was high in the gills, intestine, spleen, and anterior kidney of control group. The expression was low or absent in the muscle, heart, and liver of control group. Low level of constitutive expression of TNFα gene was found in spleen of infected (I) and immunized goldfish with FK A. hydrophila vaccine on week 1 while the other tissues are little or absence of expression. On week 2 immunized goldfish with FK A. hydrophila vaccine, the TNFα gene expression was high in anterior kidney whereas low in the muscle, gills, intestine, spleen, and heart tissues. The similar results and higher expression were found on week 4 immunized goldfish with FK A. hydrophila vaccine (Figure 7).
The relative expression ratio of TNFα gene
The relative expression was high in the muscle of control and immunized groups. However, in the gills, intestine, and spleen, the relative expression were high all the groups. The anterior kidney relative expression was higher infected goldfish after weeks 2 and 4 of immunization FK A. hydrophila vaccine group then the control group. But the relative expression was found low in all groups of the heart and liver tissues (Figure 8).

Figure 8: The percentage of relative TLR mRNA expression by semi-quantitative analysis obtained relative to β–actin gene expression in different tissues of goldfish (mean ± SE, n = 3) sampled at 1, 2, and 4 week of post-challenged or immunized with formalin-killed A. hydrophila vaccine. Diagram represents the ratio of TLR mRNA/β-actin. M: muscle, G: gills, I: intestine, S: spleen, K: kidney, H: heart, L: liver.
Discussion
Mononuclear phagocytes of mammals have been identified as the primary producers of TNFα [43]. The present results and those of others suggested that the same was true in fish [10,18,19]. The higher mRNA levels of TNFα of goldfish were observed in MAF-activated mature macrophages, compared to monocytes. Monocytes appeared to be major producers of TNFα induced ROI as shown in this study. In contrast, goldfish mature macrophages produced nitrogen intermediates in response to different stimuli [44,45]. It has been reported that mammalian TNFα isoforms were differentially induced by LPS or GM-CSF in monocytes compared to the monocyte-derived macrophages [46]. These mammalian isoforms are distinct from those reported in fish, since they are generated by post-transcriptional or translational modifications [46]. rather than by being encoded by different genes as in teleosts [10,15]. Presumably, the role of different TNFα isoforms in mammals is to finely tune the pro-inflammatory immune responses, and the fish TNFα isoforms being encoded by separate genes may have a similar role [29].
Recent studies have been demonstrated that protective immunity on measuring Ab levels as well as cell-mediated immunity (CMI) in fish. Phagocytes well known as the direct effector cells in the control or elimination of the pathogenic agents and are often considered playing an important role in the anti-microbial immune response via phagocytosis [47]. The productions of cytokines (CKs) by leucocytes are able to influence phagocyte functions and understood to be a key characteristic of CMI response. Graham and Secombes [48] described a method for determining the secretion of MAF from the leucocytes to stimulated T cell mitogen of rainbow trout in vitro. The present results is strongly suggested that MAF secreted by immune leucocytes, it may contribute to protective immunity against A. hydrophila. To date there was no substantial direct evidence supporting the protective roles of these CKs in the anti-microbial activities of the immunized fish. Marsden et al. [39] and Francis and Ellis [49] were confirmed that MAF could be produced by the immune HK leucocytes.
In this study, goldfish TNFα was up-regulated in response to MAF. Similarly, in trout, multiple TNFα isoforms exhibited differential expression in stimulated macrophages [15]. The TNFα-1 expression was very low levels in stimulated macrophages in trout compared to the TNFα-2 [15]. The recombinant trout TNFα-1 did not up-regulate native trout TNFα-1, while recombinant TNFα-2 induced increased expression of both native TNFα isoforms [15]. The evolutionary distances between different teleost groups of TNF isoforms may be regulated and respond differentially to various stimuli. The MAF supernatants contain a plethora of potential immunomodulating agents, the differences in expression profiles of the goldfish TNFα after activation monocyte/macrophage and their role in the regulation of inflammation remain to be elucidated.
TNFα has been fully characterized in mammals and is one of the central regulatory as well as effector cytokines in inflammation. Mammalian TNFα has been shown to promote macrophage migration, phagocytosis, and production of ROI and RNI [29]. It is confirmed in the present results and those of others [10,15,18], that the pro-inflammatory roles of TNFα have been evolutionarily conserved in teleost. For example, it has been reported in trout rTNFα induced a gradient-dependent migration response of HK leukocytes [11]. Similarly, our results indicate that TNFα induced a chemotactic response of goldfish kidney-derived macrophages. It has been established mammalian TNFα but does not induce a direct chemotactic effect; instead, it promotes chemotaxis by inducing production of chemokines by various cell types [50,51]. In contrast, in both trout and goldfish rTNFα was chemotactic to kidney-derived macrophages in a dose-dependent manner. The elucidation of precise mechanisms of goldfish TNFα-mediated chemotaxis activity differs from those of mammals. It must await further understanding of TNFα/TNFα receptor interactions in goldfish.
The present study was confirmed that MAF to be released by immune HK leucocytes when incubated with A. hydrophila. These MAF-containing supernatants could stimulate HK phagocytes and increase their production of both ROI and RNI. The ability of the supernatants from various cultures of catfish leucocytes, the production of oxygen free-radicals could be considered to be an important bactericidal pathway in fish [52]. The NO is a paramagnetic gas with a short half-life and also has significant cytotoxic effects on a variety of facultative and obligate intracellular pathogens in mammal [53]. This RNI has been produced in goldfish and trout [41]. The HK phagocytes to be stimulated by LPS or MAF released from mitogen-primed HK leucocytes [54].
Mammalian TNFα have been shown to induce reactive oxygen production by neutrophils including production of super oxide anions [55] as well as hydrogen peroxide [56]. The TNFα is one of the central cytokines responsible for induction of macrophage anti-microbial responses in higher vertebrates [57,58]. This study the goldfish TNFα also induced induce production of ROI and RNI in kidney-derived macrophages. In the present study was further confirmed, a role of NO as bactericidal activity (an effective inhibitor of fish NO synthase) in goldfish phagocytes by NG-MMLA. These reports are strongly support that NO produced by activated goldfish phagocytes that contributes to the killing of intracellular bacteria as in mammalian macrophages. Further our results indicate that the goldfish monocyte subpopulation was selectively induced to generate an ROI response by MAF or TNFα, whereas the mature macrophages were induced by MAF and TNFα to generate RNI [43,59]. The elaboration of segregated anti-microbial functions in monocytes and mature macrophages previously described in the goldfish [43,59]. In this study may be similar to those observed in higher vertebrates where activated human monocytes have been reported to produce abundant amounts of ROI, whereas monocyte-derived macrophages upon further differentiation acquired the capacity to produce RNI upon activation [11].
In the present study emphasized that a direct anti-microbial activity for A. hydrophila was also found in goldfish MAF supernatants from the cultures of immune leucocytes. It is unclear which type of activated immune leucocytes to be contributed to this bactericidal factor and its genuine function of this factor in vivo. A similar activity has been found in immune BALB/c mice MAF supernatants of cultured T cells [60]. Therefore the results in the present study, in vitro and in vivo experiments suggested that a possible overall mechanism for the immune responses of goldfish after immunization with FK A. hydrophila. The specific Ab produced against the A. hydrophila could be primarily used to prevent the virulent bacteria from attaching and penetrating the epithelium of the goldfish. The pathogen had successfully entered into the body epithelial cells under certain conditions. It leads to an enhanced and elimination of pathogens via phagocytosis [42]. The specific antibody is probably to be exclusively involved in opsonization of A. hydrophila.
It was demonstrated in the present study and elsewhere [11], that teleost rTNFα induced an enhanced phagocytic response of kidney-derived phagocytes. The flow cytometry-based phagocytosis assays were allowed the examination of TNFα-induced phagocytosis at both cell subpopulation and individual cell level. Our results showed that goldfish TNFα induced phagocytosis of kidney derived macrophages. Recently significant constitutive tissue expression of TNFα have been reported in bony and teleosts fish to be highly variable [10,11,31,61]. However, a recent report in Japanese flounders, little or no constitutive tissue expression [18]. In carp, a broad pattern of tissue expression was attributed to only one isoform (TNFα-3), while the other isoforms (TNFα-1 and TNFα-2) were constitutively expressed only in the gill [10]. The present results quantitative expression analysis was different from that reported in carp, since we observed high constitutive expression of the goldfish TNFα in most tissues examined.
The results from the present study also clearly demonstrate that goldfish leucocytes primed with bacterial antigen could show a proliferative response to the specific antigens. Further it releases many types of protective cytokines which could activate the phagocytes, by enhancing phagocytosis and bactericidal activity. The conclusion in the present study was an effective protective immunity of goldfish against A. hydrophila has to depend on the co-operation of both humoral and cellular immune responses. The findings in this study indicate that TNFα plays a central role in the inflammatory response of lower vertebrate species such as bony fish. The goldfish description of this cytokine is a potent inducer of several trademark inflammatory response pathways and as such is an important tool for further explanation of the regulatory mechanisms of inflammation in teleost’s.
References
- Biller-Takahashi JD, Urbinati EC (2014). Fish Immunology. The modification and manipulation of the innate immune system: Brazilian studies. Ann Braz Acad Sci 86:1483-1495.
- Dent AE, Bergmann-Leitner ES, Wilson DW, Tisch DJ, Kimmel R, et al (2008). Antibody-mediated growth inhibition of Plasmodium falciparum: relationship to age and protection from parasitemia in Kenyan children and adults. PLoS One 3: Article ID e3557.
- Roca FJ, Mulero I, Munoz AL, Sepulcre MP, Renshaw SA, et (2008) Evolution of the inflammatory response in vertebrates: fish TNF-α Is a powerful activator of endothelial cells but hardly activates phagocytes. J Immunol 181: 5071-5081.
- Czarniecki CW (1993) The role of tumor necrosis factor in viral disease. Antiviral Res 22: 223-58.
- Wellmer A, Gerber J, Ragheb J, Zysk G, Kunst T, et (2001). Effect of deficiency of tumor necrosis factor alpha or both of its receptors on Streptococcus pneumoniae central nervous system infection and peritonitis. Infect Immun 69: 6881-6886.
- Dubravec DB, Spriggs DR, Mannick JA, Rodrick ML (1990) Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor alpha. Proc Natl Acad Sci 87: 6758-6761.
- Goetz FW, Iliev DB, McCauley LA, Liarte CQ, Tort LB, Planas JV, et (2004). Analysis of genes isolated from lipopolysaccharide-stimulated rainbow trout (Oncorhynchus mykiss) macrophages. Mol Immunol 41: 1199-1210.
- Moreno E, Yan M, Basler K (2002) Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr Biol; 12: 1263-1268.
- Bridgham JT, Wilder JA, Hollocher H, Johnson AL (2003) All in the family: evolutionary and functional relationships among death receptors. Cell Death Differ 10: 19-25.
- Hong S, Li R, Xu Q, Secombes CJ, Wang T (2013). Two types of TNF-α exist in teleost fish: Phylogeny, expression, and bioactivity analysis of type-II TNF-α3 in Rainbow trout Oncorhynchus mykiss. J Immunol 191: 5959-5972.
- Glenney GW, Wiens GD (2007) Early diversification of the TNF superfamily in teleosts: genomic characterization and expression analysis. J Immunol 178: 7955-7973.
- Kim M.S, Hwang YJ, Yoon KJ, Zenke K, Nam YK, Kim SK, Kim KH (2009) Molecular cloning of rock bream (Oplegnathus fasciatus) tumor necrosis factor-alpha and its effect on the respiratory burst activity of phagocytes. Fish Shellfish Immunol 27: 618-624.
- Garcia-Castillo J, Pelegrin P, Mulero V, Meseguer J (2002) Molecular cloning and expression analysis of tumor necrosis factor alpha from a marine fish reveal its constitutive expression and ubiquitous Immunogenetics 54:200-207.
- Laing KJ, Wang T, Zou J, Holland J, Hong S, et (2001) Cloning and expression analysis of rainbow trout Oncorhynchus mykiss tumour necrosis factor alpha. Eur J Biochem 268: 1315-1322.
- Zou J, Wang T, Hirono I, Aoki T, Inagawa H, et al. (2002) Differential expression of two tumor necrosis factor genes in rainbow trout, Oncorhynchus mykiss. Dev Comp Immunol 26: 161-172.
- Saeij JP, Stet RJ, de Vries BJ, van Muiswinkel WB, Wiegertjes GF (2003) Molecular and functional characterization of carp TNF: a link between TNF polymorphism and trypanotolerance? Dev Comp Immunol 27: 29-41.
- Saeij JP, Verburg-van Kemenade LB, van Muiswinkel WB, Wiegertjes GF (2003) Daily handling stress reduces resistance of carp to Trypanoplasma borreli: in vitro modulatory effects of cortisol on leukocyte function and apop Dev Comp Immunol 27: 233-245.
- Hirono I, Nam BH, Kurobe T, Aoki T (2000) Molecular cloning, characterization, and expression of TNF cDNA and gene from Japanese flounder Paralychthys olivaceus. J Immunol 165: 4423-4427.
- Ordas MC, Costa MM, Roca FJ, Lopez-Castejon G, Mulero V, et al. (2007) Turbot TNFa gene: molecular characterization and biological activity of the recombinant protein. Mol Immunol 44: 389-400.
- Zou J, Cunningham C, Secombes CJ (1999) The rainbow trout Oncorhynchus mykiss interleukin-1 beta gene has a different organization to mammals and undergoes incomplete splicing. Eur J Biochem 259: 901-8.
- Hodgkinson JW, Grayfer L, Belosevic M (2015) Biology of bony fish macro Biology 4: 881-906.
- Yin Z, Lam TJ, Sin YM (1997). Cytokine-mediated antimicrobial immune response of catfish, Clarias gariepinus, as a defence against Aeromonas hydrophila. Fish Shellfish Immunol 7: 93-104.
- Hardie LJ, Chappell LH, Secombes CJ (1994) Human tumor necrosis factor alpha influences rainbow trout Oncorhynchus mykiss leucocyte responses. Vet Immunol Immunopathol 40: 73-84.
- Tafalla C, Figueras A, Novoa B (2001) Viral hemorrhagic septicemia virus alters turbot Scophthalmus maximus macrophage nitric oxide Dis Aquat Org 47: 101-107.
- Novoa B, Figueras A, Ashton I, Secombes CJ (1996) In vitro studies on the regulation of rainbow trout (Oncorhynchus mykiss) macrophage respiratory burst activity. Dev Comp Immunol 20: 207-216.
- Neumann NF, Stafford JL, Belosevic M (2000) Biochemical and functional characterisation of macrophage stimulating factors secreted by mitogen-induced goldfish kidney leucocytes. Fish Shellfish Immunol 10: 167-86.
- MacKenzie S, Planas JV, Goetz FW (2003) LPS-stimulated expression of a tumor necrosis factor-alpha mRNA in primary trout monocytes and in vitro differentiated macrophages. Dev Comp Immunol 27: 393-600.
- Tafalla C, Novoa B (2000) Requirements for nitric oxide production by turbot (Scophthalmus maximus) head kidney macrophages. Dev Comp Immunol 24: 623-31.
- Grayfer L, Walsh JG, Belosevic M (2008) Characterization and functional analysis of goldfish (Carassius auratus L.) tumor necrosis factor-α. Dev Comp Immunol 32: 532-543.
- Ordas MC, Costa MM, Roca FJ, Lopez-Castejon G, Mulero V, et al (2007) Turbot TNFa gene: molecular characterization and biological activity of the recombinant protein. Mol Immunol 44: 389-400.
- Garcia-Castillo J, Chaves-Pozo E, Olivares P, Pelegrin P, Meseguer J, et (2004) The tumor necrosis factor a of the bony fish seabream exhibits the in vivo proinflammatory and proliferative activities of its mammalian counterparts, yet it functions in a species-specific manner. Cell Mol Life Sci 61: 1331-1340.
- Harikrishnan R, Nisha Rani M, Balasundaram C (2003) Hematological and biochemical parameters in common carp, Cyprinus carpio, following herbal treatment for Aeromonas hydrophila Aquaculture 221: 41-50.
- Joseph SW, Carnahan A (1994) The isolation, identification, and systematics of the motile Aeromonas species. Annu Rev Fish Dis 4: 315-343.
- Davis JF, Hayasaka SS (1983) Pathogenic bacteria associated with cultured American eels Anguilla rostrata Le Sueur. J Fish Biol 23: 557-564.
- Amend D (1981) Potency testing of fish Dev Biol Stand 49:447–54.
- Roberson BS (1990) Bacterial agglutination. In: Stolen JS, Fletcher GC, Anderson DP, Roberson BS, van Muiswinkel, WB, editors. Techniques in Fish Fair Haven, NJ: SOS Publications 137-154.
- Marsden MJ, Hamdani SH, Secombes CJ (1995) Proliferative responses of rainbow trout, Oncorhynchus mykiss, T and B cells to antigens of Aeromonas salmonicida. Fish Shellfish Immunol 5: 199-210.
- Daly JG, Moore AR, Olivier G (1995) A colorimetric assay for the quantification of brook trout (Salvelinus fontinalis) lymphocyte Fish Shellfish Immunol 5: 265-273.
- Joerink M, Ribeiro CMS, Stet RJM, Hermsen T, Savelkoul HFJ, et (2006). Head kidney-derived macrophages of common carp (Cyprinus carpio L.) show plasticity and functional polarization upon differential stimulation. J Immunol 177: 61-69.
- Graham S, Secombes CJ (1988) The production of a macrophage-activating factor from rainbow trout Salmo gairdneri Immunology 65: 293- 297.
- Wang R, Neumann NF, Belosevic M (1994) Establishment and characteristics: goldfish and rainbow trout macrophage cell lines. Dev Comp Immunol 18 (Suppl.1), S137.
- Yin Z, Lam TJ, Sin YM (1996) The role of specific antiserum of catfish, Clarias gariepinus, as a defence against Aeromonas hydrophila. Fish Shellfish Immunol 6: 57-69.
- Savan R, Sakai M (2004) Presence of multiple isoforms of TNF alpha in carp (Cyprinus carpio ): genomic and expression analysis. Fish Shellfish Immunol 17: 87-94.
- Stafford JL, Wilson EC, Belosevic M (2004) Recombinant transferring induces nitric oxide response in goldfish and murine macrophages. Fish Shellfish Immunol 17: 171-185.
- Branch DR, Guilbert LJ (1996) Differential expression of tumor necrosis factor-α isoforms from lipopolysaccharide and cytokine-stimulated mouse mac Int J Biochem Cell Biol 28: 949-955.
- Paulnock DM (1992) The molecular biology of macrophage activation. The New York: Oxford University Press: 47-62.
- Grayfer L, Kerimoglu B, Yaparla A, Hodgkinson JW, Xie J, et al. (2018) Mechanisms of fish macrophage antimicrobial immunity. Front Immunol 9:1105.
- Francis CH, Ellis AE (1994) Production of a lymphokine (macrophage activating factor) by salmo (Salmo salar) leucocytes stimulated with outer membrane protein antigens of Aeromonas salmonicida. Fish Shellfish Immunol 4: 489-497.
- Lin SK, Kok SH, Shun CT, Hong CY, Wang CC, et al (2007) Tumor necrosis factor-alpha stimulates the expression of C–C chemokine ligand 2 gene in fibroblasts from the human nasal polyp through the pathways of mitogen-activated protein kinase. Am J Rhinol 21: 251-255.
- Chen MC, Keshavan P, Gregory GD, Klumpp DJ (2007). RANTES mediates TNF-dependent lamina propria mast cell accumulation and barrier dysfunction in neurogenic cystitis. Am J Physiol Renal Physiol 292: 1372-1379.
- Kordon AO, Karsi A, Pinchuk L (2018) Innate immune responses in fish: Antigen presenting cells and professional phagocytes. Turk J Fish Aquat Sci 18: 1123-1139.
- Woods ML, Mayer J, Evans TG, Hibbe JB (1994) Antiparasitic effects of nitric oxide in an in vitro murine model of Chlamydia trachomatis infection and an in vivo murine model of Leishmania major infection. Immunol Ser: 179-196.
- Ishibe K, Yamanishi T, Wang Y, Osatomi K, Hara, et al. (2009) Comparative analysis of the production of nitric oxide (NO) and tumor necrosis factor-alpha (TNF-alpha) from macrophages exposed to high virulent and low virulent strains of Edwardsiella tarda. Fish Shellfish Immunol 27: 386-389.
- Tsukimori K, Nakano H, Wake N (2007). Difference in neutrophil superoxide generation during pregnancy between preeclampsia and essential hyperten- Hypertension 49: 1436-1441.
- Comen E, Wojnarowicz P, Seshan VE, Shah R, Coker C, et al (2016) TNF is a key cytokine mediating neutrophil cytotoxic activity in breast cancer NPJ Breast 20: 16009.
- De Titto EH, Catterall JR, Remington JS (1986) Activity of recombinant tumor necrosis factor on Toxoplasma gondii and Trypanosoma cruzi. J Immunol 137: 1342-1345.
- Philip R, Epstein LB (1986) Tumor necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induction by itself, g-interferon and interleukin-1. Nature 323: 86-89.
- Hanington PC, Belosevic M (2007) Interleukin-6 family cytokine M17 induces differentiation and nitric oxide response of goldfish (Carassius auratus ) macrophages. Dev Comp Immunol 31: 817-829.
- Markham RB, Goellner J, Pier GB (1984) In vitro T cell-mediated killing of Pseudomonas aeruginosa. I. Evidence that a lymphokine mediates killing. J Immunol 133: 962-968.
- Nascimento DS, Pereira PJ, Reis MI, do Vale A, Zou J, et (2007) Molecular cloning and expression analysis of sea bass (Dicentrarchus labrax L.) tumor necrosis factor-α (TNF-α). Fish Shellfish Immunol 23: 701-710.
Citation: Devi G, Balasundaram C, Ramasamy H (2020) Differential Tissue Expression of TNFα Gene and their Mediated Antimicrobial Immune Response on Goldfish against Aeromonas Hydrophila. J Cell Mol Biol 4: 10.
Copyright: © 2020 2020 Devi G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.