*Corresponding Author:
Shalini Dhir,
Department of Anesthesia and Perioperative Medicine, SJHC, 268 Grosvenor Street, London, Ontario, Canada
Tel: 519 646 6000; Ext: 64219
Fax: 519 646 6116
Email: sdhir2@uwo.ca
Abstract
Background: Dexmedetomidine has been studied as a perineural adjuvant agent with the intent of extending the sensory block duration of single injection peripheral nerve blocks. We compared the analgesic effect of intravenous dexmedetomidine sedation with propofol for infraclavicular block for upper limb surgery.
Methods: One hundred ASA I-III patients booked for upper limb surgery received either dexmedetomidine loading dose of 0.5 mcg kg-1 over 10 minutes followed by intravenous infusion of 0.1 to 0.5 µg kg-1 hr-1 or propofol 50 to 100 µg kg-1 min-1. After an ultrasound-guided infraclavicular block. We tested the primary hypothesis that dexmedetomidine extends the sensory block duration. Intraoperative haemodynamic stability, motor block duration, 24h opioid use, pain scores, postoperative nausea and vomiting, satisfaction and sleep disturbances were also evaluated.
Results: Seventy-seven patients (dexmedetomidine group: 37, propofol group: 40) were analyzed. The median time for sensory block was 833 (720-1008) min in the dexmedetomidine and 722 (601-945) min in the propofol group (p=0.14). There was higher incidence of bradycardia in dexmedetomidine group during the first 15 min of infusion (p= 0.007). All other secondary outcomes were not different.
Conclusion: When compared to propofol, intravenous dexmedetomidine after infraclavicular block provided satisfactory intraoperative sedation with sensory block prolongation of approximately 2 hours.
Keywords
Analgesia; Anesthetics; Bradycardia; Dexmedetomi- dine; Nerve block; Propofol
Introduction
Dexmedetomidine, a newer α-2 adrenoceptor agonist has been studied as an adjuvant with the intent of extending the duration of analgesia of single-shot peripheral nerve blocks. Several systematic reviews and meta-analyses have shown that dexmedetomidine may prolong the peripheral nerve and neuraxial block [1,2] when used by different routes. There are a few reports on the extension of the sensory and motor block duration after spinal anesthesia [3,4] and peripheral nerve blocks with intravenous [5] or perineural [6] dexmedetomidine. Use of several other agents (intravenous or perineural) was inconclusive [7] or resulted in unacceptable side effects [8,9].
This Randomized Controlled Trial (RCT) was planned to compare intravenous dexmedetomidine effects, when given as a single sedative agent for upper limb surgery with propofol, the standard sedative for Monitored Anesthesia Care (MAC) at our centre. The primary outcome measure was the absolute increase in the sensory block dura- tion after Infraclavicular Brachial plexus block (ICB) with intravenous dexmedetomidine as the sole intraoperative sedative drug.
Methods
The Institutional Review Board of Western University (REB 104572) approved this RCT and clinicaltrials.gov registered it (NCT01981369; principal investigator: SD). We prepared this report in agreement with the guidelines of Consolidated Standards of Reporting Trials (CONSORT) [10]. Between January 2014 and January 2016, after written and informed consent, we enrolled 100 English speaking; ASA status 1-3 participants aged 18-80 years scheduled for unilateral upper limb surgery with an ICB at St. Joseph’s Hospital, London, Ontario. Exclusion criteria included contraindications to nerve block including coagulopathy, neurological deficits of the upper arm, narcotic dependence (opioid intake equivalent of 60 mg of morphine daily for more than 3 months), associated significant cardiac or respiratory disease, allergy to any study drugs, BMI>35 kg m-2 and scheduled duration of surgery greater than 3 hours.
Randomization and blinding: For this randomized, parallel-arm, prospective, double blind, superiority clinical trial, the patients were assigned to one of two groups (DEX and PROP) with an allocation ratio of 1:1. Randomization chart was generated using web-based Randomness and Integrity Services (Random.org, Dublin) by an investigator with no further study involvement. Operating room anesthesiologist, the only unblinded person in the study received a sealed opaque envelope with the concealed allocation sequence after the block was successful. The patient, anesthesiologist performing the block, operating room staff and the co-investigators were unaware of the group assignment.
Perioperative procedure
Block procedure: All blocks were performed in a dedicated block room. Routine standard monitoring and care included pulse oximetry, ECG, non-invasive blood pressure and 6-8 L min-1 oxygen via face mask. Subsequent to intravenous access on the non-operative side, all patients received midazolam 0.025-0.05 mg kg-1 for anxiolysis, if needed. An ultrasound guided single injection ICB with 0.5% ropivacaine 35 ml was done using a 13-6 MHz linear probe (HFL38, Sonosite, M-Turbo, Bothell, WA), and an in-plane paracoracoid technique. At 30 min, sensory block was tested with ice in all relevant dermatomes (C5-T1) by comparing with the contralateral limb. The grading of the sensory block was on a 3-point scale with ice (0=normal sensation, 1=partial loss of sensation to cold, touch present and 2=complete loss of cold and touch). If the ICB was incomplete, the unblocked terminal branch was blocked with 5 ml of 2% lidocaine.
Intraoperative care: Patients were moved to the operating room once the block was considered complete. Those allocated to the DEX group received an initial loading dose of intravenous 0.5 µg kg-1 dexmedetomidine (100 µg/ml preservative free dexmedetomidine hydrochloride, Precedex, Hospira Inc, Canada) over a 10-minute period followed by an intravenous infusion of 0.1 to 0.5 µg kg-1 h-1. The PROP Group received intravenous propofol infusion (Propofol, Fresenius Kabi, Canada) 50-100 µg kg-1 min-1. During the surgical procedure, vital signs and Ramsay sedation score (RSS) [11] were recorded every 15 minutes. The use of any other drugs was also noted.
Postoperative care: After surgery, patients were moved to Post Anesthesia Care Unit (PACU) where the Verbal Rating Scale (VRS) for pain at rest and movement (0=no pain at all, 10=worst possible pain), level of sensory and motor block (0=no block, 2=complete block) and presence of post-operative nausea and vomiting (PONV) (0=none, 1=mild, 2=moderate, 3=severe) were recorded. After 24 hours, all patients received a phone call from one of the investigators who asked preset questions regarding return of sensory and motor functions, pain scores, PONV, first 24h opioids use, number of times they were woken up at night due to pain and satisfaction with the anesthesia technique and pain management.
Power analysis
We calculated the sample size based on the duration of ropivacaine block (827±175 min) from an earlier study [12]. For a type 1 error of
0.05 and power of 90%, the requisite was 74 patients (37 in each arm) to detect a clinically meaningful increase in duration of sensory block of 120 min (superiority margin) between groups. In consideration of a 25% dropout rate, we increased the sample size to 50 per group [13].
Statistical analysis
We used per-protocol analysis but also added modified Intention-To-Treat (ITT) principle for the primary outcome. Patient and surgical characteristics were analyzed using descriptive statistics. For Frequency and percentage was used for dichotomous variables and medians with Interquartile Ranges (IQR) for continuous variables. We used Fisher’s exact test or chi-square test for categorical variables and unpaired t-test when comparing changes from baseline among groups. Cochrane-Armitage Trend Test and Wilcoxon two-sample tests were used to compare PONV scores, number of times patient woke up at night (as a surrogate for sleep quality), Ramsay sedation score, sensory and motor block scores, pain at rest and movement on arrival to PACU and sensory and motor recovery, total dose of opioids used and satisfaction at 24 h between groups. P ≤ 0.05 was considered statistically significant. We used SAS 9.4 (Copyright © 2002-2012 by SAS Institute Inc., Cary, NC, USA) for analysis.
Results
We assessed 115 patients for eligibility and randomized 100 patients between the two groups. Eighty-eight patients received the intervention (DEX: 42, PROP: 46) and 77 patients (DEX: 37, PROP: 40) completed follow-up on the Post-Operative Day One (POD1). The flow diagram shows patient progression through the study (Figure 1). The patient characteristics were similar and there were no statistically or clinically meaningful differences amongst the two groups (Table 1) There was a statistically higher incidence of bradycardia during the first 15 minutes of infusion in the DEX group (15 min change from baseline 8.5 vs 2.4, p=0.007, 30 min change from baseline 8.8 vs 4.3, p= 0.064, 45 min change from baseline 9.1 vs 5.0, p= 0.117, 60 min change from baseline 9.3 vs 6.3, p= 0.267). The use of vasopressors or anticholinergics between groups was not different. Both groups had similar intraoperative monitoring parameters and sedation scores with no episodes of apnea or desaturation. Rest and movement pain scores, PONV and RSS on arrival to PACU were also similar in both groups.

Figure 1: CONSORT flow chart.
Table 2 shows postoperative day 1 outcomes. The median sensory block duration in the DEX group was 833 min [IQR: 720-1008 min] compared to 722 min [IQR: 601-945 min] in the PROP group (Figure 2). This difference of 111 min between the groups was not significant, statistically (p=0.14). With ITT analysis, the median duration of sensory block for DEX group was 795 min (IQR: 582-977) compared to 720 min (IQR: 431-888) in the PROP group (p=0.21). The motor block duration after the ICB, the 24 h morphine equivalent consumption, presence of PONV and sleep disturbance did not show any significant differences between the two groups (Table 2). The overall satisfaction after POD1 was 100% for both groups.
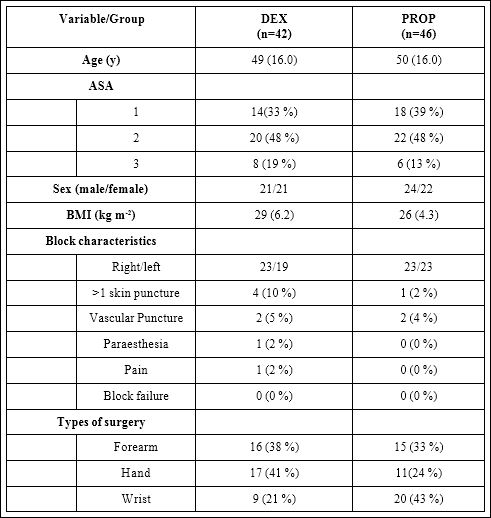
Table 1: Patients’ demographics and perioperative characteristics.
BMI: Body Mass Index
Values are represented as mean (SD) or number of subjects (%) when indicated
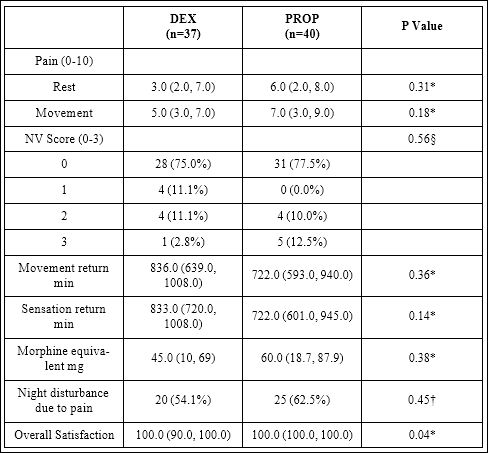
Table 2: Post-operative day 1 outcomes.
Values are represented as median (IQR) or number of subjects (%) when indicated
P value reflects: † Chi-square test, * Mann Whitney test, § Cochrane-Armitage Trend Test
Discussion
This prospective, double blind, parallel arm, randomized superiority study shows that the median duration of sensory block (primary outcome) increased by 111 minutes (15.4%) when dexmedetomidine was compared to propofol for intraoperative sedation after ICB. Though not statistically significant, the block prolongation of almost two hours was clinically relevant.

Figure 2: Sensory block duration in minutes Boxes represent median and 25th-75th percentile and whiskers extend to a maximum of 1.5 interquartile ranges. Outliers (more than 1.5 box lengths are represented by circles).
Other studies have reported similar findings where either intravenous or perineural dexmedetomidine was used as an adjuvant. On comparing to placebo, Abdallah and others found an increase in the sensory block (interscalene) duration of 4.2 hours with perineural and 3.1 hours with intravenous dexmedetomidine in patients undergoing shoulder surgery [14].
A 2017 systematic review analyzed 32 trials with 2007 patients and found that perineural dexmedetomidine prolonged the sensory block duration by 57% compared to control [6]. Other studies have shown prolonged duration of spinal block when used as a sedative with decreased need for opioids and increased patient satisfaction [15,16]. Based on these findings, it appears that the effects of dexmedetomidine may not depend on the route of administration [3,4,17].
It is proposed that blockade of ‘hyperpolarization-activated cation current’ (Ih) is the potential cause of the adjuvant effect of dexmedetomidine on the nerve block [18]. The Ih current involved in cell excitability has both central and peripheral actions. A α-2 adrenoceptor agonist can block this current and augment hyperpolarization and inhibit the action potential. In animal studies, Brummett and colleagues reversed the dexmedetomidine effect on the local anesthetic block using a Ih channel enhancer but not with the α-2 receptor antagonist [19,20]. The peripheral effects appear unclear but may be due to supraspinal and/or centrally mediated mechanisms, α-2B adrenoceptor related vasoconstrictive effects, inflammatory response decline and explicit action on a peripheral nerve [21]. Whether the aforesaid mechanisms are effective with intravenous use of the drug remains unknown. It is also postulated that sensory but not motor conduction is inhibited as motor fibres may need higher concentrations than unmyelinated C fibres [22]. However, we found an increase in the motor block by 114 minutes (15.8%) in a fashion similar to sensory block. However, this was not a primary outcome measure and study was not powered for this parameter.
Dexmedetomidine does not produce significant respiratory depression. It may offer amnesia, reduce anxiety and improve analgesia. Upper limb surgeries with blocks as the main anesthetic technique benefit from intravenous sedation during the procedure by reducing anxiety and providing greater comfort to patients [23]. We found a higher tendency of bradycardia in the dexmedetomidine group only during the first 15 minutes of infusion. This could be due to the initial loading dose. However, we did not observe any difference in the use of vasopressor or chronotropic therapy or changes in any other hemodynamic parameters. Different studies have reported similar results when both agents were compared [24]. The negative chronotropism is a well-known effect of α-2 agonists and other authors have reported it [25,26].
This superiority trial failed to find a significant difference among the two groups therefore we could have established the lesser objective of noninferiority but we did not have a prospective predefined margin for it. Nevertheless, our data may provide a quantitative estimate of the minimum estimated effect for future studies. We used per-protocol analysis that is considered conservative and convincing in a superiority trial [27] but we also obtained similar conclusions regarding the primary outcome with ITT principle.
Our study is subject to a few limitations. First, patients were moved to the operating room once the block was well established. Therefore, there was a delay of at least 30 min in starting the dexmedetomidine or propofol infusion. We are uncertain if the results would have been different if the dexmedetomidine was started at the time of the block. Second limitation was the 24h follow-up. We do not know if a longer follow up could identify other effects related to dexmedetomidine. No long-term studies regarding neurotoxicity of dexmedetomidine on the peripheral nerve exist. Future studies could address effectiveness of the adjuvants therapy and its long-term effects. In addition, sensory block duration was the primary target of this study. It was not tested at pre-specified time intervals postoperatively as the block recession happened when the patients were at home, after hours. Within the constraints of our patient care facility and the prolonged effects of the drug ropivacaine, we used patient recall to capture the return of pain and sensations as a surrogate for the sensory block duration. This was not ideal and recall bias may have been a methodological issue. Furthermore, we did not perform a cost analysis though there are reports of decrease in costs [28], and fiscal assessment is needed. Final, and above all, reported ropivacaine block duration is very variable with authors reporting anything between 4.8 and 14h [29-32]. Probable causes may include drug volume, concentration, type of block, nerve localization instrument used, known and unknown adjuvants, individual variations in techniques and other unknown factors. With such variability, it is unsure if results from one ropivacaine study with or without adjuvants could be applied to another.
Dexmedetomidine is not authorized for perineural use, anywhere in the world. ‘Off label’ use of regional anesthetic drugs may disclose neurotoxic features of the non-medicinal ingredients and until further data becomes available, it is recommended that regional anesthesiologists consider intravenous administration to increase the block duration prior to attempting perineural route [33].
In conclusion, our results suggest that intravenous dexmedetomidine provide satisfactory sedation during surgery with non-significant but clinically relevant prolongation of sensory block duration after single injection ICB.
Authors’ contributions
- Study concept and design: LMLV, PTM, SD
- Randomization: SD
- Data acquisition: LMLV, PTM
- Data interpretation, reanalysis or both: LMLV, AB, SD
- Writing and revising the manuscript: LMLV, AB, PTM, SD
- Final approval of the manuscript: LMLV, PTM, AB, SD
- Agreement to be accountable for all aspects of the work: LMLV, AB, PTM, SD
- Reproducible science: full protocol and raw data available from Dhir: sdhir2@uwo.ca
Acknowledgments
- Department of Anesthesia and Perioperative Medicine: support in the development of the study
- Larry Stitt: help with statistical analysis
- Janice Yu: for data collection and database help
- Daniel Cuillerier: for editing
Declaration of Interests
- Presented in part at the Canadian Anesthesiologist Society annual meeting, Niagara Falls, Canada, 23-26 June
- Funding: None
- Conflict of Interest: None
References
- El-Boghdadly K, Brull R, Sehmbi H, Abdallah FW (2017) Perineural Dex- medetomidine Is More Effective Than Clonidine When Added to Local Anesthetic for Supraclavicular Brachial Plexus Block: A Systematic Review and Meta-analysis. Anesth Analg 124: 2008-2020.
- Gupta K, Tyagi V, Gupta PK, Rastogi B, Jain M, et al. (2016) Monitored anesthesia care with propofol or dexmedetomidine for patients undergoing upper limb surgeries under brachial plexus blockade: a comparative Ain-Shams J Anaesthesiol 9: 563-568.
- Abdallah FW, Brull R (2013) Facilitatory effects of perineural dexmedeto- midine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth 110: 915-925.
- Kaya FN, Yavascaoglu B, Turker G, Yildirim A, Gurbet A, et (2010) In- travenous dexmedetomidine, but not midazolam, prolongs bupivacaine spinal anesthesia. Can J Anaesth 57: 39-45.
- Rutkowska K, Knapik P, Misiolek H (2009) The effect of dexmedetomidine sedation on brachial plexus block in patients with end-stage renal disease. Eur J Anaesthesiol 26: 851-855.
- Vorobeichik L, Brull R, Abdallah FW (2017) Evidence basis for using peri- neural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled Br J Anaesth 118: 167-181.
- Kirksey MA, Haskins SC, Cheng J, Liu SS (2015) Local Anesthetic Periph- eral Nerve Block Adjuvants for Prolongation of Analgesia: A Systematic Qualitative PLoS One 10: 0137312.
- Mannion S, Hayes I, Loughnane F, Murphy DB, Shorten GD (2005) Intra- venous but not perineural clonidine prolongs postoperative analgesia after psoas compartment block with 0.5% levobupivacaine for hip fracture sur- gery. Anesth Analg 100: 873-878.
- Pöpping DM, Elia N, Marret E, Wenk M, Tramèr MR (2009) Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks: a meta-analysis of randomized Anesthesiology 111: 406-415.
- Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 statement: up- dated guidelines for reporting parallel group randomised trials. BMJ 340:332.
- Jonghe BD, Cook D, Appere-De-Vecchi C, Guyatt G, Meade M, et al. (2000) Using and understanding sedation scoring systems: a systematic Intensive Care Med 26: 275-285.
- Yang CW, Kwon HU, Cho CK, Jung SM, Kang PS, et al. (2010) A compari- son of infraclavicular and supraclavicular approaches to the brachial plexus using Korean J Anesthesiol 58: 260-266.
- Sakpal TV (2010) Sample Size Estimation in Clinical Trial. Perspect Clin Res 1: 67-69.
- Abdallah FW, Dwyer T, Chan VWS, Niazi AU, Ogilvie-Harris DJ, et al. (2016) IV and Perineural Dexmedetomidine Similarly Prolong the Dura- tion of Analgesia after Interscalene Brachial Plexus Block: A Randomized, Three-arm, Triple-masked, Placebo-controlled Trial. Anesthesiology 124: 683-695.
- Rapchuk IL, Glover P (2013) Combined use of fascia iliaca block, sub- arachnoid block and dexmedetomidine sedation for patients having frac- tured femur J Anesth 27: 149-150.
- Coskuner I, Tekin M, Kati I, Yagmur C, Elcicek K (2007) Effects of dexme- detomidine on the duration of anaesthesia and wakefulness in bupivacaine epidural Eur J Anaesthesiol 24: 535-540.
- Abdallah FW, Abrishami A, Brull R (2013) The facilitatory effects of intra- venous dexmedetomidine on the duration of spinal anesthesia: a systematic review and meta-analysis. Anesth Analg 117: 271-278.
- Dalle C, Schneider M, Clergue F, Bretton C, Jirounek P (2001) Inhibition of the Ih current in isolated peripheral nerve: A novel mode of peripheral antinociception? Muscle Nerve 24: 254-261.
- Brummett CM, Williams BA (2012) Additives to Local Anesthetics for Pe- ripheral Nerve Int Anesthesiol Clin 49: 104-116.
- Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R (2011) Perineu-ral dexmedetomidine added to ropivacaine for sciatic nerve block in ratsprolongs the duration of analgesia by blocking the hyperpolarization-acti-vated cation current. Anesthesiology 115: 836-843.
- Leem JW, Choi Y, Han SM, Yoon MJ, Sim JY, et (2000) Conduction block by clonidine is not mediated by alpha2-adrenergic receptors in rat sciatic nerve fibers. Reg Anesth Pain Med 25: 620-625.
- Al-Metwalli RR, Mowafi HA, Ismail SA, Siddiqui AK, Al-Ghamdi MA, et (2008) Effect of intra-articular dexmedetomidine on postoperative anal- gesia after arthroscopic knee surgery. Br J Anaesth 101: 395-399.
- Höhener D, Blumenthal S, Borgeat A (2008) Sedation and regional anaes- thesia in the adult Br J Anaesth 100: 8-16.
- Kumar A, Sinha C, Kumar A, Kumari P (2017) The Effect of Intravenous Dexmedetomidine Compared to Propofol on Patients Hemodynamics as a Sedative in Brachial Plexus Block: A Comparative Anesth Essays Res 11: 201-205.
- Park JY (2017) Factors to bear in mind regarding the use of dexmedetomi- Korean J Anesthesiol 70: 233-234.
- Nguyen V, Tiemann D, Park E, Salehi A (2017) Alpha-2 Agonists. Anesthe- siol Clin 35: 233-245.
- Snapinn SM (2000) Noninferiority trials. Curr Control Trials Cardiovasc Med 1: 19-21.
- Maldonado JR, Wysong A, van der Starre PJA, Block T, Miller C, et al. (2009) Dexmedetomidine and the reduction of postoperative delirium af- ter cardiac Psychosomatics 50: 206-217.
- Greengrass RA, Klein SM, D’Ercole FJ, Gleason DG, Shimer CL, et al. (1998) Lumbar plexus and sciatic nerve block for knee arthroplasty: com- parison of ropivacaine and Can J Anaesth 45: 1094-1096.
- Kaur A, Singh RB, Tripathi RK, Choubey S (2015) Comparision between bupivacaine and ropivacaine in patients undergoing forearm surgeries un- der axillary brachial plexus block: a prospective randomized study. J Clin Diagn Res 9: 1-6.
- Droog W, Lin DY, Huisman JS, Franssen FA, van Aggelen GP, et al. (2017) Individual duration of axillary brachial plexus block is unpredictable: a prospective double centered observational study. Minerva Anestesiol 83: 1146-1151.
- Klein SM, Greengrass RA, Steele SM, D’Ercole FJ, Speer KP, et al. (1998) A comparison of 0.5% bupivacaine, 0.5% ropivacaine, and 0.75% ropivacaine for interscalene brachial plexus Anesth Analg 87: 1316-1319.
- Martinez V, Fletcher D (2014) Dexamethasone and peripheral nerve blocks: on the nerve or intravenous? Br J Anaesth 113: 338-340.
Citation: Lopera-Velasquez LM, Builes A, Magsaysay PT, Dhir S (2021) Compari- son of Intravenous Dexmedetomidine with Propofol on the Duration of Infraclavicular Block with Ropivacaine: A Randomized Double-Blind Controlled Trial. J Anes Perio Manag 5: 011
Copyright: © 2021 Lopera-Velasquez LM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


