*Corresponding Author:
Mohamed N Ibrahim,
Department of Diagnostic Radiology, Rotherham General Hospital, Rotherham general, England, UK
Tel: +44 07513331524
Email: mohamednabil2005@gmail.com
Abstract
Bacterial endocarditisis a complex disease that is associated with cardiac-localized infection and multiorgan complications resulting from septic emboli. The diagnosis is defined radiologically and laboratory usually with positive blood culture. We report the case of a 24 years old patient who presented with acute confusion and imaging findings for bacterial endocarditis and aortic root abscess complicated with multiple septic brain emboli and abdominal organs infarcts.
Keywords
Aortic root abscess; Prosthetic aortic valve; Renal and hepatic infarcts; Septic brain emboli; Splenic
Introduction
Septic embolism is an obstruction of a blood vessel, typically by an infected thrombus that travels through the bloodstream from a distant infectious source and blocks a blood vessel. Septic emboli result in two insults-the early embolic/ischemic insult due to vascular occlusion that may lead to infarction and the infectious insult that leads to inflammation and possible abscess formation [1].
Case Report
A 24 years old male presented to the Urgent and Emergency care center with gradual onset of sweating and high temperature for 5 days followed by acute onset of confusion. His past medical history included an aortic valve replacement 5 months ago for correction of congenital aortic stenosis. Medications are aspirin, bisoprolol, warfarin, and lansoprazole.
No known drug allergy. No relevant genetic or psychiatric history.
On examination, he was warm, sweaty, and confused. He was not oriented to the time and could not remember the events from the previous day. The Chest was clear, and heart examination revealed normal 1st and 2nd heart sounds and metallic click. Capillary refill was <2 seconds and the abdomen were soft and not tender. There was no jugular venous distension or peripheral oedema. ECG revealed sinus tachycardia with heart rate=131b/m. and noother acute changes were identified. Blood pressure was 120/70 and temperature 38.2 degree Celsius.
Bedside echocardiography did not show pericardial effusion. His blood tests were: Haemoglobin: 15 g/L (13.2-16.9), white cell count: 12.60109/L (3.7-10) Platelet: 110109/L
(150-450). Neutrophils 11.2 109/L (1.7-6.6) and C-reactive protein 256 mg/L (0-10)
PT: 93 (12.7-15.7), INR: 8.7, APTT: 42.4 (24.4-31.6). No significant growth in urine culture. A chest x-ray was requested instantly (Figure 1).
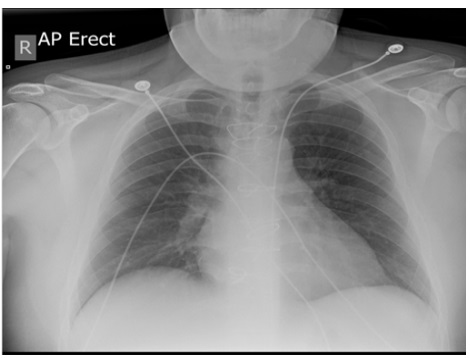
Figure 1: Chest x-ray: No lung consolidation or cavitation. Sternotomy wires and metallic aortic valve.
The urgent CT brain was normal. The patient was initially diagnosed with sepsis and IV antibiotics and fluids were administered as per local protocol. Then He was admitted to the hospital under the medical team who requested an MRI brain (Figure 2).
Based on the previous history of Aortic valve replacement and MRI brain findings, a provisional diagnosis of bacterial endocarditis and septic emboli was suggested by the radiologist. Therefore, the patient was transferred to the Coronary care unit where he was monitored for signs of fluid overload and deterioration of the Glasgow coma scale.
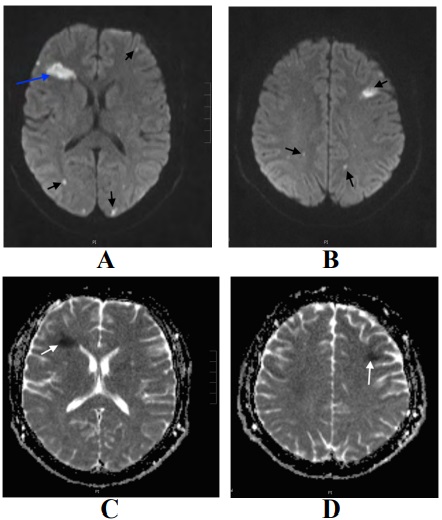
Figure 2: (A and B) MRI brain diffusion-weighted images (B value 1000) demonstrating multiple areas of diffusion restriction in both cerebral hemispheres involving white matter, subcortical areas, and cortex (black arrows). The Largest within the white matter of right frontal lobe (blue arrow) (C and D) ADC map showing signal loss confirming diffusion restriction.
CT chest (Figure 3), abdomen, and pelvis (Figure 4) were done.
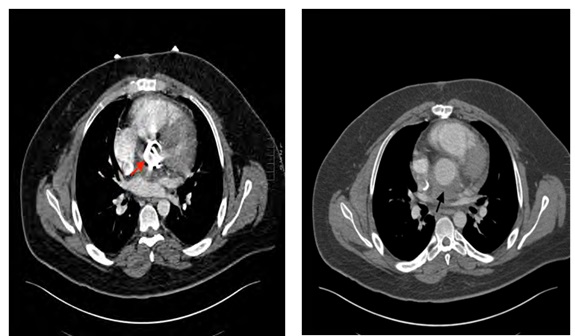
Figure 3: Post-contrast CT chest: revealing metallic aortic valve (red ar- row) and collection surrounding the aortic root denoting abscess (black arrow).
No further echocardiography or MRI heart done. Blood culture results returned the next day showing the growth of gram-negative bacilli Serratia marcescens. The microbiology team advisedIV antibiotics regimen of IV Meropenem 2 gm Tds for 5 days, Vancomycin 1.25 gm, and Linezolid 600 mg IV infusion BD for 4 days. Warfarin continued during hospitalization.
After 5 days in the Coronary care unit, the patient significantly improved and transferred to a medical ward where his inflammatory markers returned to normal. His repeated blood culture did not reveal any growth. Shortly, he was discharged with no neurological deficits and on home intravenous antibiotic for 6 weeks of Meropenem 2 gm IV Tds, Vancomycin 1.25 gm, and Linezolid 600 mg IV infusion BD. Oral medications included beta-blockers, aspirin, and warfarin.No cardiac surgery performedand the patient had outpatient cardiology clinic follow-up.
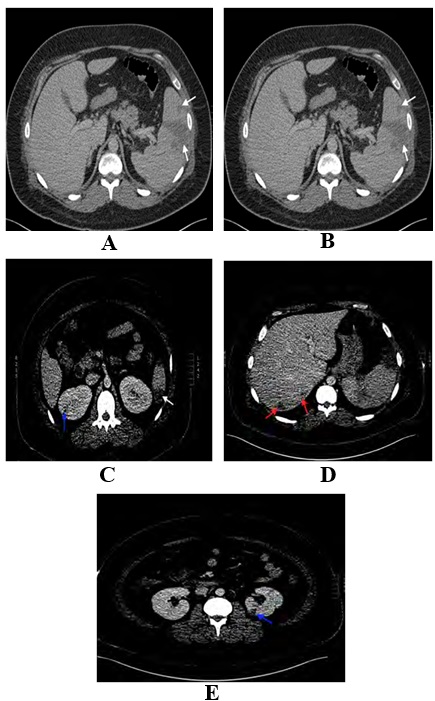
Figure 4: Post-contrast CT abdomen: Wedge-shaped areas of hypodensity reaching splenic capsule consistent with infarctions (white arrows) other smaller hypodense areas involving both renal and hepatic parenchyma in keeping with small infarcts.
Discussion
Infective Endocarditis (IE) of the aortic valve is a serious, life- threatening condition, with a mortality rate of 30% at 1 year. Aortic valve Prosthetic Valve Endocarditis (PVE) has a poor prognosis, with a reported mortality of 20-40%. This is likely due to the higher propensity for infection to extend into the perivalvular tissue compared with native valve endocarditis The risk of PVE is greatest during the 5 years after surgery [2-5].
Mechanical prosthetic valve imaging by echocardiography can be limited by artifacts caused by acoustic shadowing. IE is suspected on a prosthetic valve when there is a perivalvular mass or when valve dehiscence can be demonstrated. ECG-gated CT performs well in the identification of PVE, with one series reporting sensitivity of 93% when compared to surgical findings [6].
The major signs of PVE on CT are the presence of vegetations, thickening of the aortic root wall of>5 mm, the presence of a perivalvular abscess or pseudoaneurysm, and prosthetic valve dehiscence. The latter manifests as a rocking motion of the mechanical valve throughout the cardiac cycle, which can be demonstrated on ECG-gated cardiac CT with multiphase acquisition throughout the cardiac cycle [7].
The CT finding of a markedly thickened area around the aortic root is indicative of an aortic root abscess. Inflammatory changes and pockets of gas may be present. CT also enables crucial anatomic evaluation of the valve, coronary arteries, and aorta for preoperative planning if necessary [8].
A systematic review of the role of non-invasive imaging in the diagnosis of PVE supports the use of CT in addition to echocardiography for improving diagnostic accuracy, especially in cases of life-threatening perivalvular extension [9].
Several studies have demonstrated the ability of CT to demonstrate infective endocarditisrelated complications such as large aortic root abscesses [10].
One of the main limitations of CT in the assessment of PVE is the presence of a streak artifact caused by the high-density valve material. Artifacts caused by valve material often limit the role of Cardiac MR in the diagnosis of mechanical PVE, causing local spin dephasing. Given these limitations, 18 F-FDG PET/CT has emerged as a useful adjunct in the diagnosis of PVE. Studies by Saby et al. and Pizzi et al. demonstrated that the addition of increased tracer uptake around the prosthetic valve on 18 F-FDG PET/CT in cases of suspected PVE increased the sensitivity of the modified Duke criteria from 70 to 97% and 52 to 91%, respectively [11,12].
Septic embolic stroke usually results from vascular occlusion and corresponding degrees of ischemia and infarction, depending on vessel size, location, and collateral blood flow [13].
Cerebral arterial occlusion resulting in either infarction or transient ischemic attack accounts for 40-50% of Central Nervous System (CNS) complications of infective endocarditis [14].
The septic emboli are challenging because they involve three important cerebrovascular conditions: (a) cerebrovascular occlusions, (b) intracerebral abscess, and (c) arterial mycotic aneurysms. The main risk of neurologic complications is the absence of appropriate antibiotic therapy. It is important to note that most neurologic complications are already evident at the time of hospitalization or develop within a few days [15,16].
Septic emboli to the brain are usually diagnosed with MRI with and without gadolinium. F-FDG-PET/CT has proven its high diagnostic value for the detection of peripheral emboli in patients with infective endocarditis and cardiac device infections, substantially affecting patients’ outcomes and treatment. F-FDG-PET/CT is limited for the detection of brain foci, where CTA and/or MRI are mandatory [17].
The choice of antimicrobial therapy in the management of septic emboli targets the causative organisms, the organ involved, and the pharmacokinetics and pharmacodynamics of the available drugs. Anticoagulation has been a controversial topic in the management of infective endocarditis and septic emboli. Continuation of anticoagulation in patients with a definitive pre-existing indication should merit consideration in patients with left-sided infective endocarditis in the absence of other contraindications [18].
The spleen is a particularly easy target for embolism damages owing to the characteristicsof its anatomy and blood circulation. The occlusion of the splenic circulation, even by a sterile embolism can lead to the formation of an abscess. In endocarditis, the presence of pathogens in embolic fragments is sufficient to give rise to a splenic abscess [19].
In CT, the classic image of a splenic infarction is a triangular area with its base in the periphery; its outline is well defined but hypointense, and it is not made more obvious by the use of contrast. However, it is more common to find diffuse, hypointense, and poorly- outlined areas [20].
The involvement of the kidney in endocarditis can be caused by 3 mechanisms: focal or diffuse glomerulonephritis through the deposit of immune-complexes, renal failure, and renal abscess. The most common form of kidney involvement is embolic renal infarction [21].
The liver may also be a target of hematogenous seeding via the arteries. However, the most common cause of hepatic abscesses is related to biliary or colonic disease, gastric or duodenal surgery, local trauma, and pancreatitis [22].
As well as using clinical data, embolisms were diagnosed using abdominal CT, abdominal ultrasound, or a combination of these techniques. The diagnostic performance of both was similar. CT is considered the technique of choice owing to its greater sensitivity and the absence of artiacts in the images produced [23].
Our patient risk factor for bacterial endocarditis was the prosthetic aortic valve. Although his CT brain and bedside echocardiography were normal, prompt empirical IV antibiotics treatment decreased the risk of neurological complications and abdominal visceral abscesses.
MRI brain was diagnostic for septic emboli and CT chest, abdomen and pelvisrevealed aortic root abscess and infarcts in spleen, kidneys, and liver. The patient ideally should undergo repeated echocardiography and ECG gated cardiac CT. Appropriate Antibiotics were given according to blood culture results and the outcome was good.
Conclusion
Bacterial Endocarditis (IE) is a complex disease with cardiac involvement and multiorgan complications. Its prognosis depends on a prompt diagnosis that leads to aggressive therapeutic management combining antibiotic therapy and early cardiac surgery when indicated. Echocardiography, cardiac and extracardiac CT, cerebral magnetic resonance imaging are the main imaging modalities. Our patient demonstrates bacterial endocarditis and aortic root abscess complicated with septic cerebral emboli and abdominal organs infarcts. A Prompt diagnosis allows early treatment and good outcomes.
References
- 1. Stawicki SP, Firstenberg MS, Lyaker MR, Russell SB, Evans DC, et (2013) Septic embolism in the intensive care unit. Int J Crit Illn Inj Sci 3: 58-63.
- 2. Habib G, Badano L, Tribouilloy C, Vilacosta I, Zamorano JL, et (2010) Rec- ommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2: 202-219.
- 3. Wang A, Athan E, Pappas PA, Fowler Jr VG, Olaison L, et al. (2007) Con- temporary clinical profile and outcome of prosthetic valve endocarditis. Jama 12: 1354-361.
- 4. Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, et (2009) Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): The task force on the prevention, diagnosis, and treatment of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for infection and cancer. Eur Heart J 19: 2369-2413.
- 5. Bashore TM, Cabell C, Fowler V (2006) Update on infective endocarditis. Curr Probl Cardiol 31: 274-352.
- 6. Fagman E, Perrotta S, Bech-Hanssen O, Flinck A, Lamm C, et al. (2012) ECG-gated computed tomography: A new role for patients with suspected aortic prosthetic valve Eur Radiol 11: 2407-2414.
- 7. Grob A, Thuny F, Villacampa C, Flavian A, Gaubert JY, et al. (2014) Cardiac multidetector computed tomography in infective endocarditis: A pictorial es- Insights Imaging 5: 559-570.
- 8. Habets J, Budde RP, Symersky P, van den Brink RB, de Mol BA, et (2011) Diagnostic evaluation of left-sided prosthetic heart valve dysfunction. Nat Rev Cardiol 8: 466-478.
- 9. Habets J, Tanis W, Reitsma JB, van den Brink RBA, Mali WPTM, et (2015) Are novel non-invasive imaging techniques needed in patients with suspect- ed prosthetic heart valve endocarditis? A systematic review and meta-analy- sis. Eur Radiol 7: 2125-2133.
- 10. Lentini S, Monaco F, Tancredi F, Savasta M, Gaeta R (2009) Aortic valve infective endocarditis: Could multi-detector CT scan be proposed for routine screening of concomitant coronary artery disease before surgery? Ann Tho- rac Surg 87: 1585-1587.
- 11. Saby L, Laas O, Habib G, Cammilleri S, Mancini J, et al. (2013) Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: Increased valvular 18F-fluorodeoxyglucose uptake as a novel major J Am Coll Cardiol 23: 2374-2382.
- 12. Pizzi MN, Roque A, Fernández-Hidalgo N, Cuéllar-Calabria H, Ferrei- ra-González I, et al. (2015) Improving the diagnosis of infective endocarditis in prosthetic valves and Intracardiac devices With 18F-fluorodeoxyglucose positron emission tomography/computed tomography angiography: Initial re- sults at an infective endocarditis referral Circulation 12: 1113-1126.
- 13. Stawicki SP, Firstenberg MS, Lyaker MR, Russell SB, Evans DC, et (2013) Septic embolism in the intensive care unit. Int J Crit Illn Inj Sci 3: 58-63.
- 14. Özbek C, Yetkin U, Bademci M, Karahan N, Gürbüz A (2008) Ring annu- loplasty and successful mitral valve repair in a staphylococcal endocarditis case with bilobular saccular mycotic aneurysm at cerebral artery and frontal region infarction. Secondary to septic emboli. Archives of Medical Science 4: 94-99.
- 15. American Journal of Neuroradiology (2008) Magnetic resonance imaging of the brain and Lippincott Williams & Wilkins, Pennsylvania, USA.
- 16. Sonneville R, Mourvillier B, Bouadma L, Wolff M (2011) Management of neu- rological complications of infective endocarditis in ICU patients. Annals of Intensive Care
- 17. Mikail N, Benali K, Mahida B, Vigne J, Hyafil F, et al. (2018) 18F-FDG-PET/ CT Imaging to diagnose septic emboli and mycotic aneurysms in patients with endocarditis and cardiac device Curr Cardiol Rep 6: 14.
- 18. Davis KA, Huang G, Petty SA, Tan WA, Malaver D, et (2020) The effect of preexisting anticoagulation on cerebrovascular events in left-sided infective endocarditis. Am J Med 133: 360-369.
- 19. O’Keefe JH, Holmes DR, Schaff HV, Sheedy PF, Edwards WD (1986) Throm- boembolic splenic Mayo Clin Proc 61: 967-72.
- 20. Balcar I (1984) CT patterns of splenic infarction: A clinical and experimental Radiology 151: 723-729.
- 21. Sarriá C, Vilacosta I, San Román JA (2002) Manifestaciones clínicas de la endocarditis infecciosa. In: Vilacosta I, San Román JA (eds). Endocarditis Prous Science, Barcelona, Spain.
- 22. Brook I, Frazer EH (1998) Microbiology of liver and spleen J Med Microbiol 47: 1075-1080.
- 23. Mangoni ED, Adinolfi LE, Tripodi MF, Andreana A, Gambardella M, et al. (2003) Risk factors for major embolic events in hospitalized patients with in- fective Am Heart J 146: 311-316.
Citation:Ibrahim MN (2021) Bacterial Endocarditis and Aortic Root Abscess Com- plicated with Multiple Septic Brain Emboli and Abdominal Visceral Infarcts: Case Report and Review. J Case Repo Imag 5: 030.
Copyright: © 2021 Ibrahim MN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


