*Corresponding Author:
Esan Ayodele J,
Hematology and Blood Transfusion Department, Federal Teaching Hospital, Ido-Ekiti, Nigeria
Tel: +2348035477756
E-mail: ayodelejacob4u@gmail.com, ayodelejacob4u@yahoo.com
Abstract
Background: Anaemia decreases survival, independently accelerates disease progression, and increases mortality among HIV-infected individuals. The association between anaemia and decreased survival has been found to be independent of CD4+ T-lymphocyte count, and plasma HIV RNA concentration.
Aim: To assess the severity of anaemia using immunovirological and erythropoietin level among HIVinfected patients.
Methodology: This study was carried out at Federal Teaching Hospital, Ido Ekiti. One hundred samples each was collected from HIV positive subjects on ART and ART naïve. Six milliliters (6ml) of whole blood was collected from each consented subject, 3ml was dispensed into K2EDTA bottle for immediate analysis of Haemoglobin concentration using haematology analyzer, HIV screening was carried out using serial algorithm, CD4 count and CD8 count using flow cytometer. The remaining 3ml of blood was dispensed into plain bottle, serum was extract for the analysis of erythropoietin using ELISA and viral load using PCR.
Result: Mean values of CD4, CD8, CD4/CD8 and EPO in Hb<8.0g/ dl were lower compared to 8.0-9.9g/dl, Hb 10.0-12.9g/dl, and Hb > 13.0g/dl. Mean values of VL in Hb<8.0g/dl were higher compared to 8.0-9.9g/dl, Hb 10.0-12.9g/dl, and Hb > 13.0g/dl among ART-naïve and ART subjects.
Conclusion: Haemoglobin levels reflect rapidity of disease progression rates and independently predict prognosis, increases in haemoglobin level are predictive of treatment success. This present study showed the severity of HIV infection as haemoglobin level reduces, the rates of haemoglobin decrease is correlate with CD4 count, CD8 count, CD4/CD8 ratio, and erythropoietin decreases with increases in viral load.
Keywords
Anaemia; Erythropoietin and HIV-infected; Immunovirological; Severity
Abbreviations
ART: Antiretroviral Therapy
CD4: Cluster of Differentiation 4
CD8: Cluster of Differentiation 8
EPO: Erythropoietin
HB: Haemoglobin Concentration
K2EDTA: Di-potassium ethylene enthylene-diamine tetra-acetic acid
SD: Standard Deviation
VL: Viral Load
Introduction
Anaemia is the most common haematological complication associated with HIV, which increased as HIV disease progresses [1]. Anaemia is independently associated with decreased quality of life, accelerated HIV disease progression, and increases mortality in HIV-infected individuals. Survival time in HIV-infected individuals may be improved with recovery from anaemia [2]. The influence of HIV disease on anaemia starts, before patients are eligible for ART. Risk of anaemia increased by low CD4+ cells counts (<200 cells/mL), and higher HIV RNA levels in plasma. A low CD4 cell count has a strong independent association with anaemia even after controlling for opportunistic infections and malnutrition [3].
This association may represent anaemia caused by the HIV infection itself, which may inhibit erythropoiesis directly through infection of progenitor cells or upregulation of cytokines [4]. Erythropoietin level in HIV-infected patients showed that the levels of erythropoietin failed to rise commensurate with increasing anaemia, suggested that insufficient amounts of erythropoietin cause anaemia due to direct effect of HIV infection on marrow progenitor cells [5]. The mechanism of anaemia in this study is depressed bone marrow function by HIV infection leading to low production of erythropoietin which resulted into ineffective production of RBC [6].
Anaemia in patients with CD4 cell counts<200 cells/µL has implications for the choice of initial ART regimen, since anaemia is an adverse effect of zidovudine (AZT), use of this drug is contraindicated in HIV-patients with preexisting anaemia. Inability to achieve an increase in CD4 count in the context of sustained virological suppression is also known as immuno-virological discordance; this risk was greatly reduced with longer time spent with a suppressed viral load suggesting that maintaining a suppressed viral load is key for patients with immuno-virological discordance [7]. Viral load is associated with loss of CD4 cells, viral load>100000 copies/ml shows the probability of rapid CD4 cell count decline is very high.
VL is the earliest indicator of treatment success or failure, followed by CD4 cell count approximately a month later. Virological response and immunological failure occurs; consequently, VL should be seen in combination with CD4 cell count. Failure to decrease viral load to 400-1000 copies/ml in 4 to 8 weeks, it means there is a risk of virological failure. Low viral load (1000-5000 copies/ml) indicates slow progression, high viral load (>100000 copies/ml) indicates a high risk for rapid progression [8]. CD4 cell count response on its own can be used as an indicator of treatment failure or success. On average, a CD4 cell increase of about 150 cells/mm³ occurs in the first year in treatment-naive patients.
Failure to increase CD4 cell count more than 50 cells/mm³ during the first year of ART is considered immunological failure [9]. If the CD4 cell count does not increase for six months, adherence to treatment should be reassessed and ensured. Viremia less than 10000 copies/ml or suppression of at least 1.5 log copies/ml less than the pre-therapy value, was not associated with a decline in CD4 cell count. Successful ART is first reflected by the decrease of viral load; immunological response is a result of viral load, and thus occurs later [10].
ART monitoring is best done with viral load and CD4 count immune and virological responses of patients that had CD4 count <200 cells/ml were at increased risk of death due to occurrence of opportunistic diseases and high degree of anaemia. The aim of this study was to assess the severity of anaemia using immunovirological and erythropoietin level among HIV- infected patients.
Materials and Methods
Study design
This study was carried out at Federal Teaching Hospital, Ido Ekiti, Nigeria. One hundred samples each was collected from HIV positive subjects on ART and HIV positive subjects ART naïve. Consented subjects were re-screened for HIV infection for the purpose of the study to confirm their HIV status using serial algorithm method. Subject’s consent was sort for through an informed consent form and ethical approval was obtained from Federal Teaching Hospital, IdoEkiti.
Sample collection and sample preparation
Six milliliters (6ml) of whole blood was collected from each consented subject, 3ml was dispensed into K2EDTA bottle for immediate analysis of Haemoglobin concentration, CD4 count, CD8 count and HIV screening. The remaining 3ml of blood was dispensed into plain bottle, allowed to clot and centrifuged at 2500 revolution per minute for 5minutes to extract the serum into another plain bottles, stored at -40 0C for the analysis of erythropoietin and viral load.
Methodology
HIV screening test
Human immunodeficiency virus was diagnosed using serial algorithm method. Determine HIV-1/2 (Abbott Diagnostic Division, Belgium/Luxemburg), Uni-Gold HIV Kit (Trinity Biotech, Wicklow Bay, Ireland) and Chembio HIV ½ Stat-PakTM Assay. Patients reactive to antibody screening tests were considered positive and recruited into the study; the test was carried out according to the manufacturer’s instruction.
Haemoglobin concentration
Haemoglobin concentration was analyzed using Haematology Analyzer (Sysmex XN 350 five parts) following Manufacture’s instruction.
Analysis of CD4 and CD8 count using flow cytometry (Cyflow Counter)
Research samples for CD4 and CD8 count was prepared and run on the Partec cyflow counter (Partec flow cytometer, GMBH, Munster, Germany) according to the manual instructions.
Viral load analysis
Extracted plasma from K2EDTA sample was used to estimate HIV- RNA viral load analysis using polymerase Chain Reaction (PCR), the procedure was follow as describe in the manual.
Erythropoietin
Erythropoietin (EPO) was estimated using Enzyme-Linked ImmunoSorbent Assay (ELISA) kit, the procedure was followed as described in the manual [11].
Results
Table 1 showed comparison of CD4, CD8, CD4/CD8, VL and EPO on degree of anaemia in HIV infected subjects ART naïve. The mean ± SD of CD4 214.73 ± 91.71 in Hb<8.0g/dl was significantly lower (P<0.05) compared to 388.68 ± 49.41, 407.50 ± 59.26 and 493.00 ± 21.37 in Hb 8.0- 9.9g/dl, 10.0-12.9 g/dl and Hb ≥ 13.0 g/dl respectively (F-value50.79; P- value 0.00).
The mean ± SD of CD8 653.16 ± 193.22 in Hb<8.0g/dl was significantly lower (P<0.05) compared to 960.53 ± 110.69, 984.77 ± 93.01 and 996.33 ± 113.19 in Hb 8.0-9.9g/dl, 10.0-12.9g/dl and Hb ≥13.0g/dl respectively (F-value 33.03; P-value 0.00).
The mean ± SD of CD4/CD8 0.34 ± 0.07 in Hb<8.0g/dl was significantly lower (P<0.05) compared to 0.42 ± 0.04, 0.42 ± 0.69, and 0.53 ± 0.06 in Hb 8.0-9.9g/dl, 10.0-12.9g/dl and Hb ≥ 13.0g/dl respectively (F-value 16.88; P-value 0.00).
The mean ± SD of VL 167440.00 ±100586.00 in Hb 8.0g/dl was significantly higher (P<0.05) compared to 91887.00 ± 20948.21, 77841.00 ± 13656.77 and 75103.00 ± 2362.76 in Hb 8.0-9.9g/dl, 10.0-12.9g/dl and Hb ≥ 13.0g/dl respectively (F-value 9.85; P-value 0.00).
The mean ± SD of EPO 1.67 ± 0.47 in Hb<8.0g/dl was significantly lower (P<0.05) compared to 2.38 ± 0.73, 3.15 ± 0.37 and 3.40 ± 0.30 in Hb 8.0-9.9g/dl, 10.0-12.9g/dl and Hb ≥ 13.0g/dl respectively (F-value 52.43; P-value 0.00). Multiple comparison between Hb 8.0-9.9g/dl and 10.0-12.9g/dl shows that, mean ± SD of CD4, CD8 and CD4/CD8 in Hb 8.0-9.9g/dl were lower compared to Hb 10.0-12.9g/ dl although the difference was not significant (P>0.05). Mean ± SD of VL in Hb 8.0-9.9g/dl was significantly higher (P<0.05) compared to Hb 10.0-12.9g/dl.
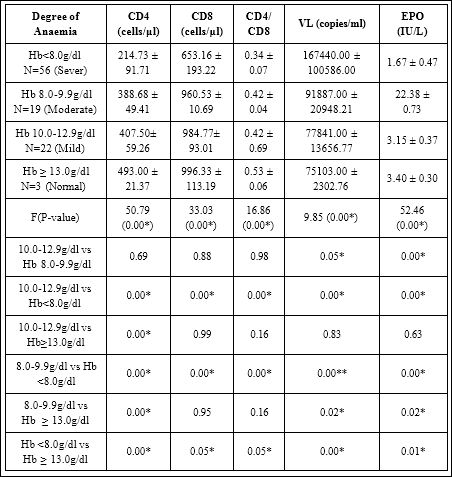
Table 1: Mean + SD Of CD4, CD8, CD4/CD8 And Epo On Degree Of Anaemia In Hiv Art-Naïve Subjects.
Mean values of CD4, CD8, CD4/CD8 and EPO in Hb<8.0g/dl were lower compared to 8.0-9.9g/dl, Hb 10.0-12.9g/dl, and Hb ≥ 13.0g/dl. Mean values of VL in Hb<8.0g/ dl were higher compared to 8.0-9.9g/dl, Hb 10.0-12.9g/dl, and Hb ≥ 13.0g/dl among ART-naïve subjects. P ≤ 0.05 was considered significant, p>0.05 was considered not significant, F-value = mean ± SD of parameters was compared using ANOVA
Table 2 showed comparison of immunological, virological and erythropoietin response on degree of anaemia in HIV-Infected subjects on ART parameters include CD4, CD8, CD4/CD8 VL and EPO. The Mean ± SD of CD4 100.00 ± 81.95 in Hb<8g/dl (Sever Anaemia) was significantly lower (P<0.05) compared to 250.50 ± 3.54, 325.63 ± 76.70 and 528.37 ± 80.54 in Hb 8.0-9.9g/dl (Moderate), Hb 10.0-12.9g/dl (Mild) and Hb > 13.0g/dl (Normal) respectively (F-value 136.79; P-value 0.00).
Mean ± SD of EPO in Hb 8.0-9.9g/dl was significantly lower (P<0.05) compared to Hb 10.0-12.9g/dl. Multiple comparison between Hb<8.0g/dl and Hb 10.0-12.9g/dl shows that, mean ± SD of CD4, CD8, CD4/CD8 and EPO in Hb <8.0g/dl were significantly lower (P<0.05) compared to Hb 10.0-12.9g/dl.
Mean ± SD of VL in Hb<8.0g/dl was significantly higher (P<0.05) compared to Hb 10.0-12.9g/dl. Multiple comparison between Hb 10.0-12.9g/dl and Hb ≥ 13.0g/dl shows that, CD4 in Hb 10.0-12.9g/dl was significantly lower (P<0.05) compared to Hb ≥ 13.0g/dl.
Mean ± SD of CD8, CD4/CD8, and EPO in Hb 10.0-12.9g/dl were lower compared to Hb ≥ 13.0g/dl although the difference was not significant (P>0.05).
Mean ± SD VL in Hb 10.0-12.9g/dl was higher compared to Hb ≥ 13.0g/dl; the difference was not significant (P> 0.05). Multiple comparison between Hb<8.0g/dl and Hb 8.0-9.9g/dl shows that mean ± SD of CD4, CD8, CD4/CD8 and EPO in Hb 8.0g/dl were significantly lower (P<0.05) compared to Hb 8.0-9.9g/dl.
Mean ± SD of VL in Hb<8.0g/dl was significantly higher (P<0.05) compared to Hb 8.0-9.9g/dl. Multiple comparison between Hb 8-9.9g/dl and Hb ≥ 13.0g/dl shows that mean ± SD of CD4 and EPO in Hb 8.0-9.9g/dl were significantly lower (P<0.05) compared to Hb ≥ 13.0g/dl, mean ± SD of VL in Hb 8-9.9g/dl was significantly higher (P<0.05) compared to Hb ≥ 13.0g/dl.
Mean ± SD of CD8 and CD4/CD8 in Hb 8-9.9g/dl were lower compared to Hb ≥ 13.0g/dl although the difference was not significant (P>0.05). Multiple comparison between Hb<8.0g/dl and Hb ≥ 13.0g/dl shows that mean ± SD of CD4, CD8, CD4/CD8 and EPO in Hb<8.0g/dl were significantly lower (P<0.05) compared to Hb ≥ 13.0g/dl. Mean ± SD of VL in Hb<8.0g/dl was significantly higher (P<0.05) compared to Hb ≥ 13.0g/dl.
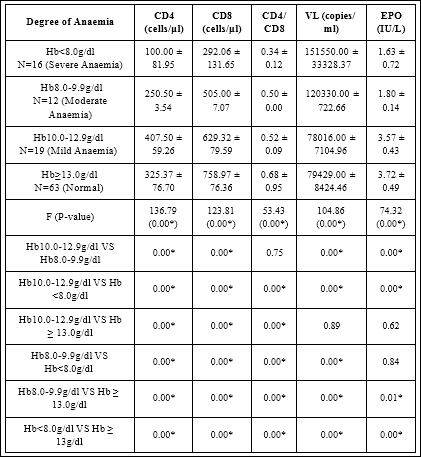
Table 2: Mean+SD Of CD4, CD8,VL and EPO On Degree Of Anaemia in HIV-In- fected Subjects On Art.
Mean values of CD4, CD8, CD4/CD8 and EPO in Hb<8.0g/dl were lower compared to 8.0-9.9g/dl, Hb 10.0-12.9g/dl, and Hb ≥ 13.0g/dl. Mean values of VL in Hb<8.0g/ dl were higher compared to 8.0-9.9g/dl, Hb 10.0-12.9g/dl, and Hb ≥ 13.0g/dl among ART subjects. P ≤ 0.05 was considered significant, p>0.05 was considered not signifi- cant, F-value=mean ± SD of parameters was compared using ANOVA
The mean ± SD of CD8 292.06 ± 131.65 in Hb<8g/dl (Sever Anaemia) was significantly lower (P>0.05) compared to 505.00 ± 7.07, 629.32 ± 79.59 and 758.97 ± 76.36 in Hb 8.0-9.9g/dl (Moderate), Hb 10.0-12.9g/dl (Mild) and Hb ≥ 13.0g/dl (Normal) respectively (F-value 123.81; P-value 0.00).
The mean ± SD of CD4/CD8 0.34 ± 0.12 in Hb<8.0g/dl was significantly lower (p<0.05) compared to 0.50 ± 0.00, 0.52 ± 0.09 and 0.68 ± 0.95 in Hb 8.0-9.9g/dl, Hb 10.0-12.9g/dl and Hb ≥ 13.0g/dl respectively (F-value 53.43; P-value 0.00).
The mean ± SD of VL 151550.00 ± 33328.37 in Hb<8.0g/dl was significantly higher (P<0.05) compared to 120330.00 ± 722.66, 78016.00 ± 7104.96 and 79429.00 ± 8427.46 in Hb 8.0- 9.9g/dl Hb 10.0-12.9 and Hb ≥ 13.0g/dl respectively (F-value 104.86; P-value 0.00).
The mean ± SD of EPO 1.63 ± 0.72 in Hb<8.0g/dl was significantly lower (P<0.05) compared to 1.80 ± 0.14, 3.57 ± 0.43 and 3.72 ± 0.49 in Hb 8.0-9.9g/dl, Hb, 10-12.9g/dl and Hb ≥ 13.0g/dl respectively (F-value 74.32; P-value 0.00). Multiple Comparison between Hb 10.0-12.9g/dl and Hb 8.0-9.9g/dl shows that, mean ± SD of CD4, CD8 and EPO in Hb 8.0-9.9g/dl were significantly lower (p<0.05) compared to Hb 10.0-12.9g/dl.
The mean ± SD of CD4/CD8 in Hb 8-9.9 was lower compared to Hb 10-12.9g/dl although the difference was not significant (P>0.05). Mean ± SD of VL in Hb8.0-9.9g/dl was significantly higher (p<0.05) compared to 10.0-12.9g/dl. Multiple Comparison between Hb<8.0g/ dl and Hb 10-12.9g/dl shows that mean ± SD of CD4, CD8, CD4/CD8 and EPO in Hb<8.0g/dl were significantly lower (p<0.05) compared to Hb 10.0-12.9g/dl.
The mean ± SD of VL in Hb<8.0/dl was significantly higher (p<0.05) compared to Hb 10.0-12.9g/dl. Multiple comparison between Hb10.0-12.9g/dl and Hb ≥ 13.0g/dl show that, mean ± SD of CD4, CD8 and CD4/CD8 in Hb10.0-12.9g/dl were significantly lower (P<0.05) compared to Hb ≥ 13.0.
Mean ± SD of VL and EPO in Hb 10.0-12.9g/dl were lower compared to Hb ≥ 13.0, the difference was not significant (P>0.05). Multiple comparison between Hb<8.0g/dl and Hb8.0-9.9g/dl shows that, mean ± SD of CD4, CD8 and CD4/CD8 in Hb<8.0g/dl were significantly lower (P<0.05) compared to Hb 8.0-9.9g/dl.
The mean ± SD of VL in Hb<8.0g/dl was significantly higher (P<0.05) compared to Hb8.0-9.9g/dl. The mean ± SD of EPO in <8g/ dl was lower compared to Hb8.0-9.9g/dl although the difference was not significant (P>0.05). Multiple comparison between Hb 8.0-9.9g/ dl and Hb ≥ 13.0g/dl shows that mean ± SD of CD4, CD8, CD4/ CD8 and EPO in Hb 8.0-9.9g/dl were significantly lower (P<0.05) compared to Hb ≥ 13.0.
The mean ± SD of VL in Hb 8.0-9.9g/dl was significantly higher (P<0.05) compared to Hb ≥ 13.0g/dl. Multiple comparison between Hb<8.0g/dl and Hb ≥ 13.0g/dl shows that, mean ± SD of CD4, CD8, CD4/CD8 and EPO in Hb<8.0g/dl were significantly lower (P<0.05) compared to Hb ≥ 13.0g/dl. The mean ± SD of VL in Hb<8.0g/dl was significantly higher (P<0.05) compared to Hb ≥ 13.0g/dl.
Discussion
The incidence of anemia was strongly and consistently associated with the progression of HIV disease as measured by CD4 count, CD8 count, CD4/CD8 ratio, viral load and erythropoietin in this study. This association is most likely explained by the increasing viral burden as HIV disease progresses, which resulted into anaemia by increased cytokine mediated myelosuppression and reduces erythropoietin level in response to anaemia in HIV-infected. This study is consistent with other study, reported that low haemoglobin level is associated with faster HIV disease progression into advanced immunodeficiency and mortality [6,12].
Prevalence of anaemia recorded in this study varies from 22% mild anaemia to 56% severe anaemia in HIV ART-naïve subjects while 19% mild anaemia to 16% severe anaemia was reported in HIV subjects on ART. Interventions of ART improved health condition of HIV subject on therapy which reduces the anaemic condition as reported in this study. similar to this study, Gedefaw reported prevalence of anaemia in ART naïve and ART HIV patients was 29.9% and 16.2% respectively, his study confirmed the effectiveness of ART in reducing HIV associated anaemia by reducing the occurrence of opportunistic infections [13].
Supporting the findings in this study, Servais reported the prevalence of anaemia in patients who are on ART was 11.7% while in ART naïve patients was 29.7% this affirmed that haematological disorders are corrected by antiretroviral therapy which also decreases the viral load [14]. From this study, there was a decrease in the blood haemoglobin levels as the HIV infection progressed, CD4 counts <200 cells mm−3 was strongly associated with severe anaemia in ART-naïve patients and could lead to rapid disease progression and decreased survival [6,15]. Supporting the findings in this study, it was suggested that as the immunity of HIV patient decreases, anaemia was more prevalent [16].
Generally, EPO level decline with advancing HIV disease as reported in this study. The severity of anaemia was found to increase with the severity of the HIV infection, the level of EPO failed to increase commensurately with the degree of anaemia in HIV infected patients, suggesting that one of the reasons for anaemia in this population was erythropoietin deficiency [6,17].
Conclusion
Anaemia has been associated with HIV progression to AIDS and shorter survival times for HIV-infected patients. Haemoglobin levels reflect rapidity of disease progression rates and independently predict prognosis, increases in haemoglobin level are predictive of treatment success. This present study showed the severity of HIV infection as haemoglobin level reduces, the rates of haemoglobin decrease is correlate with CD4 count, CD8 count, CD4/CD8 ratio, and erythropoietin decreases with increases in viral load.
References
- Semba RD, Martin BK, Kempen JH, Thorne JE, Wu AW (2005) The impact of anemia on energy and physical functioning in individuals with Arch Intern Med 165: 2229-2236.
- O’Brien ME, Kupka R, Msamanga GI, Saathoff E, Hunter DJ, et al. (2005) Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J Acquir Immune Defic Syndr 40: 219-225.
- Meidani M, Rezaei F, Maracy MR, Avijgan M, Tayeri K (2012) Prevalence, severity, and related factors of anemia in HIV/AIDS patients. J Res Med Sci 17: 138-142.
- Semba RD, Gray GE (2001) Pathogenesis of anemia during human immunodeficiency virus infection. J Investig Med 49: 225-239.
- Ajey S, Rajesh K, Aarti K, Chhaya V, Uday R, et al. (2018) Anaemia in HIV infected HAART naïve and HAART exposed children. International Journal of Contemporary Pediatrics 5: 1962-1965.
- Esan AJ, Osime EO, Oyedele TE (2020) Degree of Anaemia and Severity of HIV Infection in HIV Patients on Art and Art-Naïve. Journal of AIDS and Clinical ResearchResearch 11: 1-5.
- Zoufaly A, Cozzi-Lepri A, Reekie J, Kirk O, Lundgren J, et al. (2014) Immuno-Virological Discordance and the Risk of Non-AIDS and AIDS Events in a Large Observational Cohort of HIV-Patients in PLoS ONE 9: e87160.
- Graber (2000) Clinical outcome of patients with HIV-1 infection according to immunological and virologic response after 6 months of highly active antiretroviral therapy. Annals of Internal Medicine 133: 401-410.
- Murri R, Lepri AC, Cicconi P, Poggio A, Arlotti M, et al. (2006) Is moderate HIV viremia associated with a higher risk of clinical progression in HIV-infected people treated with highly active antiretroviral therapy. Journal of Acquired Immune Deficiency Syndrome 41: 23-30.
- Smith CJ, Sabin CA, Youle MS, Kinloch-de Loes S, Lampe FC, et al. (2004) Factors influencing increases in CD4 cell counts of HIV-positive persons receiving long-term highly active antiretroviral Journal of Infectious Diseases 190: 1860-1868.
- ALPCO (2018) Quantitative determination of erythropoietin in human serum by ELISA. Catalog Number: 21-EPOHU-E01 1-12.
- Obirikorang C, Yeboah FA (2009) Blood haemoglobin measurement as a predictive indicator for the progression of HIV/AIDS in resource-limited Journal of Biomedical Science 16: 102.
- Gedefaw L, Yemane T, Sahlemariam Z, Yilma D (2013) Anemia and risk factors in HAART naïve and HAART experienced HIV positive persons in south west Ethiopia: A comparative study. PLoS ONE 8: 72-80.
- Servais J, Nkoghe D, Schmit JC, Arendt V, Robert I, et al. (2001) HIV- associated hematologic disorders are correlated with plasma viral load and improve under highly active antiretroviral J Acquir Immune Defic Syndr 14: 221-225.
- Owiredu WK, Quaye L, Addai-Mensah O (2011) Prevalence of anaemia and immunological markers among Ghanaian HAART-naïve HIV-patients and those on HAART. African Health Sciences 11: 2-15.
- Ferede G, Wondimeneh Y (2013) Prevalence and related factors of anemia in HAART-naive HIV positive patients at Gondar University Hospital, Northwest Ethiopia. BMC Hematology 13: 1-5.
- Gatukui DK, Oyoo GO, Rajab J, Kayima J, Omonge E, et (2014) Serum erythropoietin in patients with anaemia on HAART attending the Kenyatta National Hospital, Comprehensive Care Centre. East African Journal of Pathology 1: 2-6.
Citation: Esan AJ, Osime EO, Esther OB, Titilayo EO, Kelvin O (2021) Assessment of Severity of Anaemia using Immunovirological and Erythropoietin Level among HIV Infected Patients. J Hematol Hemother 6: 017.
Copyright: © 2021 Esan AJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.