*Corresponding Author:
Martha Verghese,
Department of Food and Animal Sciences, Alabama A&M University, 4900 Meridian Street North, Normal, Alabama 35762, USA
E-mail: martha.verghese@aamu.edu
Abstract
While one out of every two adults in the U.S. is affected by a chronic disease, oxidation has been linked to a number of these diseases, including cancer and obesity. Poppy (Papaver somniferum) is a flowering plant that produces poppy seeds, which are of importance to the food, animal and cosmetic industries. Produced predominantly for its oil and use in baked goods, research suggests the presence of antioxidative phytochemicals in poppy seeds. Objective of study was to determine effects of processing on antioxidant content, potential and inhibition of metabolizing enzyme activities by blue and white poppy seeds. Total Phenolic Content (TPC), Total Flavonoid Content (TFC), free radical scavenging activity by 1,1-diphenyl-2-picryhydrazyl (DPPH), Trolox Equivalent Antioxidant Capacity (TEAC), Ferric Reducing Antioxidant Power (FRAP) and inhibition of lipase, α-amylase and α-glucosidase were evaluated in blue and white poppy seeds (raw (BR and WR), toasted (BT and WT) (150-178°C), defatted (BD and WD) extracted with water (W) and 80% ethanol (E). TPC ranged from 22.38 (WDE) -78.37mg (BTE) GAE/100g. At a concentration of 0.02mg/ml, the highest DPPH scavenging activity was observed on BTE (86.28%). BDE and WDE inhibited α-glucosidase activity significantly more than other extracts. Results from present study suggest antioxidant potential of poppy seed ‘cake’ (by-product of defatting).
Keywords
Metabolizing enzymes; Poppy; Processing
Introduction
One out of every two adults in the U.S. is affected by a chronic disease, with oxidative stress being a main main influencer of a number of chronic disease pathologies, including cancer, diabetes and obesity [1-3]. Antioxidants help to lessen the effects of oxidative stress, by neutralizing free radicals [4]. Natural antioxidants are receiving an enormous amount of attention from nutritionists, food manufacturers, medical experts and consumers due to their health benefits. Often consumed as snacks, nuts and seeds contain a myriad of health-benefitting (vitamins, minerals, alkaloids, phenolic acids) phytochemicals [5-7]. One such seed, increasing in popularity is the poppy seed.
Poppy (Papaver somniferum) is a flowering plant that produces poppy seeds, which are of importance to the food, animal and cosmetic industries. Produced predominately for its oil and use in baked goods, research suggests the presence of antioxidative phytochemicals in poppy seeds [8].
Due to the high amounts of carbohydrates and lipids, the western diet has been associated with the promotion of diseases of civilization, including obesity and diabetes. Lipase, α-glucosidase and α-amylase are enzymes that aid in the metabolism of fats (lipase) and carbohydrates (α-amylase). Pancreatic lipase, composed of 449 amino acids, is a crucial enzyme for the absorption of dietary triglycerides [9]. α-glucosidase and α-amylase (pancreas) are enzymes that play key roles in carbohydrate digestion. The inhibition of these key metabolizing enzymes could result in retardation of lipid and carbohydrate absorption; possibly reducing the risk of chronic diseases [10,11]. Though limited research has been conducted on poppy seed’s effect on obesity and diabetes, some studies do suggest the effects of other seeds on the aforementioned health conditions. A study conducted by Binita and others shows that cumimi seeds may decrease the risk of type II diabetes [12]. The possible anti-obesity effects of another seed, Phalaris canariensis, were suggested in research conducted by Gutierrez and others via the inhibition of the fat metabolizing enzyme, lipase [13].
Though seeds are consumed raw, they are commonly subjected to processing (drying, toasting) to enhance sensory attributes. Some studies suggest an increase in content of select phytochemicals upon toasting of nuts and seeds [14-15]. As poppy seeds are commonly used for their oils, defatting is the processing used to extract this product. Containing approximately 5.2% crude fat [16], after defatting, the remaining by-product, poppy seed cake, is often times discarded. The defatted seed cake may still have some functional benefits, as suggested by Yilmaz and Emir. According to these researchers, poppy seed cake contains a number of macro and micronutrients and could still be beneficial for human consumption [17].
Though research has been conducted on various poppy seeds and the differences in their oil contents, there is limited information on effects that processing may have on the antioxidant potential the blue poppy seed and its white counterpart. The aim of the study was to determine effects of processing on the antioxidant content, potential and inhibition of metabolizing enzyme activities of blue and white poppy seeds.
Materials and Methods
All chemicals were obtained from Sigma Chemical Company, St. Louis, Mo. and Fisher Scientific Company, Waltham, Mass. Poppy seeds were obtained from Belmar Spice Company, Turkey. Determination of Phytochemicals in Poppy seeds.
Sample preparation and extraction of phenolics: Extraction of poppy seeds was performed using established methods. Poppy seeds were extracted raw (unprocessed control), roasted (processed) and defatted (processed). Roasted seeds were exposed to heat (150- 178°C) for 7 mins. All seeds were ground to a powder using a Robot Coupe Blixer 4V (Ridgeland, MS). Five grams of seed powder was added to 50mL of methanol (80%), or water (25°C). Defatting of raw seeds was performed by mixing hexane and seed powder at a 4:1 ratio continuously for 90mins; after which the oil was decanted. This process was repeated 2times; the resulting seed cake was extracted with methanol or water. The mixtures were stirred for 2 hr on an orbital shaker then centrifuged at 3000 xg for 20 mins. The supernatant was collected, filtered, and evaporated to dryness. The extraction was reconstituted with respective solvent and stored at -80°C until further analysis. Table 1 shows poppy seed extracts and their respective abbreviations.
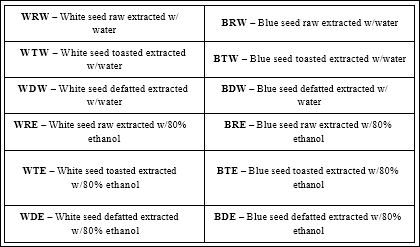
Table 1: White and Blue Poppy Seed Extract Abbreviations.
Determination of Total Phenolic and Total Flavonoid Contents in Poppy Seed Extracts. Total phenolics in seed extracts were determined by the Folin-Ciocalteau method and reported as Gallic Acid Equivalents (GAE) as described by [18]. Total flavonoids in poppy seed extracts were determined by a colorimetric methods described by [18] and reported as catechin equivalents (CAE).
Antioxidant Potential of Poppy Seed
Antioxidant activities including, Ferric Reducing Antioxidant Power (FRAP), 1,1 diphenyl 2-picrahydrazyl (DPPH) radical scavenging ability, trolox equivalent antioxidant capacity (TEAC) of seed extracts were determined.
FRAP of poppy seed extracts was determined by the methods described by [19]. DPPH radical scavenging ability of seed extracts was determined following methods described [19]. Trolox Equivalent Antioxidant Capacity (TEAC) of poppy seed extracts was performed following methods by [20] using the 2,2’-azino- bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical. Determination of Lipid and Carbohydrate Metabolizing Enzyme Inhibition. Inhibition of lipase, a-glucosidase, a-amylase by poppy seed extract was determine by methods described by [21] and [22], respectively.
Statistical Analysis
Results are presented as means ± SEM using SAS system version
9.3 ANOVA was used to determine any significant differences among the treatment groups. Significance was determined at p ≤ 0.05. The means were separated using Tukey’s Studentized Range Test.
Results and Discussion
Nuts are seeds are oftentimes consumed as snacks and as ingredients in a number food products, post processing. Whole blue poppy seeds are commonly used in the food industry as garnish for bagels, muffins and other baked goods. Oil extracted from poppy seeds is oftentimes used, but the remaining seed cake is most always discarded. The effects of processing on antioxidant content, potential and metabolizing enzyme activities of blue and white poppy seeds extracted with water and ethanol was determined. Figure 1 shows the Total Phenolic Content (TPC) of raw, roasted and defatted poppy seeds extracted with water and ethanol. Overall, white poppy seeds (raw and defatted) extracted with water and blue poppy seeds (toasted) extracted with ethanol had significantly (p ≤ 0.05) higher TPC compared to other poppy seed extracts. Toasting significantly (p ≤ 0.05) decreased phenolic yield of blue and white poppy seeds extracted with water. TPC of defatted blue and white poppy seeds was comparable to phenolic content of some of the whole seed extracts.
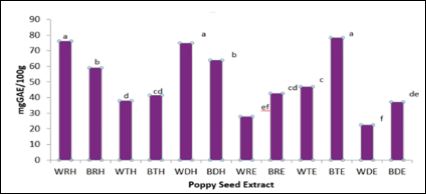
Figure 1: Total Phenolic Content of Poppy Seeds.
Bars (n=3) expressed as means ± SEM. Means within treatments without a common letter (abc) differ significantly at p ≤ 0.05.
Abbreviations: GAE – gallic acid equivalents, BRW – blue poppy seed raw extracted with water, WTW – white poppy seed toasted extracted with water, BTW – blue poppy seed toasted extracted with water, WDW– white poppy seed defatted extracted with water, BDW – blue poppy seed defatted extracted with water, WRE – white poppy seed raw extracted with 80% ethanol, BRE – blue poppy seed raw extracted with 80% ethanol, WTE – white poppy toasted extracted with 80% ethanol, BTE – blue poppy seed toasted extracted with 80% ethanol, WDE-white poppy seed defatted extracted with 80% ethanol, BDE – blue poppy seed defatted extracted with 80% ethanol.
Figure 2 shows the total flavonoid content (TFC) raw and processed poppy seeds, extracted with water or ethanol. As similarly observed in total phenolic content results, toasted blue poppy seeds extracted with ethanol (BTE) yielded significantly (p ≤ 0.05) higher TFC compared to other extracts. With few exceptions, blue poppy seed extracted yielded higher flavonoids compared to white. As seen in TPC results, blue and white poppy seed cake extracts yielded similar flavonoid content to whole seed extracts.
Table 2 displays the ferric reducing antioxidant power and trolox equivalent antioxidant capacity of poppy seeds. The ability of the seeds to reduce ferric to ferrous iron is display in the second column of this table, but neither processing nor solvent extraction had an effect on the reducing ability. The third column of table 2 displays the ability of the seeds to scavenge the ABTS free radical, compared to the scavenging ability of vitamin E analog, trolox. Extraction of defatted blue poppy seed with ethanol significantly (p ≤ 0.05) increased the scavenging ability of the seed, compared to the other extracts.
DPPH results are displayed in Figure 3. DPPH is a stable free radical that is deep purple in color. This assay measures the ability of biological samples to reduce 1,1-diphenyl-2-picryl hydrazyl radical to 1,1-diphenyl-2-picryl hydrazine [24], therefore a reduction in purple color indicates a reduction in free radicals. Aligning with antioxidant content results (TPC and TFC), BTE was able to scavenging the DPPH radical more effectively than the other extracts. Overall, ethanolic extracts of poppy seed had significantly (p ≤ 0.05) higher scavenging ability compared to their aqueous counterparts.
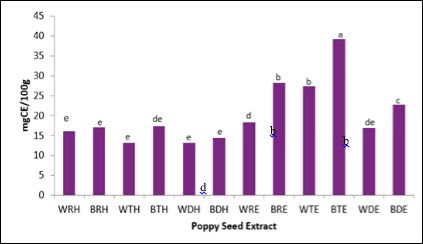
Figure 2: Total Flavonoid Content of Poppy Seeds.
Bars (n=3) expressed as means ± SEM.
Means within treatments without a common letter (abc) differ significantly at p ≤ 0.05.
Abbreviations: CE – catechin equivalents, BRW – blue poppy seed raw extracted with water, WTW - white poppy seed toasted extracted with water, BTW – blue poppy seed toasted extracted with water, WDW– white poppy seed defatted extracted with water, BDW – blue poppy seed defatted extracted with water, WRE – white poppy seed raw extracted with 80% ethanol, BRE – blue poppy seed raw extracted with 80% ethanol, WTE – white poppy toasted extracted with 80% ethanol, BTE – blue poppy seed toasted extracted with 80% ethanol, WDE – white poppy seed defatted extracted with 80% ethanol, BDE – blue poppy seed defatted extracted with 80% ethanol
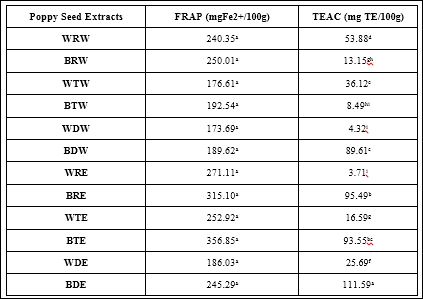
Table 2: Antioxidant Capacity of Poppy Seeds.
Bars (n=3) expressed as means ± SEM. Means within treatments without a common letter (abc) differ significantly at p ≤ 0.05.
Abbreviations: FRAP-ferric reducing antioxidant power, FE2+ - ferrous sulphate, TEAC- Trolox Equivalent Antioxidant Capacity, TE – trolox equivalents, BRW – blue poppy seed raw extracted with water, WTW – white poppy seed toasted extracted with water, BTW – blue poppy seed toasted extracted with water, WDW– white poppy seed defatted extracted with water, BDW – blue poppy seed defatted extracted with water, WRE – white poppy seed raw extracted with 80% ethanol, BRE – blue poppy seed raw extracted with 80% ethanol, WTE – white poppy toasted extracted with 80% ethanol, BTE – blue poppy seed toasted extracted with 80% ethanol, WDE – white poppy seed defatted extracted with 80% ethanol, BDE – blue poppy seed defatted extracted with 80% ethanol.
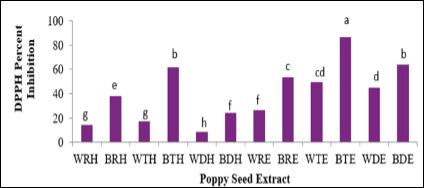
Figure 3: DPPH Radical Scavenging Ability of Poppy Seeds.
Bars (n=3) expressed as means ± SEM. Means within treatments without a common letter (abc) differ significantly at p ≤ 0.05.
Abbreviations: DPPH - 1,1 diphenyl 2-picrylhydrazyl, BRW – blue poppy seed raw extracted with water, WTW – white poppy seed toasted extracted with water, BTW – blue poppy seed toasted extracted with water, WDW– white poppy seed defatted extracted with water, BDW – blue poppy seed defatted extracted with water, WRE – white poppy seed raw extracted with 80% ethanol, BRE – blue poppy seed raw extracted with 80% ethanol, WTE – white poppy toasted extracted with 80% ethanol, BTE – blue poppy seed toasted extracted with 80% ethanol, WDE – white poppy seed defatted extracted with 80% ethanol, BDE – blue poppy seed defatted extracted with 80% ethanol.
Table 3 shows carbohydrate and lipid metabolizing enzyme inhibition by poppy seeds. The Western diet is of growing concern to researchers, as it contain a high amount of carbohydrates and fats; which contribute heavily to the pathogenesis of obesity and diabetes. Lipase and a-amylase are enzymes that aid in the metabolism of fats (lipase) and carbohydrates (α-amylase and α-glucosidase). Inhibition of the aforementioned enzymes could lead to the prevention of chronic diseases, such as diabetes and obesity. Heat treated blue poppy seeds that were extracted with water inhibited the lipase enzyme more effectively than other extracts (80.57% inhibition). Toasting of white poppy seeds extracted with ethanol (WTE) significantly increased the ability of the seed to inhibit the a-amylase enzyme (63.50% inhibition) compared to other extracts. Defatting significantly (p ≤ 0.05) increased the ability of poppy seeds to inhibit the a-glucosidase enzyme.
Discussion
TPC, TFC, DPPH radical scavenging, FRAP, TEAC and enzyme inhibition were all evaluated in various extractions of blue and white poppy seed. In the present study, processing and extraction solvent has an effect on phytochemical content and ability of poppy seeds to inhibit enzymes associated with metabolism.
The present results could relate to results by Ghafoor and others, which showed that processing of various poppy seeds had affected quality and phytochemical composition. The authors found that roasting decreased concentration of several phytochemicals in poppy seed, including vanillic acid [23]. In the present study, heat treating of poppy seeds decreased TPC and TFC with the exception of the BTE group. Blue poppy seeds may contain a similar cyaniding-based anthocyanin, responsible for the blue petals of the Himalayan poppy [24].
In the present study, defatted seed extracts had comparable antioxidant content and potential to their full-fat counterparts. The findings here align with results from Brodowska and others, where the antioxidant potential of defatted flaxseed extract was comparable (or greater than in some cases) to full-fat flaxseed extract [25].
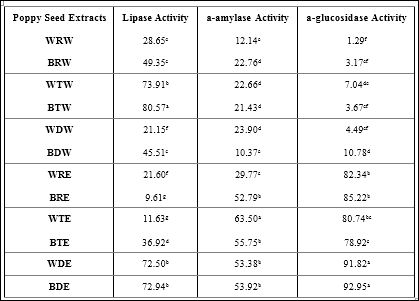
Table 3: Metabolizing Enzyme Inhibition by Poppy Seeds.
Bars (n=3) expressed as means ± SEM.
Means within treatments without a common letter (abc) differ significantly at p ≤ 0.05.
Abbreviations: BRW – blue poppy seed raw extracted with water, WTW – white poppy seed toasted extracted with water, BTW – blue poppy seed toasted extracted with water, WDW– white poppy seed defatted extracted with water, BDW – blue pop- py seed defatted extracted with water, WRE – white poppy seed raw extracted with 80% ethanol, BRE – blue poppy seed raw extracted with 80% ethanol, WTE – white poppy toasted extracted with 80% ethanol, BTE – blue poppy seed toasted extracted with 80% ethanol, WDE – white poppy seed defatted extracted with 80% ethanol, BDE – blue poppy seed defatted extracted with 80% ethanol.
Though there have been a number of studies conducted focused on the role of poppy seed meal as a fat replacer, there is limited information on the seed’s possible anti-obesity status. Results from the current study suggest that poppy seeds may have anti-obesity effects, in vitro, via the inhibition of the lipase enzyme. The results here align with similar finding from Rahman and others, which showed the ability of camelina and sophia seeds to inhibit the lipase enzyme [26].
Poppy seed extracts inhibited carbohydrate metabolizing enzymes in the present study; which could suggest implications in the prevention of diabetes. Blue and white seed cake extracted with ethanol inhibited the a-glucosidase enzyme more efficiently than other extracts in this study. Defatted residues (seed cakes) have been suggested to have beneficial effects in other studies. Sesame seed cake was shown to normalize blood glucose levels and improved glucose tolerance in rats fed a high fructose diet [27].
Conclusion
Though there are a number of products containing seeds on the market, very few utilize blue and white poppy seeds as an ingredient. The present study explored the effects of processing, variety and solvent extraction on antioxidant content, potential and metabolizing enzyme inhibition of poppy seeds. The results of the study suggest that poppy seeds may benefit consumers by improving their antioxidant status and inhibiting carbohydrate and lipid metabolizing enzymes, possibly playing a crucial role in the prevention of chronic diseases.
Though the present study explores water and ethanol as extraction solvents, mixtures of several organic solvents (ethanol, methanol, acetone) were not taken into account. Based on results from lipase, a-amylase and a-glucosidase, in-vitro studies using cell culture models could also be conducted to further determine the possible anti- diabetic and anti-obesity effects of poppy seed extracts.
Acknowledgements
Department of Food and Animal Sciences, Alabama A&M University, Normal, AL 35762. Contributed by the Agricultural Experiment Station, Alabama A&M University, Journal No. Research supported by Title III Department of Education, USDA National Institute of Food and Agriculture, [Evans-Allen] project [ALAX- 012-0412]
References
- Valko M, Rhodes C, Moncol J, Izakovic MM, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico- biological interactions 160: 1-40.
- Baynes JW (1991) Role of oxidative stress in development of complications in diabetes. Diabetes 40: 405-412.
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, et al. (2004) Increased oxidative stress in obesity and its impact on metabolic The Journal of clinical investigation 114: 1752-1761.
- Gordon MH (1990) The mechanism of antioxidant action in vitro. Food antioxidants 1-18.
- Zhang YJ, GanR Y, Li S, Zhou Y, Li AN, et al. (2015) Antioxidant phytochemicals for the prevention and treatment of chronic Molecules 20: 21138-21156.
- Bozan B, Temelli F (2008) Chemical composition and oxidative stability of flax, safflower and poppy seed and seed oils. Bioresource technology 99: 6354-6359.
- Nergiz C, Ötles S (1994) The proximate composition and some minor constituents of poppy seeds. Journal of the Science of Food and Agriculture 66: 117-120.
- Souri E, Amin G, Farsam H, Barazandeh TM (2008) Screening of antioxidant activity and phenolic content of 24 medicinal plant extracts. DARU Journal of Pharmaceutical Sciences 16: 83-87.
- Slanc P, Doljak B, Kreft S, Lunder M, Janes D, et (2009) Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 23: 874-877.
- Drent ML, Veen EA van der (1993) Lipase inhibition: A novel concept in the treatment of obesity. International journal of obesity and related metabolic disorders. 17: 241-244.
- Kenjiro T, Minami Y, Takamatsu K, Matsuoka T (2006) Inhibition of α-glucosidase and α-amylase by Journal of nutritional science and vitaminology 52: 149-153.
- Binita K, Sharma V, Yadav S (2017) The therapeutic potential of Syzygium Cumini seeds in diabetes mellitus. J Med Plants Stud 5: 212-218.
- Perez Gutierrez RM, Ahuatzi DM, Cruz Victoria T, Madrigales Ahuatzi D (2016) Inhibition by Seeds of Phalaris canariensis Extracts of Key Enzymes Linked to Obesity. Alternative Therapies in Health & Medicine 22.
- Monagas M, Garrido I, Lebrón-Aguilar R, Gómez-Cordovés MC, Rybarczyk A, et (2009) Comparative flavan-3-ol profile and antioxidant capacity of roasted peanut, hazelnut, and almond skins. Journal of Agricultural and Food Chemistry 57: 10590-10599.
- Lin JT, Liu SC, Hu CC, Shyu YS, Hsu CY, et (2016) Effects of roasting temperature and duration on fatty acid composition, phenolic composition, Maillard reaction degree and antioxidant attribute of almond (Prunus dulcis) kernel. Food chemistry 190: 520-528.
- Bozan B, Temelli F (2008) Chemical composition and oxidative stability of flax, safflower and poppy seed and seed oils. Bioresource technology 99: 6354-6359.
- Emir DD, Güneșer O, Yılmaz E (2014) Cold pressed poppy seed oils: sensory properties, aromatic profiles and consumer preferences. Grasas y Aceites 65: 29.
- Gajula D, Verghese M, Boateng J, Walker LT, Shackelford L, et al. (2009) Determination of Total Phenolics, Flavonoids and Antioxidant and Chemopreventive Potential of Basil (Ocimum basilicum L. and Ocimum tenuiflorum L.)” D. Gajula,’M. Verghese,” J. Boateng,” LT International Journal of Cancer Research 5: 130-143.
- Benzie Iris FF, JJ Strain (1999) Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods in enzymology 299: 15-27.
- Miller NJ, Rice-Evans CA (1997) Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay. Free radical research 26: 195-199.
- Mosmuller EWJ, Van Heemst JDH, Van Delden CJ, Franssen MCR, Engbersen JFJ (1992) A new spectrophotometric method for the detection of lipase activity using 2, 4-dinitrophenyl butyrate as a substrate. Biocatalysis 5: 279-287.
- Apostolidis E, Kwon YI, Shetty K (2007) Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innovative Food Science & Emerging Technologies 8: 46-54.
- Ghafoor K., Özcan MM, Fahad AJ, Babiker EE, Fadimu GJ (2019) Changes in quality, bioactive compounds, fatty acids, tocopherols, and phenolic composition in oven-and microwave-roasted poppy seeds and LWT 99: 490-496.
- Tanaka Y, Sasaki N, Ohmiya A (2008) Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. The Plant Journal 54: 733-749.
- Brodowska K, Catthoor R, Brodowska AJ, Symonowicz M, Lodyga- Chruscinska E (2014) A comparison of antioxidant properties of extracts from defatted and non-defatted flax (Linum usitatissimum) seeds. Albanian Journal of Agricultural Sciences 13: 16-23.
- Rahman MJ, Ambigaipalan P, Shahidi F (2018) Biological Activities of Camelina and Sophia Seeds Phenolics: Inhibition of LDL Oxidation, DNA Damage, and Pancreatic Lipase and α‐Glucosidase Activities. Journal of food science 83: 237-245.
- Bigoniya P, Nishad R, Singh CS (2012) Preventive effect of sesame seed cake on hyperglycemia and obesity against high fructose-diet induced Type 2 diabetes in rats. Food chemistry 133: 1355-1361.
Citation: Willis S, Verghese M (2021) Antioixidant Potential and Enzyme Inhibition of White and Blue Poppy Seeds. J Nutr Food Sci 4: 033.
Copyright: © 2021 Willis S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.


