*Corresponding Author:
Kulvinder Kochar Kaur,
Kulvinder Kaur Centre for Hu- man Reproduction 721, G.T.B. Nagar Jalandhar-144001, Punjab, India
Tel: 91-181- 9501358180
E-mail: kulvinder.dr@gmail.com
Abstract
Colorectal Cancer (CRC) represents one of the commonest cancer of the gastrointestinal tract with marked morbidity as well as mortality. Most common cause of demise in CRC is metastasis to distant sites. Hence the patients prognosis in addition to survival is influenced by this along with remains a crucial problem in the management of CRC. Long non coding RNAs (lnc RNAs) represent non coding RNAs molecules possessing more than 200 nucleotides. Aberrant expression of lnc RNAs is intricately associated with the initiation along with propagation of various kinds of diseases. That includes cancers. The studies conducted recently demonstrated that a variety of lnc RNAs play key parts in CRC metastasis, along with reverting their expression via artificial measures might diminish the malignant phenotype of metastatic CRC to certain degree. Hence here we conducted a systematic review utilizing search engine pubmed, google scholar; web of science; embase; Cochrane review library utilizing the MeSH terms like Colorectal Cancer; Epigenetics; LncRNA as biomarkers; as therapeutic targets; mode of action DNA methylation; Histonealterations ;biomarkers; epigenetic liquid biopsy; role of tea phenols; curcumin; nicotine; phosphatidylinositide 3-kinase(PI3K) /protein kinase B(AKT); Wnt/beta catenin signaling pathwaysfrom 2000 to 2021 till date. We found a total of 1800 articles out of which we selected 115articles for this review. No metaanalysis was done. Thus we summarize here the detailed modes by which lnc RNAs act as well as contribute in CRC metastasis along with how they might be utilized as biomarkers along with targets for therapy along with predict prognosis.
Keywords
Cancer metastasis; CRC; lnc RNAs; PI3K/AKT Signaling/Wnt/beta catenin signaling pathways/biomarkers
Introduction
Earlier we have reviewed on epigenetics in relation to mammalian reproduction, its role in SRY mutations, Disordered Sexual Development (DSD), in pregnancy and preeclampsia, then in relation to obesity, DM, as well as recently in Diabetic Nephropathy (DN) [1- 6]. Colorectal Cancer (CRC) is presently the 3rd commonest malignant tumor all over world. Roughly 1.8 million new cases as well as around 900,000 deaths documented worldwide every year. With an escalated incidence along with high mortality of CRC pose a big threat to health of human beings [7]. The generation of CRC is a complicated event which implicates exogenous along with endogenous factors, like signaling molecules, homeostasis, microenvironment, diet, lifestyle that possess a significant part in the CRC etiopathogenesis [8,9]. Molecular Pathological Epidemiology (MPE) has demonstrated that the diet, lifestyle possess a close association with tumorigenesis. Like smoking, consumption of red as well as possessed meat, exaggerated alcohol consumption, along with some drugs like aspirin have been validated to be correlated with the generation of CRC [10]. The pace in clinical treatment has grown rapidly along with possessing significant impact. Nevertheless, the results of therapy in patients with metastatic CRC are still not optimal as well as the 5yr survival rate in these cases is just 12% [11]. CRC metastasis is a significant factor resulting in CRC recurrence as well as demise. Hence finding the Molecular mode of CRC metastasis along with elucidation of biomarkers associated with metastasis are key for enhancement of therapy results in CRC. Long non coding RNAs (lnc RNAs) represent non coding RNAs molecules possessing more than 200 nucleotides length wise. Maximum of the need RNA polymerase II for transcription as well as are akin to messenger RNAs (mRNAs) despite the coding capacity being absent [12]. There are 5 types of lnc RNAs as per their potential association with protein coding; i) sense ii) anti sense iii) bidirectional iv) interintron v) intergenic lnc RNAs [13]. The long nucleotide chain of lnc RNAs can either generate a complicated spatial body as well as crosstalk with protein. Collection of proof points that lnc RNAs are a significant class of molecules that control the genomic events. The long nucleotide chain of lnc RNAs can generate a complicated spatial structure as well ascrossreacts with protein factors, or give a large area for the simultaneous binding of various molecules, that together take part in silencing X chromosome, genomic imprinting, besides other ones [14]. In view of lnc RNAs playing a significant part in the different parts of gene expression the association among lnc RNAs as well as tumors has gained significance as well as received attention in present research. Different lnc RNAs have been demonstrated to facilitate or repress tumor generation in various cancers. Like Zhuang et al. [15], observed that lnc RNAs GClnc1 facilitates proliferation along with invasion of bladder cancers by stimulation of MYC expression. Lnc RNAs PVT1 possess a carcinogenic part in case of prostate cancers as well as has the ability of acting as a diagnostic biomarker [16]. In case of CRC, researchers have observed that a lot of differentially expressed lnc RNAs as well as validated their significant part in controlling CRC cell proliferation, apoptosis, invasion an as well as metastasis in addition to their sensivity to radio as well as chemotherapy [17]. Like HOXB-AS3 peptide that gets encoded by lnc RNAs HOXB-AS3 has got demonstrated to hamper the generation of CRC [18]. Collection of proof pointed to lnc RNAs being significant biomarkers of CRC metastasis. It was revealed by Yue et al. [19], that lnc RNAs CYTOR can facilitate CRC metastasis via Wnt/β-catenin signaling pathway [19]. Thus lnc RNAs, can be potential treatment targets for CRC. Having reviewed role of teal polyphenols, anthrocyanins in obesity relief and associated cancers, here we chose to conduct a systematic review with regards to a lot of research with regards to role of lnc RNAs in CRCalong with as biomarkers along with avoidance of CROC metastasis.
Methods
Here we conducted a systematic review utilizing search engine pubmed, google scholar; web of science; embase; Cochrane review library utilizing the MeSH terms like Colorectal Cancer; Epigenetics; LncRNA as biomarkers; as therapeutic targets; mode of action DNA methylation; Histonealterations; biomarkers; epigenetic liquid biopsy; role of tea phenols; curcumin; nicotine; phosphatidylinositide 3-kinase(PI3K) /protein kinase B(AKT); Wnt. beta catenin signaling pathwaysfrom 2000 to 2021 till date.
Results
We found a total of 1800 articles out of which we selected 115 articles for this review. No meta-analysis was done.
Properties as well as Parts Played by lnc RNAs
Usually nc RNAs can be grouped as long chain as well as short chain nc RNAs depending on their length [20]. The 1st lnc RNAs transcript got sequence was invented in eukaryotes possesses a length of greater than 200nt as well as an mRNA like structure. Subsequent to splicing, a 7 MC cap is mostly added at the 5’ end of the lnc RNAs sequence, as well as a poly a tail is occasionally added at the 3’end [21]. Certain studies have illustrated that for certain lnc RNAs that correspond to the DNA areas are present among genes or introns, certain of which overlap with protein coding genes, whereas some lnc RNAs encode a lesser number of functional small peptides [22]. Whereas the primary structure ofan lnc RNA is its nucleotide sequence, while its functional action is based on the base pairing but that is conserved at a lower level in contrast to its higher order structure [23]. The secondary as well as tertiary structures of lnc RNAs decide their functions. The secondary structures basically include double helices, hairpins, while the tertiary structures have greater diversity, like sarcin-ricin loops. The lesser conservation of its primary structure is balanced via these structure of higher order [24,25].
Mechanistic ally the modes utilizedby lnc RNAs are i) hampering the mRNAs cleavage by generation, of complementary double stranded RNA [26], ii) altering the action of a particular protein via direct binding [28] iii) changing the cytoplasmic location of a particular protein via direct binding [27] iv) changing the expression of target genes by hampering the RNA polymerase II or via chromatin remodeling as well as histone modification [28] v) meddling with the target genes expression by starting transcription from the promoter area of the protein coding genes [29] vi) along with generation of double stranded RNA with the transcripts of protein coding genes as well as generation, of endogenous si RNA via action of Dicer [30], reviewed by us in ref [4,5] vii)working as a structural constituent by development of a nucleic-acid-protein complex [31], besides viii) working as a precursor of small RNA (like mi RNAs or pi RNAs). Usually lnc RNAs are expressed in the nucleus as well as their expression amounts are lesser in contrast to that of mRNAs [32]. Nevertheless, lnc RNAs are closely implicated in the control of different biological actions in view of their tissue particular expression, in addition to influencing disease events [33]. Further lnc RNAs can control the expression of significant genes at various levels through (Epi) genetic control as well as by mediation of transcription, post transcriptional events, translation as well as a protein modification, in the form of RNA transcribed to start with or a mature spliced. Furthermore, lnc RNAs might possess significant parts in physiological events that includes generation, tissue differentiation, and reproduction in addition to immunity, besides the generation along with growth of tumors.
Mode of action of lnc RNAs in CRC metastasis
Metastasis of tumors represents an event in which malignant cells separate from the primary tumor area as well as get shifted via the circulation to secondary, tissues/organs in which they establish colonization along with, generation of secondary tumors [34]. Invasion of tumors along with metastasis are complicated, dynamic events which classically implicate alterations in tumor microenvironment, epithelial-mesenchymaltransition (EMT), Hypoxia along with angiogenesis, besides other modes [35]. Collection of studies have demonstrated that lnc RNAs can control the metastasis of CRC, basically controlling crucial factors that concomitantly influence a lot of signaling pathways that are intricately associated with metastasis of tumors. In rest of cases lnc RNAs might sponge mi RNAs to control the target genes expression. Further lnc RNAs might bind directly to the proteins for stimulation of protein break down through influencing their phosphorylation /ubiquitination. Invasion of tumors along with metastasis influence the future prognosis of patients along with survival as well as are significant etiologies of tumors associated mortality, thus blocking these events continue to be a key problem in treatment of cancers [36].
Control of CRC metastasis via lnc RNAs by controlling signaling pathways
Complicated Controlling signaling pathways are implicated in tumor metastasis along with changes in a lot of signaling pathways in the microenvironment associated with the tumors [37]. Various pathways,that are i) Wnt/β-catenin [38], phosphatidylinositide 3-kinase (PI3K)/protein kinase B (AKT) [39], Signal Transcription And Transducer (STAT) [40], Mitogen Activated Protein Kinase (MAPK) [41] along with Notch signaling pathways [42] have significant part in the metastasis of various tumors.
Various studies have documented how Wnt/β-catenin signaling pathway is intricately associated with CRC metastasis. That lnc RNAs CYTOR that is markedly expressed in CRC generates a positive feedforward loop with β-catenin along with takes part in the control of colon cancer metastasis was demonstrated by Yue et al. [19]. In this event, cell receptors get bound to the β-catenin located in the cytoplasm followed by blocking this β-catenin phosphorylation that gets catalyzed by the enzyme casein kinase (CK)1, resulting in collection of β-catenin along with its nuclear transport. Following that, the β-catenin /TCF complex stimulates the expression of cell receptor encoding genes, thus generating a positive feed forward loop. The lnc RNAs SLCO4A1-AS1 hampers the crosstalk of β-catenin with GSKβ, hampering the β-catenin phosphorylation, as well as enhances β-catenin stableness, finally facilitating the proliferation, migration along with invasion of CRC cells [43]. Wu et al. [44], illustrated that lnc RNAs JMJD2C facilitates CRC metastasis via escalation of β-catenin signaling pathway, besides taking part in the control histone methylation at the MALAT1 promoter. Besides taking part in the β-catenin signaling pathway transduction directly, lnc RNAs can further possess a significant part in indirect control of this signaling pathway. It has been demonstrated that NEAT 1 stimulates Wnt/βcatenin signaling pathway indirectly via DDX5, as well as hence influences its carcinogenic action by modulation of DDX5 [45].
The PI3K /AKT signaling pathway further possesses a significant part in CRC metastasis, along with various lnc RNAs have been illustrated to manipulate this pathway. The expression of the lnc RNAs, Plnc RNA-1, was significantly greater in CRC tissues, as well as Plnc RNA-1, knock out decreased the transportation, migration as well as invasion significantly of CRC cells as observed by Song et al. [46]. Moreover, functional evaluation demonstrated that Plnc RNA-1 influences the generation as well as metastasis of CRC basically via the phosphatidylinositide 3-kinase (PI3K) /protein kinase B (AKT) signaling pathway. The lnc RNAs SNHG6, hampers ETS1 expression by direct targeting of its 3’-untranslated region(UTR) as well as hampering the expression of PI3K /AKT/ mTOR (mechanical target of rapamycin for stimulation of CRC invasion [47]. Additionally, Wang et al. [48], observed that lnc RNA ABO73614 facilitates the proliferation along with metastasis of CRC cells primarily by PI3K / AKT signaling pathway. The lnc RNA ST3Gal6, antisense1 (ST3Gal6AS1) gets obtained from the promoter area of the genes encoding sialyl transferase ST3Gal6, along with it manipulates α-2,3 sialylation via the ST3Gal6-AS1/ ST3Gal6 axis, thus controlling PI3K /AKT signaling as well as resulting in nuclear transfer ofFoxo1 in CRC cells [49].
Various other signaling pathways have got validated to possess a significant part in CRC metastasis. Functional, evaluation has demonstrated that the lnc RNA FEZF1-AS1, that gets upregulatedin CRC tissues, has the ability of binding to pyruvate kinase 2(PKM) protein along with, enhancing its stability. Greater Cytoplasmic amounts of PKM2 facilitates pyruvate kinase action along with, lactate generation (aerobic glycolysis), while Greater nuclear amounts of PKM2 stimulated by FEZF1-AS1 action, stimulates STAT3 signaling, that facilitates the proliferation along with metastasis of CRC cell [50]. The lnc RNA-cCSC1 can get manipulated by the properties of CRC stem cells by stimulation of Hedgehog signaling pathways as well as hence has a significant part in the CRC metastasis as illustrated by Zhou et al. [51].
Cytoskeletal reorganization is needed for migration along with invasion by tumor cells. That the lnc RNAs can directly control the Cytoskeleton in different tumors an as well as can change the Cytoskeleton through Rho/ROCKassociated signaling at the time of tumor migration. The lnc RNA EPB41L4A-AS1 gets overexpressed in CRC tissues as well as might influence proliferation, invasion along with migration by stimulation of the Rho/ROCK-associated protein kinase signaling pathway as demonstrated by Tang et al. [52]. Thus EPB41L4A-AS1 could get utilized as a new biomarker with regards to diagnosis along with targeted treatment of CRC [53]. Moreover, Tang et al. [54], studied the particular part of lnc RNAs SLCO4A1-AS1 in CRC as well as observed that its actions on cell proliferation, migration along with invasion were mainly correlated with controlling the EGFR/ MAPK pathway. Studies have illustrated that 1-α-25-(OH)2D as well as Vitamin D receptor (VDR) in CRC cells induce MEG3 expression by directly binding to the promoter of lnc RNA MEG3; MEG3 works as a tumor repressor by controlling cluster in action. Hence the VDR/ lnc RNA MEG3/cluster in signaling pathway might be a therapeutic target along with prognostic biomarker for CRC patients [55].
Lnc RNAs control CRC metastasis via sponging miRNA
Various studies have demonstrated that as lnc RNAs posseses various introns, they have the capacity of Sponging miRNA to generate competing endogenous RNAs (ce RNAs) networks. Lnc RNAs get shifted to the target cells through circulation, binding to intracellular miRNA, sponging them, along with restricting their capacity for influencing the translation of their target mRNAs; an event significant for cancer cell proliferation, invasion, migration as well as apoptosos. Hence the capacity for, sponging miRNA is a significant mode by which lnc RNAs control CRC metastasis (Figure 1) [56].
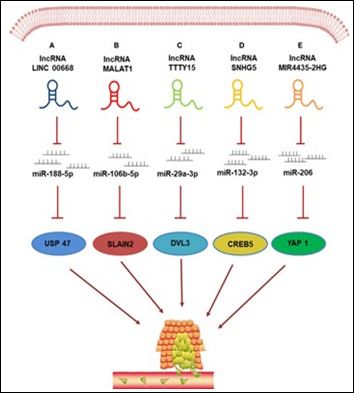
Figure 1: Courtesy ref no-56-LncRNAs regulate CRC metastasis by sponging miRNAs. (A) lncRNA LINC00668 promotes the metastasis and infiltration of CRC cells by sponging miR-188-5p and weakening its inhibiting effect on USP47 expression; (B) lncRNA MALAT1 regulates the miR-106b-5p expression by functioning as a competing endogenous RNA (ceRNA) and regulates the SLAIN2-associated microtubule mobility, leading to the CRC progression; (C) lncRNA TTTY15 functions as the ceRNA to regulate the expression of target gene DVL3 by sponging miR-29a-3p to promote CRC metastasis; (D) lncRNA-SNHG5 influences CRC cell metastasis by modulating the SNHG5/miR-132-3p/CERB5 axis. (E) lncRNA MIR4435-2HG acts as a ceRNA to promote the metastasis of CRC via upregulating YAP1 expression by sponging miR 206.
lnc RNAs LINC 00668, that gets encoded on the chromosome 18p11.31, in the form of a newly invented lnc RNAs was demonstrated by Yan et al. [57], to be correlated with Cancers. LINC 00668 gets upregulated in CRC Cancer tissues along with cells, besides that studies have illustrated that has the capacity for binding to miR 1885-p in CRC cells. Hence LINC 00668 might have a carcinogenic part in CRC by sponging miR 188-5-p along with upregulating USP47 expression. Shan et al. [58], observed that lnc RNASNHG7 controls GALT1 amounts by stimulation of miR-216b as well as has a carcinogenic part in CRC generation. MIR17HG facilitates CRC by inducton of nuclear factor κ (NFκB)/RELA expression, as documented by Xu et al. [59], besides competitively Sponging miR 375. Lnc RNASNHG5 has been illustrated to influence proliferation, metastasis along with migration by controlling the expression of miR-132-3p/ CREB5 [60]. Lnc RNA-CRNDE manipulates CRC propagation as well as chemotherapy resistance by controlling the expression amounts of miR 181a-5p as well as the action of Wnt/β-catenin signaling pathway [61]. Upregulation of Lnc RNA-HNF1A-AS1, observed in Colon Cancer tissues is intricately associated with clinical staging, vascular invasion, and metastasis to lymph nodes along with distant metastasis. Additionally, HNF1A-AS1, controls the expression of miR-34a by working once RNA, thus hampering the expression of miR-34a/SIRT1/ p53 feedback loop as well as stimulation of the Wnt signaling pathway for facilitating the generation of Colon Cancer [62]. Initially lnc RNAMIR4435-2HG was first observed in lung tissues where it works as a ce RNA along with sponge miR 206 for upregulation of expression of YAP1. This MIR4435-2HG facilitates the CRC generation along with metastasis through the miR 206/ YAP1axis [63]. Yang et al. [64], conducted a functional evaluation, that demonstrated, that knocking out of lnc RNA-FTX significantly hampered the with proliferation, migration, along with invasion of the CRC cells. Further evaluation illustrated that FTX had the capacity of directly crosstalk with miR215 as well as hampering its expression, hence avoiding the metastasis of CRC. The expression of lnc RNA-TUG1 in CRC cells is aberrantly escalated, while the expression of miR-600 is downregulated in CRC tissues, cell lines as well as metastatic tissues. Furthermore, TUG1 hampers the migration invasion, along with EMT of CRC cells by competition with miR-600 [65].
A role that had not been earlier appreciated for LncRNA- ZDHHC8P1/, was demonstrated by Li et al. [66] by controlling the propagation as well as metastasis of CRC Via targeting the miR 34a along with positing a viable strategy for therapy of late stage metastatic CRC subjects. Longnon coding RNA SNHG1 expression gets upregulated in case of CRC subjects in human beings. SNHG1 possess the properties of sponging miR-154-5p via crosstalk with EZH2. Hence it decreases its capacity of hampering the expression of cyclin D2 (CCND2). While in the nucleus SNHG1 directly can crosstalk with polycomb repressive complex (PRC2) along with manipulates Histone methylation at the promoters of kruppel like factor2 (KLF2) as well as cyclin dependent kinase inhibitor 2B (CDKN2B) [67]. Zhuang et al. [68], conducted in vivo, in vitro experimental studies where they illustrated that lnc RNAs MALAT 1 facilitates metastasis of CRC, basically through the lnc RNAs MALAT 1/ miR-106b-5p / SLAIN 2axis. Aberrant upregulation of lnc RNAs TTTY15(testis specific transcript Y-linked 15) expression is observed in CRC tissues where it works as a ce RNA as well as thus sponges regulating miR-29a-3p for controlling the expression of the target gene DVL3, that influences the proliferation, as well as metastasis of CRC [69]. The outcomes of these conducted in vivo, in vitro experimental studies have revealed that an innovative oncogenic lnc RNA RNARP-11-757G1.5 that gets overexpressed in CRC tissues controls the expression of YAP1 by sponging miR-139-5p as well as hampering its action, thus facilitating the metastasis along with invasion in CRC [70].
Lnc RNAs control CRC metastasis via protein binding
Akin to molecular escorts, lnc RNAs directly bind to transcription factors along with generate RNA- protein-DNA tertiary complexes that control the transcription of downstream target genes implicated in the CRC metastasis (Figure 2). Mechanistically lnc RNAs work by 2 modes that take place in various parts of the cells. In the nucleus, lnc RNAs can synchronize with or acts antagonists of transcription factors, thus controlling the transcription of metastasis associated genes. In the cytoplasm, lnc RNAs have the capacity of binding to protein as well as change their post-translational modifications for stimulation of protein breakdown; with these proteins significant for cancer these actions can influence tumor metastasis.
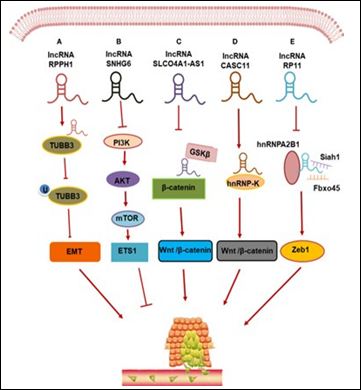
Figure 2: Courtesy ref no-56-LncRNAs regulate CRC metastasis through protein binding. (A) lncRNA RPPH1 interacts with β-III tubulin (TUBB3) to prevent its ubiquitination and induces epithelial-mesenchymal transformation (EMT) of CRC; (B) lncRNA SNHG6 activates the endogenous colorectal cancer invasion pathway by down-regulating the expression of phosphoinositol 3-kinase (PI3K)/protein kinase B (AKT)/rapamycin mechanical target (mTOR); (C) lncRNA SlCO4a1-AS1 stabilized β-catenin by impairing the interaction of β-catenin with GSKβ, thereby activating Wnt/β-catenin signaling in CRC cells; (D) lncRNA CASC11 promotes CRC cell proliferation and metastasis by interacting with hnRNP-K protein and activating the WNT/β-catenin signaling; (E) lncRNA RP11 is involved in the CRC development by forming the RP11/hnRNPA2B1/mRNA complex, which accelerates the mRNA degradation of two E3 ligases Siah1 and Fbxo45 and prevents the proteasomal degradation of Zeb1 to increase its nuclear accumulation.
Particularly LncRNA-SATB2-AS1 is downregulated in CRC tissues. Evaluation of the mode of SATB2-AS1, was demonstrated to directly bind to WDR5 as well as GADD 45A as well as cis activation of SATB2 transcription by manipulating Histone H3 lysine4 (trimethylation) (H3 K4m3) as well as DNA demethylationin the SATB2-AS1 promoter area [71]. In case of intestinal cancer cells, the lnc RNA-RP11/hnRNPA2B1 (protein)/ mRNA complex exaggerated the breakdown of Siah1 as well as Fbx045 mRNA, both of which encode ubiquitin E3 ligases, thus avoiding the proteasomal breakdown of Zeb1, a transcription factor correlated with EMT was documented by Wu et al, [72]. The post-translational upregulation of Zeb1 is key for RP11 stimulated spread of intestinal Cancer cells.
LncRNA-CPS-ITI has the capacity of blocking hypoxia-induced autophagy through hampering of HIF-α amounts, thus avoiding EMT along with metastasis in CRC [73]. It has been illustrated in recent studies that LncRNA RPPH1 can crosstalk with β-III tubulin (TUBB3) for avoiding ubiquitination, that stimulates EMT along with facilitates CRC metastasis [74]. LncRNA LUCAT 1 was illustrated to facilitate proliferation, apoptosis, migration along with invasion of CRC cells, in vivo, as well as in vitro. Evaluation, demonstrated that LUCAT 1 binds to UBA52, that encodes ubiquitin, ubiquitin the 60S ribosomal protein L40 (RPL40). Via binding to UBA52, LUCAT 1 aims at the ribosomal protein L40/MDM2/p53 pathway for facilitation of tumorigenesis as well as stimulate CRC cell cycle arrest as well as apoptosis [51]. An lnc RNA that is markedly expressed in CRC SNHG1 facilitates CRC cell proliferation, mobility, as well as EMT in vitro. SNHG1 facilitates CRC propagation by hampering EPHA7-modulated negative control via an event based on the transcription factor EZH2. SNHG1 escalates the stableness of EZH2 mRNAby crosstalk with the RNA binding protein FUS along with SPONGING miR-186b-5p- stimulated silencing as well as escalating EZH2 expression in CRC [75]. Ding et al. [76], observed that combined lnc RNACRNDE as well as EZH2, a crucial constituent of PRC2, hampered the expression of 2 downstream target genes dual specific-phosphatase (DUSP5) along with CDKN1A that has a significant part in CRC proliferation as well as metastasis. LINCO1413 binds to hn/RNP-K along with nuclear transfer of YAP1 (associated protein 1) TAZ, hence controlling the expression of ZEB1 in CRC cells as well as facilitating cancer metastasis [77]. An upregulation of lnc RNA CASC11 in CRC gets association with CRC escalation as well as metastasis as it influences its actions by crosstalk with hnRNP-K protein along with stimulation of the Wnt/ β-catenin signaling pathway as demonstrated by Zhang et al. [78]. Studies have illustrated that LINCO1354 getting over expressed in CRC led to enrichment of genes associated with the Wnt/β-catenin signaling pathway. In case of CRC LINCO1354 basically crosstalks with hnRNP-D for controlling the stableness of β-catenin mRNA along with stimulation of Wnt/β-catenin signaling pathway [79]. The lnc RNA ROR has been newly invented lnc RNA as well as Li et al. [80], illustrated that knockout of the lnc RNA ROR gene significantly escalated the protein amounts of p53 along with its target genes, while over expression of ROR had the reverse action. Hence it is concluded that the amounts of p53 protein has a negative association with ROR, which might take part in the CRC propagation via the p53 signaling pathway.
Importance of Lnc RNAs in Metastasis Clinically
Various studies have documented that lnc RNAs influence significant biological actions in the CRC metastasis. Hence from the Clinical Importance point of view the maximal application that is practical regarding lnc RNAs is their capacity to act as biomarker for diagnosing CRC metastasis just at initiation. For enhancing the pace along with ease of CRC diagnosis, these differentially expressed lnc RNAs can be found in metastatic as well as non metastatic samples like blood as well as urine. Additionally, certain lnc RNAs intricately associate with the sensitivity to radiotherapy as well as chemotherapy that might aid in generation of innovative treatments possessing a higher effectiveness with regards to the clinical treatments of metastatic CRC.
A big problem that is correlated with the present diagnostic biomarkers for CRC is their absence of enough specificity as well as sensitivity that can result in false positive or false negative outcomes. In the current yrs. various studies have demonstrated that certain lnc RNAs can be isolated in the blood, serum urine as well as other body fluids of the subjects presenting with cancer [81]. The utilization of these lnc RNAs can be done as biomarkers for as early diagnosis as feasible along with anticipation of prognosis of the patients [37,43,46,49,50,51,54,60-86,95,97]. Like lnc RNAsRP11-296E3.2 that has a very high expression in case of metastatic CRC, is correlated with short overall survival (OS). With regards to sensitivity as well as specificity in diagnosis of CRC metastasis, RNAsRP11-296E3.2 was better in contrast to CEA in plasma [80]. Xu et al. [87] observed that the plasma amounts of four lnc RNAs ZFAS1, SNHG1, LINC00909, as well as LINC 00654 had an amount that was significantly lesser in the postoperative CRC samples. These 4 lnc RNAs in association together had a great diagnostic efficacy in early CRC. Various studies have documented that lnc RNATINCR has an influence on the PI3K/AKT / mTOR signaling pathway by sponging of miR-7-5p as well as possessing a significant part in facilitation of CRC. Additionally, in contrast to healthy controls, plasma amounts of lnc RNATINCR had a significant escalation in CRC subjects that points to their capacity for earlier diagnosis [88]. An association evaluation by Pan etal. [89], demonstrated that in early CRC subjects, plasma amounts of lnc RNA PVT1 were significantly greater in contrast CEA, that pointed that PVT1 possesses much greater capacity as a biomarker for early CRC detection. A reduction in the lnc RNA-ATB expression possess a significant influence on the propagation of Colon Cancer by changing the epithelial biomarker expression like E-cadherin. An associated clinical evaluation illustrated that there was a significant escalation of plasma amounts of lnc RNA-ATB in Colon Cancer 1mth following surgery, pointing that it might be efficacious in early detection of CRC [90]. Ye et al. [91], documented that the amounts of lnc RNA-GNAT-1 in plasma of CRC subjects is associated with Tumor Node Metastasis (TNM) staging, whereas the Receiver Operating Characteristics curve (ROC) illustrated that the plasma lnc RNA-GNAT-1 possesses a moderate to good detection ability for CRC.
Lnc RNAs have been illustrated to play a part in lymph node metastasis, lung metastasis, and bone metastasis along with brain metastasis that are correlated with various cancers [92]. A markedly expressed Lnc RNA in CRC is CCAT2 that is intricately associated with TNM Stage, since CCAT2 amounts are escalated from Stage I to IV. Escalated CCAT2 expression is intricately correlated with poor cell differentiation along with depth as well as invasion, lymph node metastasis, distant metastasis, vascular infiltration, as well as escalated TNM LINC 00858 Staging, along with might be linked to liver metastasis [93]. The expression amounts of LINC 00858 are significantly greater, in CRC tissues in contrast to nearby tissues as well as greater LINC 00858 expression is linked to TNM Staging, lymph node metastasis, along with the histological grade. Silencing hampers the proliferation, migration as well as invasion of CRC cells as well as stimulates apoptosis [94]. MEIF2-AS1 expression amounts are intricately associated with tumor histological grade, lymphatic along with distant metastasis, TNM Staging, in addition to vascular infiltration [95]. Escalated Lnc RNA BANCR expression in CRC is correlated with lymph node metastasis, as well as in OS of subjects with escalated BANCR expression is smaller [96]. Chen et al. [97] divided 115 CRC subjects into 2 groups depending on the median Lnc RNA XIST expression amounts along with evaluation of these groups demonstrated that XIST expression was intricately associated with tumors size, histological grade, distant metastasis, TNM Staging. Akin to that the expression of lnc RNASNHG3 was significantly upregulated in case of CRC tissues in addition to SNHG3 expression had a positive association with the escalated clinical Staging as well as with distant metastasis [98].
Lnc RNA represent a significant group of molecules, in the human transcriptome. They play a significant part besides in various physiological events but further in different disease events that includes cancer generation as well as metastasis A lot of lnc RNAs are tumor particular along with their expression might change sensitivity to radiotherapy as well as chemotherapy. Hence they are anticipated to be of utility as novel therapeutic targets [99].
Lnc RNA MALAT 1 that was initially observed to be differentially expressed in case of patients with non-small cell lung cancer, is further significantly over expressed in CRC. Decreased expression of MALAT 1 can hamper the propagation as well as metastasis of CRC along with enhance the sensitivity of cancer cells to 5-FU. This gives a newer divergence for planning, fashioning as well as generation, of innovative treatments for metastatic CRC [100]. Additionally, MALAT 1 was observed to have significant upregulation in CRC tissues in addition to cells receiving treatments from oxyplatin [101]. Further it facilitates ant oxidative response primarily through miR- 324-3p/ADAM 17 Axis along with escalated sensitivity to oxyplatin [101]. An experiment planned for selection of lnc RNAs associated with oxyplatin resistance, Sun et al. [102]. Found that lnc RNAs CRNDE, H19, UCA1 as well as HOTAIR influence the sensitivity to oxyplatin. Escalated expression of HOTAIR is correlated with enhanced tumor nodules along with metastatic stages, besides poor prognosis of CRC. Downgulated lnc RNA POUF1P4 decreased the sensitivity of metastatic CRC cells to cetuximab, as illustrated by Peng et al. [103], as well as might serve as a potential new therapy for metastatic CRC. Wang et al. [104], demonstrated that the LINC00473 expression amounts were significantly greater, in a group of drug resistant patients in contrast to non-drug resistant patients as well as knockouts of LINC00473 rectified paclitaxel stimulated cytotoxicity, hampered cell viability along with colony generation, stimulated apoptosis, as well as reduced the capacity of tumor cells for migration along with invasion. Further Ogunwobi et al. [105], reviewed with the role of lnc RNAs for utility as anticipating biomarkers aid clinical decision making, like the existence of KRAS gene mutations predicting advantage from Epidermal Growth factor Receptor (EGFR) inhibiting antibodies. Nevertheless, few biomarkers have been translated into clinical practice emphasizing the requirement for further evaluation. They reviewed a range of protein, DNA along with RNA-based biomarkers under evaluation with regards to diagnostic, anticipating as well as prognostic characteristics for CRC. Specifically, long non-coding RNAs (lncRNA), have been evaluated as biomarkers in a range of cancers including colorectal cancer. They explored particularly evaluate the potential role of lncRNA plasmacytoma variant translocation 1 (PVT1), an oncogene, as a diagnostic, prognostic, along with therapeutic biomarker in colorectal cancer [105]. Jung et al. provided the mode by which the Korean plant artemis acts through JNK system in inhibition of CRC lines [106]. Further Muieinol-Romay as well as Diaz-Lagares [107], reviewed how in the era of precision oncology, liquid biopsy has presented as a major strategy to classify the circulating tumor elements present in body fluids, including cell-free DNA and RNA, circulating tumor cells, and extracellular vesicles. This non-invasive tool has allowed the identification of relevant molecular alterations in CRC patients, including some indicating the disruption of epigenetic mechanisms. Epigenetic alterations found in solid and liquid biopsies have shown great utility as biomarkers for early detection, prognosis, monitoring, and evaluation of therapeutic response in CRC patients. Here, we summarize current knowledge of the most relevant epigenetic mechanisms associated with cancer development and progression, and the implications of their deregulation in cancer cells and liquid biopsy of CRC patients. In particular, we describe the methodologies used to analyze these epigenetic alterations in circulating tumor material, and we focus on the clinical utility of epigenetic marks in liquid biopsy as tumor biomarkers for CRC patients. We also discuss the great challenges and emerging opportunities of this field for the diagnosis and personalized management of CRC patients [107]. Lin et al. [107], tried to identify an immune-related lncRNA signature for the prospective evaluation of prognosis in these patients. Gene expression and clinical data of colon cancer patients were accessed from The Cancer Genome Atlas (TCGA). Immune-related lncRNAs were isolated by a correlation evaluation among IRGs and lncRNAs. In total, 447 samples were divided into a training cohort (224 samples) as well as testing cohort (223 samples). Univariate, lasso and multivariate Cox regression analyses identified an immune-related nine-lncRNA signature intricately associated with OS in colon cancer patients in the training dataset. A risk score formula involving nine immune- related lncRNAs was generated to evaluate the prognostic value of the lncRNA signature in the training dataset. Colon cancer subjects with a high risk score had poorer OS than those with a low risk score. A multivariate Cox regression analysis confirmed that the immune- related nine-lncRNA signature could be an independent prognostic factor in colon cancer patients. The results were further validated in the testing cohort and the entire TCGA cohort. Furthermore, a gene set enrichment analysis demonstrated various pathways with significant enrichment in the high- and low-risk groups that might be of utility in formulating clinical approaches along with getting insight in the underlying modes. Finally, a quantitative real-time polymerase chain reaction assay observed that the nine lncRNAs were significantly differentially expressed in colon cancer cell lines. The results of this study indicate that this signature has important clinical implications for improving predictive outcomes and guiding individualized treatment in colon cancer patients. These lncRNAs could be potential biomarkers affecting the prognosis of colon cancer [108].
Discussion
Thus CRC metastasis gets stimulated by different factors in vivo, along with in vitro. Of the in vivo factors, alteration in tumors cell adhesion with the adjacent cells along with extracellular matrix, EMT, as well as the impairment of different motor proteins facilitate the CRC metastasis. Various signaling pathways like the Wnt/β-catenin, as well as PI3K /AKT signaling pathways possess a significant part in the CRC metastasis. Lnc RNAs further work as the ceRNA for controlling the downstream target genes getting expressed or constituents of CRC metastasis-correlated signaling pathway to influence CRC metastasis. Via epidemiological studies it has been demonstrated that CRC metastasis is intimately associated with various factors in vitro. Like Tea Polyphenols (TP’s), can influence anti-inflammatory, antioxidant or pro oxidant actions for facilitation of apoptosis as well as work at a lot of levels for hampering CRC growth as well as metastasis [109]. ii) In CRC cells upregulation of expression of UCA1 as well as hypoxia inducible factor-alpha (HIFα), is stimulated by Nicotine as well as facilitates proliferation, along with metastasis of CRC cells [110]. Additionally, subjects having a family history of Colorectal Cancer as well as inflammatory bowel disease (IBD) have a greater probability of generation, of Colorectal Cancer in contrast to subjects without this family history of these diseases [9]. Evaluation of the association of diet, lifestyle, along with the risk of CRC initiation with the idea of molecular epidemiology, as well as making it clear how much is the key exposure time period would aid us in getting better insight regarding how these factors influence the CRC initiation along with etiopathogenesis. Getting clear insight on CRC initiation along with generation will further aid in getting to know the clinical results [111]. Evaluation of the actions of in vitro factors as well as analyzing the mode of particular to CRC metastasis, isolation of significant molecules implicated in CRC etiopathogenesis would aid in the early clinical detection along with ideal therapy of CRC subjects.
Occasional strategies are present with regards to screening for CRC as well as maximum biomarkers brought in utilization for CRC detection, like CA199 get differential expression in a lot of cancers. Thus there is an absence of sensitivity as well as a specificity with regards to CRC detection. Escalated proof has illustrated that aberrant expression of the lnc RNAs in human tissues as well as serum possesses the capacity for early diagnosis in addition to anticipation of patient’s prognosis. Like DANCR expression was lesser in serum samples of post-operative patients in contrast to patients with recurrence. Furthermore, serum DANCR expression possessed a significant association with TNM Staging [112].
Our insight with regards to the modes behind the CRC along with treatment results have enhanced importantly with the considerable advances in research. Nevertheless, in metastatic CRC along with treatment results as well as mortality rates continue to be not satisfactory. Hence a requirement at war footing is required to generate newer therapeutic targets for metastatic CRC. Animal dependent studies have demonstrated that lnc RNAs possess significant part in metastatic CRC along with can get utilized in the form of therapeutic targets for metastatic CRC. Once lnc RNAs get silenced, CRC cells illustrate greater radio sensitivity, hence making it a potential target as far as clinical therapy is concerned for metastatic CRC [113]. Wu et al. [114], generated a xenograft mouse model as well as found that deletion of Lnc RNAPVT1 in addition to over expression of miR-16-5p has the capacity of reducing the tumor volume to the least. Utilization of Lnc RNAPVT1-miR-16-5p/ Vascular endothelial growth factor A (VEGFA)/VEGF receptor 1 (VEGFR1)/AKT Axis, Lnc RNAPVT1 has a direct implication in the propagation of CRC along with represents the potential therapy for CRC. Animal experimentation conducted by Yao as well as Li [115], illustrated that MIR 600HGhas the capacity for hampering tumor generation. In contrast to Lnc RNA MIR 600HG by itself alone, its combination with oxaliplatin resulted in significant hampering of CRC stem cells metastasis along with tumor growth.
Conclusions
Thus Colorectal cancer (CRC) continues to be the commonest etiology of death worldwide, despite progress made in its diagnosis along with management via surgery, chemotherapy, radiotherapy, and immunotherapy. Novel therapeutic agents have improved survival in both the adjuvant and advanced disease settings, although with an escalated risk of toxicity and cost. However, metastatic disease continues to have a poor long-term prognosis and significant problems persist in view of two late stage diagnosis along with treatment failure.
Despite Lnc RNAs, displaying a great capacity with regards to clinical utilization, these deficiencies are still present in Lnc RNAs research i) the precise mode behind the actions of different Lnc RNAs in CRC is still ill understood, that emphasizes the requirement for future research in its initiation along with generation of CRC ii As far as their utilization in the form of biomarkers for CRC, the heterogeneity of Lnc RNAs expression might make it tough to obtain a correct diagnosis. iii) Occasional experiments only have been conducted in animals for validation of therapy results. Hence limited results make it tough to corroborate the utility of Lnc RNAs as diagnostic along with therapeutic markers .Thus it is necessary to evaluate the association among Lnc RNA as well as CRC, for getting a robust base for the future utilization in CRC diagnosis as well as therapy. Nevertheless, research on lnc RNAs in human cancers is anticipated to provide main breakthrough regarding early diagnosis, risk evaluation, along with treatment in the coming future.
References
- Kulvinder KK, Allahbadia GN, Singh M (2016) An Update on Epi- genetics in Mammalian Reproduction with Emphasis on HumanRe- production; J Clin Epigenetics 4:20
- Kulvinder KK, Allahbadia GN, Singh M (2018) An Update on Genetics of Disorders of Sexual Development Along with Signal Transduction Pathways-Clinical implications: A Review Int J Genet Sci 4: 1-12.
- Kulvinder KK, Allahbadia GN, Singh M (2016) Gonadal Dysgene- sis-with Special Emphasis on the Molecular Mechanisms of SRY Mu- tations in Disorders of Sex Development (DSD) Reulting in Female Sex Reversal in 46XY Hereditary Genet 5: 164.
- Kulvinder KK, Allahbadia GN, Singh M (2020) How does Epigenetics Regulate Development of Placenta and Placental Pathologies like PreEclampsia (PE), Intrauterine growth Restriction (IUGR)-With Main emphasis on Advances in Bioengineering and Biomedical Science Research 2020.
- Kulvinder KK, Allahbadia GN, Singh M (2015) An Update on microR- NA’s and Metabolic Regulation with Future Therapeutic Potentials Re- garding Diagnosis and Treatment of Obesity, MetabolicSyndrome and Other Related J Health Med Informa.
- Kulvinder KK, Allahbadia GN, Singh M (2021) Potential role of Epi- genetic Modulation in prevention or therapy for Diabetic Kidney Dis- ease-still a dream or a reality-A Systematic Review’’under review. In J Diabetes Management and Nephropathy.
- Nie H, Wang Y, Liao Z, Ou C (2020) The function and mechanism of circular RNA’s in Gastrointestinal Cell Prolif 53: e12815.
- Ogino S, Chen AT, Fuchs CS, Giovanucci E (2011) Molecular patholog- ical epidemiology Colorectal neoplasia: An emerging transdisiplinary and inter disciplinary field Gut 60: 397-411.
- Hughes LAE, Simons C, Van de Brandt PA, VanEngeland M, Wei- jenberg MP (2017) Lifestyle, diet, and Colorectal Cancer according to (Epi) genetic instability: Current evidence and future directions of molecular pathological epidemiology. Curr Colorectal Cancer Rep 13: 455-469.
- Ogino S, Nowak JA, Hamada T, Milner DA, Nishihara R (2019) In- sights into pathogenic interactions among environment, host and Tu- mor at the crossroads of molecular pathology and epidemiology. Annu Rev Pathol 14: 83-103.
- Allemani C, Matsuda T, DiCarlo V, Harewood R, Matz M, et al. (2018) Global surveillance of trends in cancer survival 2000-14(CON- CORD-3): Analysis of individual records for 37513025 patients diag- nosed with one of 18 cancers from 322-population based registries in 71 Lancet 391: 1023-1075.
- Marchese FP, Raimondi I, Huarte M (2017) The multidimensional mechanisms of longnon coding RNA Genome Biol 18: 206.
- Ponting CP, Oliver PL, Reik W (2009) Evolution and functions of long non coding RNAs. Cell 136: 629-641.
- Kopp F, Mendell JT (2018) Functional classification and experimental dissection of long non coding Cell 172: 393-407.
- Zhuang C, Ma Q, Zhuang C, Zhang F, Gui Y (2019) Lnc RNAs GClnc1 promotes proliferation and invasion of bladder cancer through activa- tion of FASEB J 33: 11045-11059.
- Yang J, Li C, Mudd A, GuX (2017) Lnc RNAs PVT1 predicts prognosis and regulates Tumor growth in prostate Biosci Biotechnol Bio- chem 81: 2301-2306.
- He X, Li S, YuB, Kuang G, Wu Y, et al. (2019) Upregulation of LINC 00467 promotes tumorigenesis in Colorectal Cancer. J Cancer 10: 6405-6413.
- Huang JZ, Chen M, Gao XC, Zhu S, Huang H, et al. (2017) A peptide encoded by a putative lnc RNAs HOXB-AS3 suppresses Colon Can- cer Mol Cell 68: 171-84.e176.
- Yue I, Liu C, Sun H, Liu M, Song C, et al. (2018) A positive feed- forward loop between lnc RNAs CYTOR and Wnt/β-catenin signaling promotes metastases of Colon Mol Ther 26: 1287-1298.
- Beerman I, Piccoli mt, Viereck J, Thum T (2016) Non-coding RNAs in development and disease: Background, mechanism, and therapeutic Physiol Rev 96: 1297-325.
- Huang G, Zhu H, Wu S, Cui M, XuT (2019) Lnc RNAs can bea proba- ble mechanism and a novel target for diagnosis and therapy in Fragile X Front Genet 10: 446.
- Matsumoto A, Pasut A, Matsumoto M, Yamashi R, Fung J, et (2017) mTORc 1 and muscle regeneration are regulated by LINC 00961-en- coded by SPAR poly peptide. Nature 541: 228-232.
- Anderson DM, Anderson KM, Chang CL, Mukarewich CA, Nelson BR, et al. (2015) A micro peptide encoded by a putative long non-coding RNA regulates muscle Cell 160: 595-606.
- Qian X, Zhao J, Yeung PY, Zhang QC, Kwok CK (2019) Revealing lnc RNAs structures and interaction by sequencing based approaches. Trends Biochem Sci 44: 33-52.
- Quin JJ, Chang HY (2016) unique feautures of long non-coding RNAs biogenesis and Nat Rev Genet 17: 47-62.
- Fang Y, Full wood MJ (2016) Roles functions and mechanisms of long non-coding RNAs in Genomics, Proteomics, Bioinf 14: 42-54.
- Alvarez-Dominiquez JR, Lodish HF (2017) Emerging mechanisms of longnon coding RNAs function during normal and malignant haemato- Blood 130: 1965-1975.
- Batista PJ, Chang HY (2013) Longnon coding RNAs: Cellular address codes in development and disease. Cell 152: 1298-1307.
- Schmitz KM, Mayer C, Postpeka A, Grummt I (2010) Interaction of non coding RNA with the rDNA promoter mediates recruitment DNMT3b and silencing of rRNA Genes Dev 24: 2264-2269.
- DjupedaL I, Ekwall K (2009) Epigenetics: Heterochromatin meets Cell Res 19: 282-295.
- Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, et (2010) Long non coding RNA as a modular scaffold of Histone modification complexes. Science 329: 689-693.
- Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, et (2012) Landscape of transcription in human cells. Nature 489: 101-108.
- Wilusz JE , Sunwoo H, Spector DI (2009) Long non coding RNAs: Functional surprises for the RNA Genes Dev 23: 1494-1504.
- Ou C, Sun Z, Li X, Ren W, Qin Z (2017) MiR-590-5p, a density sensi- tive micro RNA inhibits tumorigenesis by targeting YAP1 in Colorectal Cancer Lett 399: 53-63.
- Sun Z, Ou C, Liu J, Chen C, Zhou Q, et al. (2019) YAP1induced MALAT 1 promotes epithelial-mesenchymal transition and angiogen- esis by sponging miR 126-5-p in Colorectal Cancer. Oncogene 38: 2627-2644.
- Wang Y, Nie H, He X, Liao Z, Zhou Y, et (2020) The emerging role of super-enhancer-derive non coding RNAs in human cancer. Thera- nostics 10: 11049-11062.
- Ou C, Sun Z, He Z, LiX, Fan Z, et al. (2020) Targheting YAP1/LINC 00152/FSCN 1 signaling axis prevents the progression of Colorectal Adv Sci (Weinh) 7: 1901380.
- Lu Y, Zhao X, Liu Q, Li C, Graves-Deal R (2017) long non coding RNA MIR100HG-deriver miR-100 and miR-125 b mediate cetuximab resis- tance via Wnt/β-catenin Nat Med 23: 1331-1341.
- Huang JL, Cao SW, Ou QS, Yang B, Zheng SH, et (2018) The long non coding RNA PTTG3P promotes cell growth and metastasis via up- regulating PTTG1 and activating PI3K/AKT signaling in hepatocellular carcinoma. Mol Cancer 17: 93.
- Xu S, Kong D, Chen Q, Ping Y, Pang D (2017) Oncogenic long non coding RNA landscape in breast cancer. Mol Cancer 16: 129.
- Peng WX, Huang JG, Yang L, Gong AH, Mo YY (2017) Linc-RoR promotes MAPK/ERK signaling and confers estrogen-independent growth of breast Mol Cancer 16: 161.
- Trimarchi T, Bilal E, Ntziachristos P, Fabri G, Dalla-Favera R, et al. (2014) Genome wide mapping and characterization of Notch regulated long non coding RNAs in acute Cell 158: 593-606.
- Yu J, Han Z, Sun Z, Wang Y, Zheng M, et al. (2018) Lnc RNAs SL- CO4A1-AS1 facilitates growth and metastasis of Colorectal Cancer through β-catenin dependent Wnt JExp Clin Cancer Res 37: 222.
- Wu X, Li R, Song Q, Zhang C, Jia R, et al. (2019) JMJD2C promotes Colorectal Cancer metastasis via regulating histone methylation of MALAT1 promoter and enhancing β-catenin signaling JExp Clin Cancer Res 38: 435.
- Zhang M, Weng W, Zhang Q, Wu Y, Ni S, et al. (2018) The lnc RNAs NEAT 1 activates Wnt/β-catenin signaling pathway and promotes Col- orectal Cancer progression via interacting with J Haematol On- col 11: 113.
- Song W, Mei JZ, Zhang M (2018) Long non coding RNA Plnc RNA-1 promotes Colorectal Cancer cell progression by regulating the PI3K / AKT signaling Oncol Res 26: 261-268.
- Meng S, Jian Z, Yan X, Li J, Zhang R (2019) Lnc RNAs SNHG6 inhibits cell proliferationand metastasis by targeting ETS1 via the of PI3K / AKT/mTOR pathway in Colorectal Cancer. Mol Med Res 20: 2541-2548.
- Wang Y, Kuang H, Xue J, Liao I, Yin F, et al. (2017) Lnc RNAs ABO73614 regulates proliferation and metastasis of Colorectal Cancer cells via the PI3K /AKT signaling pathway. BioMed Pharmacotherap 93: 1230-1237.
- Hu J, Shan Y, Ma J, Pan Y, Zhou H, et al. (2019) Lnc RNA ST- 3Gal6-AS1/ ST3Gal6 axis mediates Colorectal Cancer progression by regulating α-2,3 sialylation via PI3K /AKT Int J Cancer 145: 450-460.
- Bian Z, Zhang J, Li M, Feng Y, Zhang I (2018) Lnc RNA FEZF1-AS1 promotes tumor proliferation and metastasis in Colorectal Cancer by regulating PKM2 Clin Cancer Res 24: 4808-4819.
- Zhou H, Xiong Y, Peng L, Wang R, Zhang H, et al. (2020) Lnc RNA cCSC1 modulates along with Cancer stem cell properties in Colorectal Cancer via activation of the Hedgehog signaling pathway. J Cell Bio- chem Sci 121: 2510-2524.
- Tang Y, He Y, Zhang P, Wang J, Fan C, et (2018) Lnc RNAs regulate the Cytoskeleton and related Rho/ROCK-signaling in Cancer metasta- sis. Mol Cancer 17: 77.
- Bin J, Nie S, Tang Z, Kang A, Fu Z, et (2020) Long non coding RNA EPB41L4A-AS1 functions as an oncogene by regulating Rho/ROCK pathway in Colorectal Cancer. J Cell Physiol 236: 523-535.
- Tang R, Chen J, Tang M, Liao Z, Zhou I, et al. (2019) Lnc RNA SL- CO4A1-AS1 predicts poor prognosis and promotes proliferation and metastasis via the EGFR/MAPK pathway in Colorectal Cancer. Int J Biol Sci 15: 2885-2896.
- Zhu Y, Chen P, Gao Y, Ta N, Zhang Y, et (2018) MEG3 activated by Vitamin D inhibits Colorectal Cancer cells proliferation and migration via regulating clusterin. EBio Medicine 30: 148-157.
- Liao Z, Nie H, Wang Y, Luo J, Zhou J, et al. (2021) The emerging Landscape of Long non coding RNAs in Colorectal Cancer Front Oncol 11: 641343.
- Yan S, Yue Y, Wang J, Li W, Sun M, et (2019) LINC00668, promotes tumorigenesis and progression through sponging miR 188-5-p and up- regulating USP47 in Colorectal Cancer. Eur J Pharmacol 858: 172464.
- Shan Y, Ma J, Pan Y, Hu J, Liu B, et (2018) lnc RNASNHG7 spong- es miR-216b to promote proliferation and liver metastasis of Colorectal Cancer through up regulating GALNT1. Cell Death Dis 9: 722.
- Xu J, Meng Q, Li X, Yang H, Xu J, et al. (2019) Longnon coding RNA MIR17HG promotes Colorectal Cancer progression via miR 17-5p. Cancer Res 79: 4882-4895.
- Zhang M, Li Y, Wang H, Yu W, Lin S, et (2019) Lnc RNA-SNHG5 af- fects cell proliferation, metastasis and migration of Colorectal Cancer through regulating miR-132-3p/CREB5. Cancer Biol Ther 20: 524-536.
- Han P, Li JW, Zhang BM, Lv JC, Li YM, et al. (2017) The Lnc RNA- CRNDE promotes Colorectal Cancer cell proliferation and chemore- sistance via miR 181a-5p mediated regulation of Wnt/β-catenin signal- Mol Cancer 16: 9.
- Fang C, Qiu S, Sun F, Li W, Wang Z, et al. (2017) Long non coding RNA HNF1A-AS1 mediated repression of miR-34a/SIRT1/p53 feed- back loop promotes the metastasis progression of Colon Cancer by functioning as a competing endogeneous RNA. Cancer Lett 410: 50-62.
- Dong X, Yang Z, Yang H, Li D, Qiu X (2020) Long non coding RNA MIR4435-2HG promotes Colorectal Cancer proliferation and metasta- sis through mi 206/ Front Oncol 10: 160.
- Yang Y, Zhang J, Chen X, Xu X, Cao G, et al. (2018) Lnc RNA- FTX sponges miR-215 and inhibits phosphorylation of vimentin for promot- ing Colorectal Cancer Gene Ther 25: 321-330.
- Sun J, Hu J, Wang G, Yang Z, Zhao C, et al. (2018) LncRNA-TUG1 promoted KIAA199 expression via miR-600 to accelerate cell metas- tasis and epithelial-mesenchymal transition in Colorectal JExp Clin Cancer Res 37: 106.
- Li C, Liu T, Zhang Y, Li Q, Jin LK (2019) LncRNA-ZDHHC8P1 pro- motes the progression and metastasis in Colorectal Cancer by target- ing Eur Rev Med Pharmacol Sci 23: 1476-1486.
- Xu M, Chen X, Lin K, Zeng K, Liu X, et (2018) The Longnon coding RNA SNHG1 regulates Colorectal Cancer cell growth through interac- tions with EZH2 and miR-154-5p. Mol Cancer 17: 141.
- Zhuang M, Zhao S, Jiang Z, Wang S, Sun P, et al. (2019) MALAT 1sponges miR-106b-5p topromote the invasion and metastasis of Colorectal Cancer via SLAIN 2 enhanced microtubules mobility. E Bio Medicine 41: 286-298.
- Zheng XY, Cao MZ, Ba Y, Li YF, Ye JL (2020) Lnc RNA testis specific transcript Y-linked 15(TTTY15) promotes proliferation, migration and invasion of Colorectal Cancer cells via regulating miR-29a-3p/DVL3 Cancer Biomark.
- Zhu X, Bu F, Tan T, Luo Q, Zhu J, et al. (2020) Longnon coding RNA RP-11-757G1.5 sponges miR-139-5pand up regulates YAP1 thereby promoting the proliferation and liver, spleen metastasis of Colorectal JExp Clin Cancer Res 39: 207.
- Xu M, Xu X, Pan B, Chen X, Lin K, et al. (2019) LncRNA-SATB2-AS1 inhibits tumor metastasis and affects the tumor immune cell micro- environment in Colorectal Cancer by regulating SATB2. Mol Cancer 18:135.
- Wu Y, Yang X, Chen Z, Tian L, Jiang G, et al. (2019) M(6)A-induced LncRNA-RP11 triggers the dissemination of Colorectal Cancer cells via upregulation of Mol Cancer 18: 87.
- Zhang W, Yuan W, Song J, Wang S, Gu X (2018) LncRNA-CPS-ITI suppresses EMT and metastasis of Colorectal Cancer by inhibiting hypoxia-induced autophagy through inactivation of HIF-1alpha. Bio- chemie 44: 21-27.
- Liang ZX, Liu HS, Wang FW, Xiong L, Zhou C, et al. (2019) LncRNA RPPH1 promotes Colorectal Cancer metastasis by interacting with TUBB3 and by promoting exosome mediated macrophage M2 polar- Cell Death Dis 10: 829.
- Di W, Weinan X, Xin L, Zhiwei Y, Xinyue G, et al. (2019) Longnon coding RNA SNHG1 facilitates Colorectal Cancer metastasis through targeting EZH2 regulated EPHA7. Cell Death Dis 10:514.
- Ding J, Li J, Wang H, Tian Y, Xie M, et (2017) Long non coding RNA CRNDE promotes Colorectal Cancer cell and affects the Wang tumor immune cell proliferation via epigenetically silencing DUSP5/ CDKN1A expression. Cell Death Dis 8: e2997.
- Ji L, Li X, Zhou Z, Zheng Z, Jin L, et (2020) LINCO1413/ hnRNP-K/ ZEB1 Axis accelerates cell proliferation and EMT in Colorectal Can- cer via inducing YAP1/TAZ1 translocation. Mol Ther Nucleic Acids 19: 546-561.
- Zhang Z, Zhou C, Chang Y, Zhang Z, Hu Y, et al. (2016) Long non coding RNA CASC 11 interacts with hnRNP-K and activates the Wnt/β-catenin pathway to promote growth and metastasis in Colorec- tal Cancer Lett 376: 62-73.
- Li J, He M, Xu W, Huang S (2019) LINCO1354 interacting with hn- RNP-D contribute to the proliferation and metastasisin Colorectal Cancer through activating Wnt/β-catenin signaling pathway. JExp Clin Cancer Res 38:161.
- Li H, Jiang X, Niu X (2017) Long non coding RNA Reprogramming (ROR) promotes cell proliferation in Colorectal Cancer via affecting Med Sci Monit 23: 919-928.
- Sarfi M, Abbastabar M, Khalili E (2019) Long non coding RNA bio- marker-based cancer J Cell Physiol 234: 6971-6986.
- Cheng Y, Wu J, Qin B, Zou BC, Wang YH, et al. (2020) CREB1-in- duced lnc RNA LEF1-AS1 contribute to Colorectal Cancer progression via the miR-489/DIAPH1 Biochim Biophys Acta 526: 678-684.
- Yu X, Wang D, Wang X, Sun S, Zhang Y, et (2019) CXCL2 /CXCR4 promotes inflammation-driven Colorectal Cancer progression through activation of Rho A LOC441461 (STX17-AS1 signaling by sponging miR-133a/3p. JExp Clin Cancer Res 38: 32.
- Feng W, Li B, Wang J, Zhang H, Liu Y, et al. (2020) Long non coding RNA LINC 00115 Contributes to the progression of Colorectal Can- cer by targeting miR-489/3p via the PI3K /AKT/ mTOR Front Genet 11: 567630.
- Yang MH, Zhao L, Wang L, Ou-Yang W, Hu SS, et al. (2019) Nuclear lnc RNA HOXD-AS1 Suppresses Colorectal Carcinoma growth and metastasis via inhibiting HOXD3-induced integrin beta3transcriptional activating and MAPK/AKT Mol Cancer 18: 31.
- Jiang H, Wang Y, Ali M, Wang H, Duan Z, et (2017) Long non cod- ing RNACRNDE stabilized by hnRNPUL2accelerates cell proliferation, and migration in Colorectal Carcinoma via activating Ras/ MAPK sig- naling pathway. Cell Death Dis 8: e2862.
- Xu W, Zhou G, Wang H, Liu Y, Chen B, et al. (2020) Circulating lnc RNA SNHG11 as a biomarker for early diagnosis and prognosis of Colorectal Int J Cancer 146: 2901-2912.
- Yu S, Wang D, Shao Y, Zhang T, Xie H, et al. (2019) Lnc RNATIN- CR overexpression contributes to Colorectal Cancer progression by sponging miR-7-5p. Aging (Albany NY) 11: 1389-1388.
- Pan X, Cheng R, Zhu X, Cai F, Zheng G, et al. (2019) Prognostic significance and diagnostic value of overexpressed lnc RNA PVT1 in Colorectal Clin Lab 65: 2279-2288.
- Yue B, Qiu S, Zhao S, Liu C, Zhang D, et (2016) Lnc RNA-ATB me- diated E-cadherin repression promotes the progression of Colon Can- cer and predicts poor prognosis. J Gastroenterol Hepatol 31: 595-603.
- Ye C, Shen Z, Wang B, Li Y, Li T, et (2016) A novel Long non coding RNA lnc RNA-GNAT-1 is low expressed in Colorectal Cancer and acts as a tumor Suppressor through regulating RKIP NF-kappa B Snail cir- cuit. JExp Clin Cancer Res 35: 187.
- Liu Y, Egranov SD, Yang L, Lin C (2019) Molecular mechanisms of Longnon coding RNAs mediated cancer metastasis. Genes Chromo- somes Cancer 58: 200-207.
- Zhang I, Jiang Y, Zhu J, Wu T, Ma I, et al. (2017) Overexpression of Long non coding RNA Colon Cancer associated transcript2 is associ- ated with advanced tumor progression and poor prognosis in patients with Colorectal Oncol Lett 14: 6907-6914.
- Sha QK, Chen L, XiJ Z, Song H (2019) Longnon coding RNA LINC 00858 promotes cell proliferation, migration and invasion by acting as a ceRNA of miR-22-3p in Colorectal Artif Cells Nano Med Bio- technol 47: 1057-1066.
- Li C, Tan F, Pei Q, Zhou Z, Zhou Y, et (2019) Non coding RNAMF12- AS1 promotes Colorectal Cancer proliferation, migration and invasion through miR-574-5p/MYCBP axis. Cell Prolif 52: e12632.
- Guo Q, Zhao Y, Chen J, Hu J, Wang S, et al. (2014) BRAF activated Longnon coding RNAscontributes to Colorectal Cancer migration by inducing epithelial-mesenchymal Oncol Lett 8: 869-875.
- Chen DL, ChenLZ, Lu YX, Zhang DS, Zeng ZL, et (2017) Long non coding RNA XIST expedites metastasis and modulates epithelial-mes- enchymal transition in Colorectal Cancer. Cell Death Dis 8: e3011.
- Dacheng W, Songhe L, Weidong I, Shutao Z, Jingjing L, et al. (2020) Lnc RNA-SNHG3 promotes the growth and metastasis of Colorectal Cancer by regulating miR-539/RUNX 2 BioMed Pharmacotherap 25: 111039.
- Bermudez M, Aguilar-Medina M, Lizaragga Vrdugo E, Avendano Felix M, Silva-Benitez E, et (2019) Lnc RNAs as regulators of autophagy and drug resistance in Colorectal Cancer. Front Oncol 9: 1008.
- Tang D, Yang Z, Long F, Luo L, Yang B, et al. (2019) Inhibition of MALAT1reduces tumor growth and metastasis and promotes drug sensitivity in Colorectal Cell Signal 57: 21-28.
- Fan C, Yuan Q, Liu G, Zhang Y, Yan M, et al. (2020) Longnon coding RNA MALAT1regulates oxaliplatin via miR -324-3p/ADAM 17 Axisin Colorectal Cancer Cancer Cell Int 20: 473.
- Sun F, Liang W, Qian J (2019) The identification of CRNDE,H19,UCA1 and HOTAIR as the key lnc RNAs involved in oxaliplatin or irinotecan resistance in the chemotherapy of Colorectal Cancer based on inte- grative bioinformatics Mol Med Rep 20: 3583-3596.
- Peng K, Liu R, Yu Y, Liang L, Yu S, et al. (2018) Identification and validation of cetuximab, resistance associated Longmont coding RNA biomarkers in metastatic Colorectal Cancer. BioMed Pharmacotherap 97: 1138-1146.
- Wang L, Zhang X, Sheng L, Qiu C, Luo R (2018) LINC00473 promotes the taxol resistance via miR -15a in Colorectal Biosci Rep 38: BSR2018790.
- Ogunwobi OO, Mahmood FF, Akingboye A (2020) Biomarkers in Col- orectal Cancer research: Current research and future prospects. Int J Mol Sci 21: 5311.
- JooJung E, Paramanatham A, Kim HJ, Shin SC, King GS, et (2021) Artenisia annuapolyphenol induced cell deathis ROS Indepenently en- hanced by inhibition of JNK in HCT 116 in Colorectal Cancer cells. Int J Mol Sci 22: 1366.
- Lin Y, Pan X, Chen Z, Lin S, Chen S (2020) Identification of an immune related nine lnc RNA Sinatures Predictive of overall survival in Col- orectal Front Genet 11: 318.
- Muieinol-Romay L, Diaz –Lagares A (2021) Epigenetic landscape of liquid biopsy in Colorectal Cancer. Front Cell Dev Biol 9: 622459.
- Wang XT, Cui WQ, Pan D, Jiang M, Chang B, et al. (2020) Tea poly- phenols and their chemo preventive and therapeutic effects on Col- orectal World J Gastroenterol 26: 562-597.
- Fu Y, Zhang Y, Cui J, Yang G, Peng S, et (2020) SNPrs 12982687 affects binding capacity of lnc RNA UCA1 with miR -873-5p:involve- ment in smoking triggered Colorectal Cancer progression. Cell Com- mun Signal 18: 37.
- Rescigno T, Micolucci L, Tecce MF, Capasso A (2017) Bioactive nutri- ents and nutrigenomics in age related Molecules 22: 105.
- Shen X, Xue Y, Cong H, Wang X, Fan Z, et (2020) Circulating lnc RNA DANCR as a potential auxillary biomarker for the diagno- sis and prognostic prediction of Colorectal Cancer. Biosci Rep 40: BSR20191481.
- Liu R, Zhang Q, Shen L, ChenS, He J, et al. (2020) Longnon coding RNA lnc R 1 regulates DNA damage repair and radiation sensitivity of CRC cells through NHEJ Cell Biol Toxicol 36: 493-507.
- Wu H, Wei M, JiangX, Tan J, XuW, et al. (2020) Lnc RNAPVT1 pro- motes tumorigensis of Colorectal Cancer by stabilizing miR -16-5p and interacting with the VEGFA/VEGFR1/AKT Axis. Mol Ther Nucleic Acids 20: 438-450.
- Yao Y, Li N (2020) MIR 600HG suppresses metastasis and enhances oxali platin chemo sensitivity by targeting ALDH1A3 in Colorectal Can- cer. Biosci Rep 40: BSR20200390.
Citation: Kaur KK, Allahbadia G, Singh M (2021) An Update on Long Non Coding RNAs as Prospective Targets for Improving Prognosis of Colorectal Cancer by Acting as Biomarkers for Early Detection of Metastasis, Getting Targeted for Inhibition of the MiaRNA they Interact with that Promote Progession Along with Predicting Prog- nosis-A Systemic Review.J Cell Mol Bio 5: 014.
Copyright: © © 2021 Kaur KK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.