*Corresponding Author:
Vladimir Zaichick,
Radionuclide Diagnostics Department, Medical Radiological Research Centre Korolyev, Russia
Tel: +7 (48439) 60289
E-mail: vzaichick@gmail.com
Abstract
The prostate gland is subject to various disorders.The etiology and pathogenesis of these diseases remain not well understood. Moreover, despite technological advancements, the differential diagnosis of prostate disorders has become progressively more complex and controversial. It was suggested that the tin (Sn) level in prostatic tissue plays an important role in prostatic carcinogenesis and its measurement may be useful as a cancer biomarker. These suggestions promoted more detailed studies of the Sn content in the prostatic tissue of healthy subjects. The present study evaluated by systematic analysis the published data for Sn content analyzed in prostatic tissue of “Normal” glands. This evaluation reviewed 2033 studies, all of which were published in the years from 1921 to 2020 and were located by searching the databases PubMed, Scopus, ELSEVIER-EMBASE, Cochrane Library, and the Web of Science. The articles were analyzed and “Median of Means” and “Range of Means” were used to examine heterogeneity of the measured Sn content in prostates of apparently healthy men. The objective analysis was performed on data from the 18 studies, which included 783 subjects. It was found that the range of means of prostatic Sn content reported in the literature for “Normal” gland varies widely from 0.019 mg/kg to <0.9 mg/kg with median of means 0.054 mg/ kg on a wet mass basis. Finally, because of small sample size and high data heterogeneity, we recommend other primary studies be performed.
Keywords
Biomarkers; Human prostate; Normal prostatic tissue; Tin
Introduction
The prostate gland is subject to various disorders and of them chronic prostatitis, Benign Prostatic Hyperplasia (BPH), and Prostate Cancer (PCa) are extremely common diseases of ageing men [1-3]. The etiology and pathogenesis of these diseases remain not well understood. A better understanding of the etiology and causative risk factors are essential for the primary prevention of these diseases.
In our previous studies the significant involvement of Trace Elements (TEs) in the function of the prostate was found. [4-15]. It was also shown that levels of TEs in prostatic tissue, including tin (Sn), can play a significant role in etiology of PCa [16-20]. Moreover, it was demonstrated that the changes of some TE levels and Zn/ Sn ratios in prostate tissue can be used as biomarkers [21-27].
It was indicated low levels of Sn in human prostatic tissue (0.74 mg/kg of wet tissue) in studies published more than 65 years ago [28]. This finding allowed conclude that the prostate gland accumulates Sn, because the level of metal in prostates was almost three orders of magnitude higher the blood serum reference level (0.001 mg/L) and more than 7 times higher the liver reference level [29]. Furthermore, experimental data identified that Sn compounds should be considered as genotoxic carcinogens [30]. These findings promoted more detailed studies of the Sn content of prostatic tissue of healthy subjects, as well as of patients with different prostatic diseases, including BPH and PCa.
The effects of TEs, including Sn, are related to their concentration. Recorded observations range from a deficiency state, through normal function as biologically essential components, to an imbalance, when excess of one element interferes with the function of another, to pharmacologically active concentrations, and finally to toxic and even life-threatening concentrations [31-33]. In this context, for example, low dose of Sn has some useful effects on health [34,35], but significant exposure to this metal and its compounds may result in adverse health effects in different organs or tissues, including malignancy [30,36-38]. However, it still remains unclear what precise mechanism is responsible for Sn genotoxicity [30].
By now, a few studies have reported the Sn content in tissue of “Normal” and affected glands. However, further investigation has been considered necessary to provide a practical reference data of Sn levels in prostate norm and disorders, because the findings of various studies indicate some discrepancies.
The present study addresses the significance of Sn levels in prostatic tissue as a biomarker of the gland’s condition. Therefore, we systematically reviewed all the available relevant literature and performed a statistical analysis of Sn content in tissue of “Normal” glands, which may provide valuable insight into the etiology and diagnosis of prostate disorders.
Materials and Methods
Data sources and search strategy
Aiming at finding the most relevant articles for this review, a thorough comprehensive web search was conducted by consulting the PubMed, Scopus, ELSEVIER-EMBASE, Cochrane Library, and the Web of Science databases, as well as from the personal archive of the author collected between 1966 to 2020, using the key words: prostatic trace elements, prostatic Sn content, prostatic tissue, and their combinations. For example, the search terms for Sn content were: “Sn mass fraction”, “Sn content”, “Sn level”, “prostatic tissue Sn” and “Sn of prostatic tissue”. The language of the article was not restricted. The titles from the search results were evaluated closely and determined to be acceptable for potential inclusion criteria. Also, references from the selected articles were examined as further search tools. Relevant studies noted for the each selected article were also evaluated for inclusion.
Eligibility criteria
Inclusion criteria: Only papers with quantitative data of Sn prostatic content were accepted for further evaluation. Studies were included if the control groups were healthy human males with no history or evidence of urological or other andrological disease and Sn levels were measured in samples of prostatic tissue.
Exclusion criteria: Studies were excluded if they were case reports. Studies involving subjects that were Sn occupational exposed, as well as persons from Sn contaminated area were also excluded.
Data extraction
A standard extraction of data was applied, and the following available variables were extracted from each paper: method of Sn determination, number and ages of healthy persons, sample preparation, mean and median of Sn levels, standard deviations of mean, and range of Sn levels. Abstracts and complete articles were reviewed independently, and if the results were different, the texts were checked once again until the differences were resolved.
Statistical analysis
Studies were combined based on means of Sn levels in prostatic tissue. The articles were analyzed and “Median of Means” and “Range of Means” were used to examine heterogeneity of Sn contents. The objective analysis was performed on data from the 18 studies, with 783 subjects.
Results and Discussion
Information about Sn levels in prostatic tissue in different prostatic diseases is of obvious interest, not only to understand the etiology and pathogenesis of prostatic diseases more profoundly, but also for their diagnosis, particularly for PCa diagnosis and PCa risk prognosis [26,27,31]. Thus, it dictates a need for reliable values of the Sn levels in the prostatic tissue of apparently healthy subjects, ranging from young adult males to elderly persons.
Possible publications relevant to the keywords were retrieved and screened. A total of 2033 publications were primarily obtained, of which 2015 irrelevant papers were excluded. Thus, 18 studies were ultimately selected according to eligibility criteria that investigated Sn levels in tissue of normal prostates (Table 1) and these 18 papers [9,13,14,26,28,39-53] comprised the material on which the review was based. A number of values for Sn mass fractions were not expressed on a wet mass basis by the authors of the cited references. However, we calculated these values using the medians of published data for water - 83% [54-58] and ash - 1% (on a wet mass basis) contents in normal prostates of adult men [41,55-57].
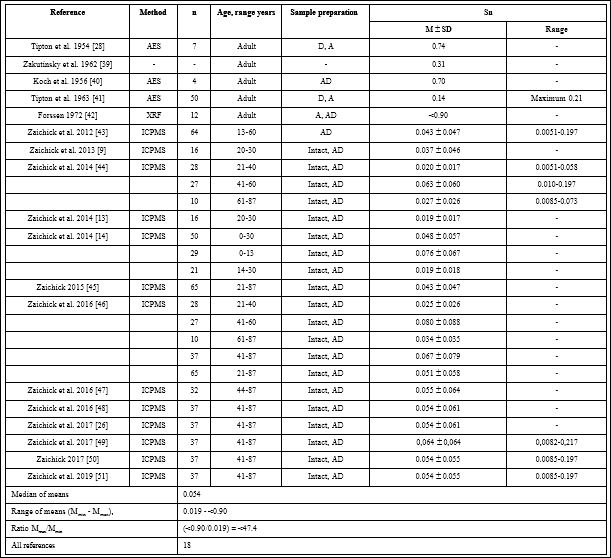
Table 1: Reference data of Sn mass fractions (mg/kg wet tissue) in “Normal” human prostatic tissue.
M-arithmetic mean, SD-standard deviation of mean, Med.-Median, AES-atomic emission spectrometry, XRF-X-ray fluorescence analysis, ICPMS-inductively coupled plasma mass spectrometry, D-drying at high temperature, A-ashing, AD-acid digestion.
Table 1 summarizes general data from the 18 studies. The retrieved studies involved 783 subjects. The ages of subjects were available for 13 studies and ranged from 0–87 years. Information about the analytical method and sample preparation used was available for 17 studies. One study determined Sn levels by X-ray fluorescence analysis (XRF), three-by atomic emission spectrometry (AES), and thirteen-by inductively coupled plasma mass spectrometry (ICPMS ) (Table 1). All methods used were the destructive analytical techniques, because they require sample ashing or acid digestion.
Figure 1 illustrates the data set of Sn measurements in 18 studies during the period from 1954 to 2020.
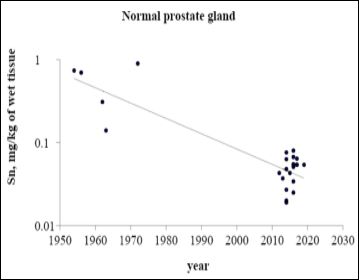
Figure 1: ta on Sn content in normal prostate tissue reported from 1954 to 2020
The range of means of Sn mass fractions reported in the literature for “Normal” prostatic tissue varies widely from 0.019 mg/kg [13] to <0.99 mg/kg [42] with median of means 0.054 mg/kg of wet tissue (Table 1). This variability of reported mean values can be explained a priori by a dependence of Sn content on many factors, including analytical method imperfections, differences in “Normal” prostate definitions, possible non-homogeneous distribution of Sn levels throughout the prostate gland volume, age, ethnicity, diet, smoking, alcohol intake, consuming supplemental trace elements, and others. Not all these factors were strictly controlled in the cited studies. For example, in some studies the “Normal” prostate means a gland of an apparently healthy man who had died suddenly, but without any morphological confirmation of “Normality” of his prostatic tissue. In other studies the “Normal” prostate means a non-cancerous prostate (but hyperplastic and inflamed glands were included) and even a visually normal prostatic tissue adjacent to a prostatic malignant tumor. Some researchers used as the “Normal” prostate the glands of patients who died from acute and chronic non-prostatic diseases including subjects who had suffered from prolonged wasting illnesses. In some studies whole glands were used for the investigation while in others the Sn content was measured in pieces of the prostate. Therefore published data allowed us to estimate the effect of only some different factors on Sn content in “Normal” prostate tissue.
Analytical method
The trend line of Sn content data in “Normal” prostate (Figure 1) showed that an improvement of analytical technologies during last 50 years impacted significantly on the mean and variability of reported values. In our opinion, the leading cause of inter-observer variability was an insufficient sensitivity of analytical techniques and a lack of quality control of results in old studies published in 50-70s.
In all reported papers destructive analytical methods were used. These methods require ashing or acid digestion of the samples at a high temperature. There is evidence that use of this treatment causes some quantities of TEs to be lost [31,58,59]. On the other hand, the Sn content of chemicals used for acid digestion can contaminate the prostate samples. Thus, when using destructive analytical methods it is necessary to allow for the losses of TEs, for example when there is complete acid digestion of the sample. Then there are contaminations by TEs during sample decomposition, which require addition of some chemicals. It is possible to avoid these problems by using non-destructive methods, but up to now there are no analytical methods which allow to quantify Sn content in “Normal” prostate without acid digestion of the samples at a high temperature. It is, therefore, reasonable to conclude that the quality control of results is very important factor for using the Sn content in prostatic tissue as biomarkers.
Age
In a few studies a significant increase in Sn content with increasing of age was shown by the comparison of different age groups or the Pearson’s coefficient of correlation between age and Sn content in prostate tissue [43-46]. The most detailed investigations of age dependence of prostatic Sn were done by Zaichick and Zaichick [46]. For example, a strongly pronounced tendency for an age-related increase of Sn mass fraction was observed in the prostate for the third to sixth decades [46]. In prostates of 41-60 year old men, the mean Sn mass fraction was 3 times greater than that in the prostates of 21- 40 year old males. Thus, the accumulated information, studied by us from reported data, allowed a conclusion that there is a significant increase in Sn mass fraction in “Normal” prostate from age 21 years to the sixth decade.
Androgen-independence of prostatic Sn levels
There was not found a significant difference between Sn levels in prostates of teenagers before puberty and of post-pubertal teenagers and young adults [9,13,14]. These findings allowed us to conclude that the Sn content in “Normal” prostates does not depend on the level of androgens, and vice versa. However, studies on the association between the Sn content in “Normal” prostates and the level of androgens in blood were not found.
Sn intake
The general population can be exposed to low levels of Sn primarily through consumption of food and ingestion of drinking water from Sn-lined cans [60-62] and to a lesser degree through inhalation of ambient air [63]. One may also be exposed to Sn through skin contact with Sn-lined cans, bronzes or other Sn alloys, paints, some plastics, and many other things contained Sn or Sn compounds [63].
Sn contents in dairy products, meat, fish, poultry, eggs, vegetables, fruits and fruit juices, nuts, beverages, and other foods not packaged in metal cans are generally about 1-2 mg/kg of food [64,65]. Higher concentrations are found in canned foods as a result of dissolution of the Sn coating or Sn plate, the levels depending largely on the type and acidity of the food, the presence of oxidants, the duration and temperature of storage and the presence of air in the can headspace [65]. Exposure to some Sn compounds (organotins) can occur by eating seafood from coastal waters [66]. Average dietary Sn intakes among the non-consumers of canned foods are about 3 mg/day but among the canned food consumers one order of magnitude higher [37,62-64].
Concentration of Sn in waters of different types such as tap water, household wells, groundwater, and surface waters (rivers, lakes, and oceans) variate very widely but the use of organotin biocides can produce significantly elevated levels [65]. Mean oncentrations of Sn in in the USA tap water samples were in range of 0.0011–0.0022 mg/L (maximum 0.0030 mg/L), however levels of <0.042–0.295 mg/L were found in 37 different bottled mineral waters [67].
The background level of Sn in air is about 0.00001 mg/m3, increasing to 0.0003 mg/m3 in urban areas and to 0.005 mg/m3 near industrial emissions [65].
Sn is sometimes included in homeopathic medications and in over-the-counter dietary supplements.
Sn content in body fluids, tissues and organs
It is known that Sn is accumulated primarily in liver, kidney, and bone [37,38,68]. For example, mass fraction of this metal in liver and whole blood of the Reference Man is 0.100 mg/kg of wet tissue and 0.001 mg/L, respectively [29]. The median of prostatic Sn content means obtained in the present review (0.054 mg/kg of wet tissue) almost equals the metal level in liver and over 50 times higher the whole blood level. Thus, we can conclude that the prostate is also a target organ for Sn.
All natural chemical elements of the Periodic System, including Sn, present in all subjects of biosphere [31,69,70]. During the long evolutional period intakes of Sn in organisms were more or less stable and organisms were adopted for such environmental conditions. Sn minerals have been known and used in relative small amounts since ancient times. For example, Sn minerals were used to make Sn alloy such as bronze. The situation with using Sn began to change after the industrial revolution, particularly, over the last 100 years. The primary use of Sn is in construction and food industries. It is present in brass, bronze, pewter, and some soldering materials. Metallic Sn is used to line cans for food, beverages, and aerosols, for the manufacturing of electrically conducting films; and to produce flat window glass surfaces. Indium Sn oxide (ITO) is widely used as a transparent conducting electrode in photoelectron devices. Some Sn inorganic compounds are also used in toothpaste, perfumes, soaps, food additives and dyes. Sn organic compounds are used to make plastics, food packages, plastic pipes, agricultural fungicides and pesticides, antifouling paints (including antifoulant and molluscicide - antifouling agents for ships), glass coatings, pest repellents, and heat stabilizers. Moreover, stannous chloride (SnCl2) is a food additive [30,38,60,62,63].
Thus, inorganic Sn is ubiquitously distributed in environment and food, water, and air everywhere contain this element. In addition to the abundant natural sources of Sn, there are a large number of industrial sources of Sn to the soil (through atmospheric emissions originating from residues from coal, oil, and gas combustion, urban refuse, Sn mine tailings, smelter slag, waste,), water (through irrigation and industrial liquid waste, livestock dips, and wastewater sludge application), and air (Sn may be released from metal smelters and from combustion of fossil fuels) contamination [71]. For example, in the 1970s the anthropogenic emission of Sn was estimated as 43,000 tons annually [72]. From the polluted environment this metal is subsequently introduced into the food chain and for the general population, the food is the main source of exposure to inorganic Sn [73].
Sn is an important product in the world economy. The annual world production of Sn has been growing slowly in recent years and reached about 268,000 tons in 2003 [73]. More than 22 countries produce Sn, but the 6 largest producers in 2001 were China (36%), Indonesia (23%), Peru (17%), Brazil (6%), Bolivia (6%), and Australia (4%) [74]. Significant quantities of the metal are also produced from smelters in Malaysia and Thailand [73]. Since the use of Sn is linked to the rapidly developing modern technology, we can assume that over the years, the need of industry in this metal has increased significantly and would continue to increase in the future.
As mentioned above, an ingestion of inorganic Sn by humans can cause a variety of disorders, such as stomachache, anemia, and liver and kidney problems [36-38]. Breathing or swallowing, or skin contact with some Sn organic compounds can effect on the brain and nervous system work and inorganic or organic Sn compounds placed on the skin or in the eyes can produce skin and eye irritation [36- 38,73]. Furthermore, some inorganic and organic Sn compounds are cytotoxic, genotoxic and can produce cancer in animals [30,73,75]. Precise molecular mechanisms by which this metal causes healthy cells to transform to malignant states have yet to be fully defined [30].
Thus, according our study for unpolluted areas there are no information could explain the variability of published means for “Normal” prostatic Sn levels from 0.019 mg/kg to <0.90 mg/kg of wet tissue. Moreover, prostate tissue Sn contents showed large variations among individuals, but sources of the variation remain unknown. It is, therefore, reasonable to assume from data of our study that inaccuracy of analytical technologies employed caused so great variability of published means for prostatic Sn levels. This conclusion was supported the fact that the Certified Reference Materials for quality control of results were not used in old studies.
There are some limitations in our study, which need to be taken into consideration when interpreting the results of this review. The sample size of each study was sometimes relatively small (from 4 to 65), and a total of 783 “Normal” prostates were investigated from all 18 studies. As such, it is hard to draw definite conclusions about the reference value of the Sn content in “Normal” prostate as well as about the clinical value of the Sn levels in “Normal” prostates as a biomarker.
Conclusion
The present study is a comprehensive study regarding the determi- nation of Sn content in “Normal” human prostates. With this knowl- edge Sn levels may then be considered as a biomarker for the recog- nition of prostate disorders. The study has demonstrated that level of Sn in “Normal” prostates increases with age and depends on many unknown factors. Because of the uncertainties we have outlined, we recommend other primary studies be performed.
References
- Nickel JC (2011) Can Urol Assoc J 5: 306-315.
- Lim KB (2017) Epidemiology of clinical benign prostatic hyperplasia. Asian J Urol 4: 148-151.
- Rawla P (2019) Epidemiology of Prostate Cancer. World J Oncol 10: 63-89.
- Avisyn AP, Dunchik VN, Zhavoronkov AA, Zaichick V, Sviridova TV (1981) Histological structure of the prostate and its zinc content at various ages. Archiv Anatomy Gistology and Ebriology (Leningrad) 81: 76-83.
- Zaichick V (2004) INAA and EDXRF applications in the age dynamics assessment of Zn content and distribution in the normal human J Radioanal Nucl Chem 262: 229-234.
- Zaichick V, Zaichick S (2013) The effect of age on Br, Ca, Cl, K, Mg, Mn, and Na mass fraction in pediatric and young adult prostate glands investigated by neutron activation Appl Radiat Isot 82:145-151.
- Zaichick V, Zaichick S (2013) INAA application in the assessment of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn mass fraction in pediatric and young adult prostate glands. J Radioanal Nucl Chem 298: 1559-1566.
- Zaichick V, Zaichick S (2013) NAA-SLR and ICP-AES application in the assessment of mass fraction of 19 chemical elements in pediatric and young adult prostate glands. Biol Trace Elem Res 156: 357-366.
- Zaichick V, Zaichick S (2013) Use of neutron activation analysis and inductively coupled plasma mass spectrometry for the determination of trace elements in pediatric and young adult Am J Analyt Chem 4: 696-706.
- Zaichick V, Zaichick S (2014) Relations of bromine, iron, rubidium, strontium, and zinc content to morphometric parameters in pediatric and nonhyperplastic young adult prostate Biol Trace Elem Res 157: 195-204.
- Zaichick V, Zaichick S (2014) Relations of the neutron activation analysis data to morphometric parameters in pediatric and nonhyperplastic young adult prostate Advances in Biomedical Science and Engineering 1: 26-42.
- Zaichick V, Zaichick S (2014) Relations of the Al, B, Ba, Br, Ca, Cl, Cu, Fe, K, Li, Mg, Mn, Na, P, S, Si, Sr, and Zn mass fractions to morphometric parameters in pediatric and nonhyperplastic young adult prostate glands. BioMetals 27: 333-348.
- Zaichick V, Zaichick S (2014) Androgen-dependent chemical elements of prostate gland. Androl Gynecol Curr Res 2: 2.
- Zaichick V, Zaichick S (2014) The distribution of 54 trace elements including zinc in pediatric and nonhyperplastic young adult prostate gland tissues. Journal of Clinical and Laboratory Investigation Updates 2: 1-15.
- Zaichick V, Zaichick S (2015) Differences and relationships between morphometric parameters and zinc content in nonhyperplastic and hyperplastic prostate glands. Br J Med Med Res 8: 692-706.
- Schwartz MK (1975) Role of trace elements in cancer. Cancer Res 35: 3481-3487.
- Zaichick V, Zaichick S (1999) Role of zinc in prostate cancerogenesis. In: Mengen und Spurenelemente. 19. Arbeitstagung. Jena Friedrich- Schiller-Universitat 104-115.
- Zaichick V, Zaichick S, Wynchank S (2016) Intracellular zinc excess as one of the main factors in the etiology of prostate cancer. J Anal Oncol 5: 124-131.
- Zaichick V, Zaichick S, Rossmann M (2016) Intracellular calcium excess as one of the main factors in the etiology of prostate cancer. AIMS Mol Sci 3: 635-647.
- Fukuda H, Ebara M, Yamada H, Arimoto M, Okabe S, et ( 2004) Trace elements and cancer. JMAJ 47: 391-395.
- Dunchik, V, Zherbin E, Zaichick V, Leonov A, Sviridova T (1980) Method for differential diagnostics of prostate malignant and benign tumours. Russian patent (Author’s Certificate No 764660, priority of invention 27.10.1977). Discoveries, Inventions, Commercial Models, Trade Marks 35:13.
- Zaichick V, Sviridova T, Zaichick S (1997) Zinc in the human prostate gland: Normal, hyperplastic and cancerous. Int Urol Nephrol 29: 565-
- Zaichick V, Sviridova T, Zaichick S (1997) Zinc in human prostate gland: Normal, hyperplastic and J Radioanal Nucl Chem 217: 157- 161.
- Zaichick S, Zaichick V (2012) Trace elements of normal, benign hypertrophic and cancerous tissues of the human prostate gland investigated by neutron activation J Appl Radiat Isot 70: 81-87.
- Zaichick V, Zaichick S (2016) Ratios of selected chemical element contents in prostatic tissue as markers of malignancy. Hematol Med Oncol 1: 1-8.
- Zaichick V, Zaichick S (2017) Trace element levels in prostate gland as carcinoma’s markers. J Cancer Ther 8: 131-145.
- Zaichick V, Zaichick S (2017) Ratios of Zn/trace element contents in prostate gland as carcinoma’s markers. Cancer Rep Rev 1: 1-7.
- Tipton JH, Steiner RL, Foland WD, Mueller J, Stanley M (1954) USAEC- ORNL-Report-CF-54-12-66.
- Iyengar GV (1998) Reevaluation of the trace element content in reference men. Radiat Phys Chem 51: 545-560.
- Tabei Y, Sugino S, Nakajima Y, Horie M (2018) Reactive oxygen species independent genotoxicity of indium tin oxide nanoparticles triggered by intracellular degradation. Food Chem Toxicol 118: 264-271.
- Zaichick V (2006) Medical elementology as a new scientific J Radioanal Nucl Chem 269: 303-309.
- Hunter P (2008) A toxic brew we cannot live without. Micronutrients give insights into the interplay between geochemistry and evolutionary EMBO Rep 9: 15-18.
- López-Alonso M (2012) Trace minerals and livestock: Not too much not too little. International Scholarly Research Notices 2012: Article ID
- Mertz V (1974) The newer essential trace elements, chromium, tin, vanadium, nickel and silicon. Pros Nutr Soc 33: 307-313.
- Weber G (1985) The importance of tin in the environment and its determination at trace levels. Fresenius’ J Anal Chem 321: 217-224.
- Schäfer SG, Femfert U (1984) Tin-a toxic heavy metal? A review of the Regul Toxicol Pharmacol 4: 57-69.
- Winship KA (1988) Toxicity of tin and its compounds. Adverse Drug React Acute Poisoning Rev 7: 19-38.
- Jaiswal AK, Bisht K, Ch ZA, DeyA, Sharma DK (2019) TIN toxicity with analytical aspects and its International Journal of Forensic Science 2: 78-83.
- Zakutinsky DI, Parfyenov YuD, Selivanova LN (1962) Data book on the radioactive isotopes toxicology. State Publishing House of Medical Literature, Moscow.
- Koch HJ, Smith ER, Shimp NF, Connor J (1956) Analysis of trace elements in tissue. I. Normal tissue. Cancer 9: 499-511.
- Tipton IH, Cook MJ (1963) Trace elements in human Part II. Adult subjects from the United States. Health Phys 9:103-145.
- Forssen A (1972) Inorganic elements in the human I. Occurrence of Ba, Br, Ca, Cd, Cs, Cu, K, Mn, Ni, Sn, Sr, Y and Zn in the human body. Annales medicinae Experimentalis et Biologie (Finland) 50: 99-162.
- Zaichick S, Zaichick V, Nosenko S, Moskvina I (2012) Mass fractions of 52 trace elements and zinc trace element content ratios in intact human prostates investigated by inductively coupled plasma mass Biol Trace Elem Res 149: 171-183.
- Zaichick V, Zaichick S (2014) Use of INAA and ICP-MS for the assessment of trace element mass fractions in adult and geriatric J Radioanal Nucl Chem 301: 383-397.
- Zaichick V (2015) The variation with age of 67 macro- and microelement contents in nonhyperplastic prostate glands of adult and elderly males investigated by nuclear analytical and related Biol Trace Elem Res 168: 44-60.
- Zaichick V, Zaichick S (2016) Age-related changes in concentration and histological distribution of 54 trace elements in nonhyperplastic prostate of adults. Int Arch Urol Complic 2: 019.
- Zaichick V, Zaichick S (2016) Prostatic tissue levels of 43 trace elements in patients with BPH. Br J Med Med Res 15: 1-12.
- Zaichick V, Zaichick S (2016) Prostatic tissue levels of 43 trace elements in patients with prostate Cancer and Clinical Oncology 5: 79-94.
- Zaichick V, Zaichick S (2017) Chemical Element Contents in Normal and Benign Hyperplastic Prostate. Ann Mens Health Wellness 1: 1006.
- Zaichick V (2017) Differences between 66 Chemical Element Contents in Normal and Cancerous Prostate. Journal of Analytical Oncology 6: 37-56.
- Zaichick V, Zaichick S (2019) Comparison of 66 chemical element contents in normal and benign hyperplastic prostate. Asian J Urol 6: 275-289.
- Isaacs JT (1983) Prostatic structure and function in relation to the etiology of prostatic cancer. Prostate 4: 351-366.
- Leissner KH, Fjelkegård B, Tisell LE (1980) Concentration and content of zinc in the human prostate. Invest Urol 18: 32-35.
- Woodard HQ, White DR (1986) The composition of body tissues. Br J Radiol 59: 1209-1218.
- Arnold WN, Thrasher JB (2003) Selenium concentration in the Biol Trace Elem Res 91: 277-280.
- Schroeder HA, Nason AP, Tipton IH, Balassa JJ (1967) Essential trace metals in man: zinc. Relation to environmental cadmium. J Chronic Dis 20: 179-210.
- Saltzman BE, Gross SB, Yeager DW, Meiners BG, Gartside PS (1990) Total body burdens and tissue concentrations of lead, cadmium, copper, zinc, and ash in 55 human cadavers. Environ Res 52: 126-145.
- Zaichick V (1997) Sampling, sample storage and preparation of biomaterials for INAA in clinical medicine and occupational and environmental In: Harmonization of health related environmental measurements using nuclear and isotopic techniques. IAEA, Vienna, 123-133.
- Zaichick V (2004) Losses of chemical elements in biological samples under the dry aching process. Trace Elements in Medicine 5: 17-22.
- Blunden S, Wallace T (2003) Tin in canned food: a review and understanding of occurrence and effect. Food Chem Toxicol 41: 1651-
- Shimbo S, Watanabe T, Nakatsuka H, Yaginuma-Sakurai K, Ikeda M (2013) Dietary tin intake and association with canned food consumption in Japanese preschool children. Environ Health Prev Med 18: 230-236.
- Yang HR, Kim ES, Ko YS, Jung K, Kim JH, et al. (2015) Food intake survey of kindergarten children in Korea: Part 2 increased dietary intake of tin possibly associated with canned foods. Environ Health Prev Med 20: 302-306.
- Gadogbe M, Bao W, Wels BR, Dai SY, Santillan DA, et (2019) Levels of tin and organotin compounds in human urine samples from Iowa, United States. J Environ Sci Health A Tox Hazard Subst Environ Eng 54: 884-890.
- Sherlock JC, Smart GA (1984) Tin in foods and the diet. Food Addit Contam 1: 277-282.
- Inorganic Tin in Drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality WHO/SDE/WSH/03.04/115 (2004) World Health Organization, Geneva.
- Olmedo P, Pla A, Hernández AF, Barbier F, Ayouni L, et al. (2013) Determination of toxic elements (mercury, cadmium, lead, tin and arsenic) in fish and shellfish samples. Risk assessment for the Environ Int 59: 63-72.
- Allen HE, Halley-Henderson MA, Hass CN (1989) Chemical composition of bottled mineral water. Arch Environ Health 44: 102-116.
- Yoo YC, Lee SK, Yang JY, In SW, Kima KW, et al. (2002) Organ distribution of heavy metals in autopsy material from normal Korean. J Health Sci 48: 186-194.
- Vernadsky VI (1978) Living Matter, Nauka,
- Zaichick V, Ermidou-Pollet S, Pollet S (2007) Medical elementology: A new scientific discipline. Trace Elem Electroly 24: 69-74.
- Pacyna JM, Pacyna EG (2001) An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ Rev 9: 261-298.
- Lantzy RJ, Mackenzie FT (1979) Atmospheric trace metals: Global cycles and assessment of man’s impact. Geochim Cosmochim Acta 43: 511-525.
- Tin and inorganic tin compounds. Concise International Chemical Assessment Document 65 (2005) World Health Organization, Geneva.
- ATSDR (2003) Toxicological profile for tin and compounds (update). Draft for public comment. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
- Cagnoli M, Alama A, Barbieri F, Novelli F, Bruzzo C, et al. ( 1998) Synthesis and biological activity of gold and tin compounds in ovarian cancer cells. Anticancer Drugs 9: 603-610.
Citation: Zaichick V (2021) A Systematic Review of the Tin Content of the Normal Human Prostate Gland. J Cell Mol Onco 3: 006.
Copyright: © 2021 Zaichick V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.