*Corresponding Author:
Rahman MM,
Fisheries and Marine Resource Technology Discipline, Khulna University, Khulna-9208, Bangladesh
Tel: +880 1764697000
Email: mrahmankufmrt@gmail.com
Abstract
Among many aquaculture species, carp is the most popular one especially in South-East Asia because of its highly favorable culture conditions, rapid growth, easy culture technique, high market demand and consumers’ acceptability. Brood stock quality, egg and sperm quantity and quality, and fitness of larvae are the primary and prime issues in carp industry, because without healthy and well fingerlings nothing will be successful. Therefore, the present study has been conducted to explore how different phenotypic traits of two different sized males (big vs. small) can influence the hatching success (direct benefits of females) in hatchery reared common carp (Cyprinus carpio). 50 mature males of two sized groups (i.e. 25 big males >1 kg and 25 small males <1 kg) have been sorted for this study. Some common females having almost the same weight (2-2.5 kg) have also been chosen as control females for the experiment. The results have found significant differences between two male groups in some selected phenotypic traits (standard length, body area, color area in body, pectoral fin and caudal fin). The study has also revealed that bigger males have produced significantly higher number of hatchlings than their counter group. Thus, the overall results provide an important suggestion to the hatchery owners to prefer the bigger males in order to produce higher number of fry for greater benefits.
Keywords
Body size; Breeding; Fertilization; Fish hatchery; Phenotypic traits
Introduction
Today, aquaculture is a well-established technology and economic activity in the world. It provides a dynamic source of food, employment, recreation and economic benefits all over the world. Bangladesh is considered one of the most suitable countries in the world especially for freshwater aquaculture because of its favorable resources and geo-ecological conditions. Recently, Bangladesh has positioned forth in inland open water harvesting and fifth in fish culture in world ranking having a production of 3.78 million MT fish of which 1.19 million MT came from only carp production in 2015-2016 [1,2].
Carp culture is the most popular form of aquaculture practice in Bangladesh because of its suitable culture environment, fast growth, easy to culture, high market demand and consumers’ acceptability [3]. Carp culture mainly depends on several factors such as broodstocks management, seed production, larvae rearing, culture systems, nutrition, disease control, etc. Among these variables, broodstocks collection, rearing and management, and then production of good quality sperm, eggs and fry are the primary and prime issues, because without healthy and well fingerlings nothing will be successful. But now-a-days due to the degradation of ecological systems, natural resources of good quality carp seeds are destroyed. Therefore, hatchery is now the main source of carp seed production.
Eggs and sperm are the main elements in hatcheries for fish breeding to produce larvae. Egg and sperm quality is significantly important for the production of high quality fish larvae and for economical utilization of hatcheries [4]. Good quality eggs and sperm can increase fish production and thereby fulfill the market demand of fish and fish protein. Good quality eggs have been defined as the ability of the egg to be fertilized and subsequently develop into a normal embryo, while good sperm quality as its ability to successfully fertilize an egg and subsequently allow the development of a normal embryo [5]. Poor quality of egg and sperm is one of the major constraints in the expansion of aquaculture especially in hatcheries for good quality larvae production.
The common carp (Cyprinus carpio) originated in European rivers around the Black sea and the Aegean basin [6]. It is one of the few fish species that have a global distribution [7]. Some research have already been carried out with common carp that show how age of broodstock can influence the reproductive traits and fertilization, about induced breeding of this species, chromosome set manipulation and sex control, how growth hormone gene can be transferred in this species, genetics and breeding, etc., [8-15]. Unfortunately no specific study has been conducted to investigate the effects of broodstocks’ phenotypic traits (e.g. standard length, body weight, colour patterns, etc.,) on hatching success of a fish species especially the commercially important common carp. Therefore, the present study has been carried out to explore the influence of males’ phenotypic traits on hatching success using common carp (C. carpio) as an experimental species with a special research design.
Methods and Materials
Source and maintenance of broodstocks
The sexually mature common carps (C. carpio) were collected from a commercial carp hatchery, the South Bay (Pvt) Ltd. hatchery, West Shiromoni, Khulna, Bangladesh which maintains breeding protocols to avoid associated inbreeding problems. 50 mature males of two sized groups (hereafter called two treatment groups) were sorted for this study where 25 individuals of bigger than 1 kg and another 25 individuals of less than 1 kg were included into two different treatment groups and each treatment had two replications (Figure 1). Some common females having almost same weight (2-2.5 kg) were also collected from the hatchery for the experiment. Then each group was reared separately under the same environmental conditions providing optimum level of diet up to couple of days.

Figure 1: The overall experimental design of this study.
Artificial propagation
The artificial propagation technique (also called ‘hypophysation’) was applied at the base of pectoral fin by using the recommended doses of Pituitary Glands (PG) for induced spawning [16]. Common females were administered two times at the rate of 1.5 mg PG/kg body weight for first time and approximately after 6 hours, the second dose was injected at the rate of 6.0 mg PG/kg body weight. The experimental males were administered only a single dose when the females were injected their second dose at the rate of 1 mg PG/kg body weight. Then they were allowed to become ready for artificial spawning after another 6 hours.
Measurements of phenotypic traits
After collecting sperm and eggs from each male and female, individual’s photograph was taken along with their ID using a digital camera (Canon DS126621). Then standard length (distance in cm from tip of the snout to the posterior end of the last vertebra) and body area (area covered from tip of the snout to the last mark of caudal peduncle) of each fish were measured from the digital photographs using ImageJ software (https://imagej.nih.gov/ij/index.html). Similarly, red colored area in body, pectoral fin and caudal fin were also measured.
Determination of hatching rate
First, eggs were collected into a bowl from a common female by hand stripping and the bowl was shaken to mix the eggs well. Then exactly 1 mL of eggs was collected with a marked syringe for each replication of every treatment. For example, there were two replications for each treatment and therefore, two bowls were ready for each treatment to put 1 mL of eggs in each bowl. Then sperm were collected into a bowl from each male by hand stripping and from the total sperm pool exactly 0.5 mL of sperm was collected with a marked syringe and mixed with the eggs of each replication. Meanwhile, required amount of pre-prepared urea and NaCl solution (20 L water + 100 g urea + 80 g NaCl) was added to each replication bowl and continuously stirred with a clean chicken feather in order to remove the adhesiveness of eggs. Finally, the externally added solution (urea and NaCl mixer) was discarded to collect the fertilized eggs. Then 25-30 mL powder milk solution was added to the fertilized eggs to increase the calcium level of eggs and also to remove the adhesiveness completely. The fertilized egg masses were then transferred to previously labeled plastic containers (2 L) filled with sufficient aerated water. Then they were allowed for incubation up to 38-40 hours at ambient temperature (27-29°C). After two days, the total number of hatchlings and also unhatched eggs in each replication were counted using a white colour plastic spoon. Thus, the comparative hatching rate between two treatment groups was determined.
Statistical analyses
All analyses were performed using ‘R’ version 3.4.3 [17]. The descriptive statistics (means, SD, SEs, etc.) were calculated with the ‘psych’ package, and normality and homogeneity were tested using the ‘one way tests’ package. The appropriate transformation was applied to yield normal distribution for any non-normally distributed trait.
The Welch two-sample t-test was performed to explore the variation in phenotypic traits (e.g. standard length, body area, body colour area, red colour area in pectoral and caudal fin) between two male groups (i.e. big vs. small male). Correlations among all interested traits were also done by using the ‘Performance Analytics’ package. A multiple linear regression analysis was performed where hatching rate was included as response variable and all the measured phonotypic traits were put as independent variables. Finally, a repeated measure ANOVA model was also applied using the ‘car’ package where hatching rate was included as a response variable, treatment and the male-by-female interaction as fixed factors and female body weight as a random effect (to account for exact amount of eggs came from the same female). All graphs were made with the ‘ggplot2’ package.
Results
Variation in phenotypic traits
The Welch two-sample t-tests have shown that bigger males (>1 kg) have significantly longer standard length (F1,43= -7.24, P<0.001), lager body area (F1,43= -9.07, P<0.001), and significantly more colour area in body (F1,43= 6.08, P<0.001), pectoral fin (F1,43= 5.85, P<0.001) and caudal fin (F1,43= -7.86, P<0.001) than the smaller males (<1 kg) (Table 1).
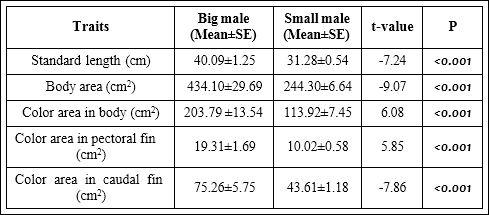
Table 1: Variation in phenotypic traits between two size groups of male common carp.
Significant P-values are marked in bold and italic fonts.
Correlation among phenotypic traits
The correlation analysis has shown very strong relationship among the different measured phenotypic traits (Table 2).

Table 2: Correlations among different measured phenotypic traits of male common carp.
Significant P-values are marked in bold and italic fonts.
Hatching rate comparison
The multiple regression analysis has revealed that hatching rate (%) was dependent only on male body weight where it was increased or decreased with the increase or decrease of male body weight, while it was not dependent on any other measured phenotypic trait (Table 3). Finally, the repeated measure ANOVA model has shown that the bigger males produced significantly higher number of hatchlings than
their counter male group (F1,46= 7.88, P<0.05, Cohen’s f= 0.41 and Figure 2). The model has also shown no effect of female weight (F1,46= 0.95, P=0.33) and treatment-by-female interaction (F1,46= 1.12, P=0.29) on hatching rate. Thus, it has been confirmed that female weight, and the interaction of male and female have no effect on hatching rate in this experiment.
Discussion
The study has shown that bigger males (>1 kg) have significantly longer standard length, larger body area, more colour area in body, pectoral and caudal fin than the smaller males (<1 kg) (Table 1). The overall results have also revealed that bigger males have the ability to produce significant number of hatchlings than the smaller males (Table 3 and Figure 2).

Table 3: Multiple regression analysis to explore whether the hatching rate of male depends on different measured phenotypic traits.
Significant P- values are marked in bold italic fonts.
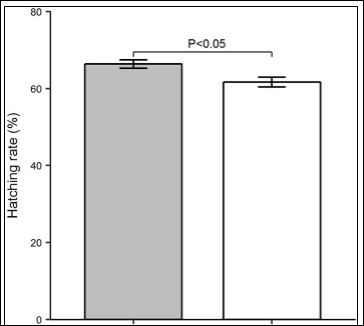
Figure 2: Variation in hatching percentage (mean±SE) between big and small male common carp.
Several studies have shown that body size is a fundamental trait that can affect many aspects of fish performance such as courtship behaviors, sperm quality and quantity, egg number and size, offspring number and fitness, etc. [18-26]. In the present study, males were selected based on their weights where the bigger males were significantly longer (i.e. standard length) having larger body size and colour area than the smaller males. The findings have revealed that bigger males had significantly more colour area in body, pectoral and caudal fins than the smaller males. Similar kinds of result were also found by other studies [18,27-29]. Smith, Phillips found that bigger males of European bitter ling (Rhodeus amarus) possessed comparatively more colour area than the smaller males, while Rahman, Kelley demonstrated that male guppy (Poecilia reticulata) fed with higher amount of diet grew larger and had more colour patterns than their counter group [18,27]. In another study, Serrano-Meneses, Córdoba-Aguilar found that large males of ruby spots (Hetaerina americana) had significantly more wing pigmentation than smaller ones. Evans, Bisazza revealed that female guppy (P. reticulata) exhibited preferences for males having relatively larger orange spots in their bodies [28,29]. Meanwhile, some studies have shown that males with relatively larger body area and colour patterns may have larger testis, produce faster and more viable sperm [30-35]. Evidences have also revealed that relatively larger and colorful males have higher parentage success than their less conspicuous counterparts [36-39].
The main outcome of this present study having higher hatching rate of larger males corroborates some other studies [26,28,40-42]. According to Pujolar, Locatello, the highest fertilization success of territorial males of grass goby (Zosterisessor ophiocephalus) was found from individuals with the largest body size [26]. There is a positive relationship between body size and fertilization success in territorial males of the grass goby (Z. ophiocephalus). In another study, Huang and Chang revealed that hatching rates were significantly positively correlated to standard length of parental males of paradise fish (Macropodus opercularis) (hatching rate: r = 0.813, P<0.001) [40]. Jones and Hutchings conducted an experiment with Atlantic salmon (Salmosalar) where they found the influence of male body size on parr fertilization success significantly in a single treatment having no anadromous male [41]. Thus they suggest that parr body size is an important predictor of the probability of an individual being involved in spawning. In an experiment with three-spined stickleback (Gasterosteus aculeatus), Largiader, Fries found an increased fertilization success of larger territorial males in comparison with smaller ones [42]. Large body size is advantageous for male ruby spots (Hetaerina americana) since it enhances territory tenure, fighting rate, wing pigmentation and mating success [28]. Levitan found in sea urchin (Diadema antillarum) that body size is a good indicator of gonad volume and gamete release across densities [43].
Previous few studies, however, found no correlation between body size and fertilization success [44-46]. In a study, Petersen, Warner revealed that there was no correlation between the size of a male and fertilization effectiveness in the blue head wrasse (Thalassoma bifasciatum) [44]. Spence and Smith did not find male size to be related to reproductive success in zebra fish (Daniorerio) while using males ranging between 33.8 and 37.4 mm. Rakitin, Ferguson found that male body size did not affect the reproductive success in Atlantic cod (Gadus morhua L.) which might be due to the effect of tank size on the courtship performance of captive males [45,46].
Conclusion
Size-dependent variation of phenotypic traits and reproductive fitness were investigated in some animals. However, very few studies revealed the correlation of phenotypic traits with the fertilization success in a commercially important carp species, the common carp (Cyprinus carpio). Therefore, this study has been conducted to explore whether some phenotypic traits of male common carp can provide an indication to confirm the fertilization success in this species. It has been concluded from the present study that the selected traits such as standard length, body area, colour area in body, pectoral fin and caudal fin are significantly different between two male groups (i.e. >1 Kg vs. <1 Kg). The study has also revealed that bigger males produced significantly higher number of hatchlings than their counter group. The overall results provide an indication of direct benefits for females (i.e. reproductive purpose) to prefer the bigger males as their mating partners, and also provide a suggestion to the commercial farmers to choose big sized males for higher fry production. To have more confirmation about this issue, still some research should be carried out to explore the use of bigger males and females based on their phenotypic traits for higher fertilization success and thereby, good offspring production in some other commercially important fish species.
Acknowledgement
We thank Md. Habibur Rahman and all the staffs of South Bay (Pvt) Ltd. Hatchery for their assistances in fish maintenance, husbandry and breeding. We also thank the Khulna University Research Cell for funding to carry out this experiment.
Competing Interests
The author declares no competing interests.
References
- http://www.flid.gov.bd/
- Fisheries Resources Survey System (2017) Yearbook of Fisheries Statistics of Bangladesh 2015-2016. Fisheries Resources Survey System, Department of Fisheries 33: 1-56.
- Penman DJ, Gupta MV, Dey MM (2005) Carp Genetic Resources for Aqua- culture in Asia. WorldFish, Penang, Malaysia.
- Coban D, Kamaci HO, Suzer C, Yildirim S, Arda G, et al. (2011) Effect of Some Morphometric Characteristics on Egg Quality in Common Dentex, Dentex dentex (Linnaeus, 1758). Turk J Fish Aquat Sci 11: 425-431.
- Bobe J, Labbe C (2010) Egg and Sperm Quality in Fish. Gen Comp Endo- crinol 165: 535-548.
- Freyhof J, Kottelat M (2008) Cyprinus carpio. The Iucn Red List of Threat- ened Species, Cambridge, UK.
- https://www.fishbase.de/
- Aliniya M, Khara H, Noveiri SB, Dadras H (2013) Influence of Age of Com- mon Carp (Cyprinus carpio) Broodstock on Reproductive Traits and Fertiliza- Turk J Fish Aquat Sci 13: 19-25.
- Vandeputte M (2003) Selective Breeding of Quantitative Traits in the Com- mon Carp (Cyprinus carpio): A Review. Aquat Living Resour 16: 399-407.
- Drori S, Ofir M, Levavi-Sivan B, Yaron Z (1994) Spawning Induction in Com- mon Carp (Cyprinus carpio) Using Pituitary Extract or Gnrh Superactive An- alogue Combined with Metoclopramide: Analysis of Hormone Profile, Prog- ress of Oocyte Maturation and Dependence on Temperature. Aquaculture 119: 393-407.
- Gomelsky B (2003) Chromosome Set Manipulation and Sex Control in Com- mon Carp: A Review. Aquat Living Resour 16: 408-415.
- Wu G, Sun Y, Zhu Z (2003) Growth Hormone Gene Transfer in Common Carp. Aquat Living Resour 16: 416-420.
- Hulata G (1995) A Review of Genetic Improvement of the Common Carp (Cyprinus carpio L.) and Other Cyprinids by Crossbreeding, Hybridization and Selection. Aquaculture 129: 143-155.
- Kirpitchnikov VS, Billard R (1999) Genetics and Breeding of Common Editions Quae, France.
- Bakos J, Gorda S (1995) Genetic Improvement of Common Carp Strains Using Intraspecific Hybridization. Aquaculture 129: 183-186.
- Aktar N, Nazrul IM (2015) Induced Breeding Practices of the Fish Hatcheries in the North-Western Region of Bangladesh. J Aquacul Mar Biol 2: 48.
- http://www.r-project.org/
- Rahman MM, Kelley JL, Evans JP (2013) Condition-Dependent Expression of Pre- and Postcopulatory Sexual Traits in Guppies. Ecol Evol 3: 2197-2213.
- Clotfelter ED, Curren LJ, Murphy CE (2006) Mate Choice and Spawning Success in the Fighting Fish Betta splendens: The Importance of Body Size, Display Behavior and Nest Size. Ethology 112: 1170-1108.
- Rahman MM, Turchini GM, Gasparini C, Norambuena F, Evans JP (2014) The Expression of Pre- and Postcopulatory Sexually Selected Traits Reflects Levels of Dietary Stress in Guppies. PLoS One 9: 105856.
- O’Dea RE, Jennions MD, Head ML (2014) Male Body Size and Condition Affects Sperm Number and Production Rates in Mosquitofish, Gambusia holbrooki. J Evol Biol 27: 2739-2744.
- Locatello L, Rasotto MB, Adriaenssens B, Pilastro A (2008) Ejaculate Traits in Relation to Male Body Size in the Eastern Mosquitofish Gambusia hol- brooki. J Fish Biol 73: 1600-1611.
- Marteinsdottir G, Begg AG (2002) Essential Relationships Incorporating the Influence of Age, Size and Condition on Variables Required for Estimation of Reproductive Potential in Atlantic Cod Gadus morhua. Mar Ecol Prog Ser 235: 235-256.
- Uusi-Heikkilä S, Wolter C, Meinelt T, Arlinghaus R (2010) Size-Dependent Reproductive Success of Wild Zebrafish Danio rerio in the J Fish Biol 77: 552-569.
- Sogard SM, Berkeley SA, Fisher R (2008) Maternal Effects in Rockfishes Se- : A Comparison among Species. Mar Ecol Prog Ser 360: 227-236.
- Pujolar JM, Locatello L, Zane L, Mazzoldi C (2012) Body Size Correlates with Fertilization Success but Not Gonad Size in Grass Goby Territorial Males. PLoS One 7: 46711.
- Smith C, Phillips A, Polačik M, Reichard M (2014) Male Coloration Signals Direct Benefits in the European Bitterling (Rhodeus amarus). Environ Biol Fishes 97: 335-341.
- Serrano-Meneses MA, Córdoba-Aguilar A, Méndez V, Layen SJ, Székely T (2007) Sexual Size Dimorphism in the American Rubyspot: Male Body Size Predicts Male Competition and Mating Success. Anim Behav 73: 987-997.
- Evans JP, Bisazza A, Pilastro A (2004) Female Mating Preferences for Co- lourful Males in a Population of Guppies Subject to High Predation. J Fish Biol 65: 1154-1159.
- Gage MJG, Macfarlane CP, Yeates S, Ward RG, Searle JB, et al. (2004) Spermatozoal Traits and Sperm Competition in Atlantic Salmon: Relative Sperm Velocity is the Primary Determinant of Fertilization Curr Biol 14: 44-47.
- Awata S, Heg D, Munehara H, Kohda M (2006) Testis Size Depends on So- cial Status and the Presence of Male Helpers in the Cooperatively Breeding Cichlid Julidochromis ornatus. Behav Ecol 17: 372-379.
- Shiel BP, Sherman CDH, Elgar MA, Johnson TL, Symonds MR (2015) In- vestment in Sensory Structures, Testis Size, and Wing Coloration in Males of a Diurnal Moth Species: Trade-Offs or Correlated Growth? Ecol Evol 5: 1601-1608.
- Locatello L, Rasotto MB, Evans JP, Pilastro A (2006) Colourful Male Guppies Produce Faster and More Viable Sperm. J Evol Biol 19: 1595-1602.
- Young MJ, Simmons LW, Evans JP (2010) Pre- and Post-Mating Sexual Se- lection Both Favor Large Males in a Rainbowfish. Behav Ecol Sociobiol 64: 915-925.
- Pitcher TE, Rodd FH, Rowe L (2007) Sexual Colouration and Sperm Traits in Guppies. J Fish Biol 70: 165-177.
- Evans JP, Zane L, Francescato S, Pilastro A (2003) Directional Postcopula- tory Sexual Selection Revealed by Artificial Insemination. Nature 421: 360-363.
- Petrie M, Tim H, Carolyn S (1991) Peahens Prefer Peacocks with Elaborate Trains. Anim Behav 41: 323-331.
- Ligon RA, Hill GE (2010) Sex-Biased Parental Investment Is Correlated with Mate Ornamentation in Eastern Bluebirds. Anim Behav 79: 727-734.
- Duryea MC, Bergeron P, Clare-Salzler Z, Calsbeek R (2016) Field Estimates of Parentage Reveal Sexually Antagonistic Selection on Body Size in a Pop- ulation of Anolis Lizards. Ecol Evol 6: 7024-7031.
- Huang WB, Chang C-C (2011) Effects of Parental Care and Body Size on the Reproductive Success of the Paradise Fish Macropodus opercularis (L.) in a Small Area. Zool Stud 50: 401-408.
- Jones MW, Hutchings JA (2001) The Influence of Male Parr Body Size and Mate Competition on Fertilization Success and Effective Population Size in Atlantic Salmon. Heredity 86: 675-684.
- Largiadèr CR, Fries V, Bakker TC (2001) Genetic Analysis of Sneaking and Egg-Thievery in a Natural Population of the Three-Spined Stickleback (Gas- terosteus aculeatus L.). Heredity 86: 459-468.
- Levitan DR (1991) Influence of Body Size and Population Density on Fertil- ization Success and Reproductive Output in a Free-Spawning Invertebrate. Biol Bull 181: 261-268.
- Petersen CW, Warner RR, Shapiro DY, Marconato A (2001) Components of Fertilization Success in the Bluehead Wrasse, Thalassoma bifasciatum. Behav Ecol 12: 237-245.
- Spence R, Smith C (2006) Mating Preference of Female Zebrafish, Danio rerio, in Relation to Male Dominance. Behav Ecol 17: 779-783.
- Rakitin A, Ferguson MM, Trippel EA (2001) Male Reproductive Success and Body Size in Atlantic Cod Gadus morhua L. Mar Biol 138: 1077-1085.
Citation: Rahman MM, Asaduzzaman S, Hasan MMA, Ahsan MN, Rahman SM (2018) A Comparative Hatching Performance between Big and Small Males of Hatchery Reared Common Carp (Cyprinus carpio). J Aqua Tech Deve 1: 001.
Copyright: © 2018 Rahman MM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


