*Corresponding Author:
Delia Teresa Sponza,
Faculty of Engıneerıng, Department of Envıronmental Engıneerıng, Department of Envıronmental Scıences, Dokuz Eylül University, Izmir, Turkey
E-mail: delya.sponza@deu.edu.tr
Abstract
A pre-treatment step was used with a 40 micron pore size cartridge before FO experiments. FO membrane was made from commercial cellulose triacetate (CTA). The variation of the increasing of water flux (5,12 and 25 L/m2.h) on the draw solid concentrations and effects of operating times (40,70,90 and 130 min) on the water flux were studied in FO membran. The variation of recovery percentage versus time and the effects of flow rates (50-400 L/h) on the rejection efficiency and on the removals of the pollutant in leather industry were studied in FO. RO experiments were performed in a spiral wounded membrane. Effect of increasing pressures (3, 8, 14 and 25 bar) and operating times (25,45,85 and 125 min) on the permeate flux were studied at a temperature of 21 C. At higher draw solution at constant pressure both rejection and water flux increased in FO. The recovery percentage both in distilled water and in leather industry versus operating time in FO. Flow rate flux decreased slightly throughout 80 min of operation, then it reached at aplateau at Jw values of 360 and 540 L/m2.h and 265 L/ m2.h, respectively. The maximum COD, turbidity, conductivity, TS, SS, sulphate, chloride and chromium and colour removals were 90%,89%,91%,91%, 91%,88%,90%,87% and 91%, respectively, in the permeate of the FO at 25 bar pressure while the removals of these parameters varied between 98% and 99% in RO at a trans membrane pressure of 30 bar. The highest permeate flux was detected as 781 and 760 L/h.m2 after 50 min in distilled water and in leather industry respectively. The permeate of the RO meet with the Discharge Standards of Water Quality for Irrigation water. Chromium, and gelatine were recovered from the RO retentate/concentrate. In order to treat 10 m3 leather industry wastewater the total cost was calculated as 1,01 USD.
Keywords
Collagene; Cromium; Draw solution; Feed solution; Forward Osmosis (FO); Gelatine; Permeate flux; Recovery; Ressure; Retentate; Reverse Osmosis (RO)
Introduction
During leather processing, various tanning agents are used along with huge quantity of fresh water where 90% of the used water is discharged as effiuent [1]. Often this generated wastewater does not receive effective treatment as demanded by effiuent discharge limits prior to discharge to the en-vironment in absence of strict compliance mechanism [2,3]. The tannery effiuent is generally characterized by high turbidity, foul smell and a range of high strength toxic chemicals represented by high COD (Chemical Oxygen Demand). The major contaminants were chromium, sulphide, volatile organic compounds, suspended solids and huge amount of inorganic solid wastes [4-6]. Leather production is a water intensive industry. Water usage is 15 to 40 m3 of water for the production of 1 ton of wet-salted raw hides and 110-260 liters . In many countries water has become an insufficient commodity and the costs for water supply and discharge increases regularly. In addition, its availability depends on the variability and seasonal variation of the climatic conditions. It is foreseeable that in the future these dynamics will probably become more serious. Large volume of wastewater discharge with high levels of chemical and organic pollutants poses serious threat to the surface water environment to the river bodies [7]. To protect surface water bodies from the onslaught of hazardous tannery wastewater, evolution of efficient and low cost treatment technology is the need of the hour [8]. The polluting components in the effiuent have the potential to adversely affect human health resulting in skin irritations, eye diseases, kidney failure and a range of gastrointestinal problems [9]. Detailed analysis of typical tannery wastewater reveals that tannery wastewater is characterized by high total dissolved solids (21,300 mg/L), total suspended solids (1250 mg/L) [10]. In concentional treatment processes such as biological treatment, phenton processesand adsorption process did not remove effectively the pollutants present at high concentrations in the leather industry [11]. Among the novel treatment processes the FO membrane process, water permeation occurs spontaneously through a semi-permeable membrane, being driven by the chemical potential difference (osmotic gradient) of a high-concentration Draw Solution (DS) and relatively low-concentration Feed Solution (FS) [12]. FO process can have the advantages of reduced capital and operational costs owing to a low energy consumption and low fouling because an additional hydraulic pressure is not required [13,14]. In a previous study, an FO process using osmotic pressure exhibited a rejection rate of the COD similar to that obtained with an RO process using hydraulic pressure [15]. It was investigated the rejection of pharmaceutically active compounds by an FO process as a function of the pH. They reported that the rejection of the compounds could be affected by the charge of molecules in solution, which could be changed depending on the pH of the FS and pKa of the molecules [15,16]. In another study, the effects of the DS and membrane materials on the removal of COD were evaluated on an FO membrane. It was reported that the high Reverse Salt Flux (RSF) of NaCl hindered the adsorption and diffusion of the COD in the FO membrane pore [17-19]. Although the removal processes by means of RO technology is prolific, scarce is being published about its application in leather treatment, focusing only on meeting irrigation standards and with no deep analysis of the RO operating conditions [20-22]. Most of these existing studies, typically based on batchwise operation, suffer from loss of membrane performance due to fouling problems, too. Pressure-driven membrane technology is nowadays considered a potential solution for wastewater recycling and reuse and shows stable and predictable treatment efficiency and performance. In particular, reverse osmosis (RO) has proven its effectivity to remove ions and organic chemicals [23-25]. However those processes are not competent in reduction of total dissolved in-organics (TDS). Hence many attempts were made to attain zero-discharge to save the environment. RO advanced method solve the problem of dissolved solids in the effiuents. Pilot studies have been carried out for removal of chromium from tannery wastewaters using RO mem-brane system and found high concentration of NaCl affected chromium separation as well as percent recovery of permeate [26-28]. Reverse Osmosis membrane rejection is influenced by interaction between effiuent composition and membrane properties [23,24]. The smoother surfaces with irregular ambiguous nodules lead to higher water fluxes and lower rejections, whereas rough surfaces with uniform distinct nodule structures contributed to higher rejections [25,27]. The electrostatic interactions and molecular sieving were important rejection mechanisms for membranes [26,27]. RO reject disposal without treating leads to environmental impacts. RO not only to improve the quality of the recycled chromium and salts recovery [29]. Metals, lipidic substances, gellatine, collagen and other impurities could presence in recovered chromium using traditional method combining alkaline precipitation of chromium [28].
In this study, the pollutants from a leather industry waste water (COD, COD dissolved, total solids, chloride, sulphides and chromium) were removed by using a sequental FO/ RO sequential membrane process at different operational conditions (water flux(5-25 L/m2.h), operating time (40-130 min), pressures (3-25 bar) and flow rates (50- 400 L/h). Some economical substances such as gelatine, collagen and cromium were recovered from the retentates of FO and RO.
Material and Methods
FO and RO membranes and membrane processes
FO membrane including cellulose triacetate thin film composite was used in this study. This membrane is composed of an asymmetric cellulose triacetate and has a thickness is 45μm and an average surface roughness on the active layer of 27 nm. The values of the water permeability, solute permeability and structure parameter (S) of this membrane were 0.650 Lm−2h−1 bar−1, 1.056 × 10−7 m s−1, and 250μm, respectively. A AFC 99 membrane with an average working pressure and internal diameter of 49 m2 and 9,3mm was used. The length and the effective volume of the RO membrane were 31.20 cm and 1.10 m2, respectively. The lab-scale RO experiments were run in a bench- scale cross flow filtration unit equipped with membrane modules (flat). The operating pressure was y adjusted with a spring loaded pressure-regulating valve on the retentive and monitored by a digital pressure gauge. It was made from stainless steel and has permeate and concentrate outlets.
Experimental and operational conditions
In the lab-scale FO and RO experiment, the initial volumes of the FS and DS were 300 mL, and each experiment was operated until the permeation volume reached at 70 mL In the DS, 0.9 M sodium chloride (NaCl) and this concentration was selected for the same initial flux in the deionized water through the operation of FO and RO.
The variation of increasing of water flux (5,7,9,12,15,17,18 and 20 L/m2.h) on the draw solid concentrations and effects of operating times(30, 60, 80, 90 and 100min) on the water flux were studied in FO. The variation of recovery percentage versus time and the effects of flow rates (30-220 L/h) on the rejection efficiency and on the removals of the pollutant removals (COD, turbidity, pH, conductivity, Total Solids (TS) Suspended Solids (SS), sulphate, chlorides, chromium and colour) were studied in FO. Effect of increasing pressures (4, 8, 16 and 20 bar) and operating times (10,20,30,60,80 and 100 min)on the permeate flux were studied at a temperature of 25 C in RO.
Pre-treatment
A pre-filtrationwas performed with a 25 micron pore size cartridge before FO experiments
Analytical procedures
The physicochemical parameters were analysed by following the methods as detailed in standard methods for analysis of water and wastewater [30]. The quantification of collagen and gelatine from the retantates of RO was measured as per Lowry’s method using bovine serum albumin as the standard at λ 660 nm using an UV- visible spectrophotometer [31]. Chrome recoveries from the RO retentates was performed with hydrolysis of collagen-chromium complexes according to procedure given by Westerhoff et al. [28]. The RO retentates was mixed with 32 mg/l sodium carbonate at 70°C temperature at a pH of 10. The hydrolysate composed from 47% Chromium, 21% collagen and 19% gelatine according to a dried retentates [32-37].
Results and Dıscussıon
Pollutant concentrations in Leather wastewater
Characterization of raw textile industry wastewater.
Variation of Water flux versus DS concentration in deion- ized water and in leather industry wastewater in FO
During this process, water transports from the feed solution to the draw solution across a semi-permeable membrane. The unwanted components will be effectively rejected by the membrane. The driving force for FO is the osmotic pressure gradient across the semi- permeable membrane and no external pressure is required [38]. Water flux as a function of DS concentration is illustrated in Table 1a for the FO membrane. The water flux decreases as DS concentration decreases because of the decreasing osmotic pressure difference between the DS and the reactor solution. Water flux through FO membrane is the hightest membrane. As a result, water flux is a function of DS concentraton Table 1b.
Effects of operating time on the flow rate of membrane durıng continous operation of distilled water and leather waste water in FO
Table 2 shows the effect of operating time on the flow rate through the operation of menbrane with distilled ana leather wastewater by increasing time. Flowrate flux is decreased during the first hour. The water flux dıd not exhibited a downward after the first hour of operating time ana remained as ıs an plateau.. The reason of this behavior is that the pure water move from the contaminated water (feed solution) through the membrane to the draw solution, and this flux leads sligthly to reduce the concentration of draw solution and then dıd not reduce the osmotic pressure which represents a driving force for the pure water transfer from the solution has high osmotic pressure to a high osmotic pressure [39-41]. Also, the flux for the leather feed solution is greater than the deionized water is high do to higher concentration of dissolved pollutant concentration in it. The high concentrationin the leather leads to increase slıgtly the osmotic pressure of feed solution, and thus did not reduce the driving force to move water across the membrane. As a result by increasing the operating time, the fouling rate was not increased on the surface of membrane, and ends with high rate of the water through the membranes.

Table 1a: Showed that the pollution load of leather concentrations was high.
Variation of recovery percentage versus increasing operat- ing time in distilled and leather industry wastewater in FO
Table 3 illustrates the increasing of recovery rate of distilled water and leather wastewater by increasing operation time. This increase is in accordance with the % recovery equation (Equation 5). A significant relationship between the product rate and the recovery percentage was calculated with Equation 5.
Recovery % = (product volume / feed vessel volume) * 100) (5)
In the continuous operation of FO after 80 min, the recovery percentage of pure water was 66% while the leather wastewater has a recovery of 65%. This slightly difference is due to the concentration of the leather did not decrease the flux and the recovery percentage.
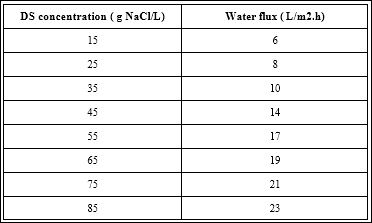
Table 1b: Effects of operating time on the flow rate of membrane during treatment distilled water and leather wastewater.
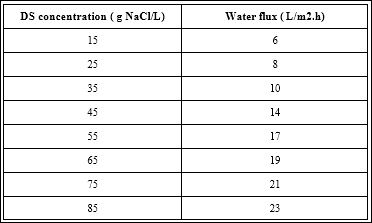
Table 2: Effects of operating time on the flow rate of membrane during treatment dis- tilled water and leather wastewater.
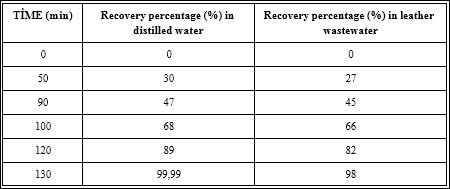
Table 3: Variation of recovery percentage versus increasing operating time in distilled and leather industry wastewater in FO.
Effect of feed flow on the rejection of pollutants in leather industry and deonized water in FO at a constant pressure of 18 bar
The results of this study showed the complete rejection of theNaCl 99,99% ( Table 4a) in distilled water was achieved at a cross flow rate of 400 L/h at the highest water flux of 280 L/m2 h . The complete rejection of pollutants (99%) in leather wastewater was detected at a flow rate of 310 L/ m2 h (Table 4b).

Table 4a: Effect of feed flow on the rejection of deonized water.
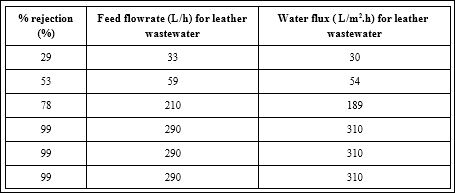
Table 4b: Effect of feed flow on the rejection of pollutants in leather industry.
Effects of increasing pressure on the pollutant rejection and water flux in FO
Table 5 shows a positive correlation between applied pressure and the water flux as the pressure was increased from 5 bar upto 25 bar. In solution-diffusion mechanism, solute flux decreases with increase in solvent flux and this is reflected in increased rejection [42,43]. Uncoupled nature of solute and solvent fluxes under this mechanism explains this opposite behaviour of solute and solvent fluxes following an increase in operating pressure.
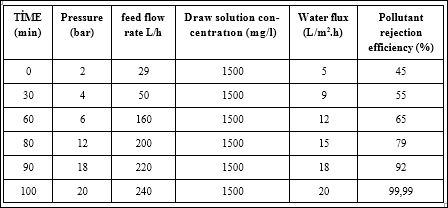
Table 5: Effects of applied pressure on rejection and flux at a draw solution of 1500 mg/l NaCl.
Table 6 shows the removals of all pollutants present in leather industry. Slightly high removals (85-89%) efficiencies was detected at a water flux of 200 L /m2.h compared to 100 L/m2.h water flux in FO.
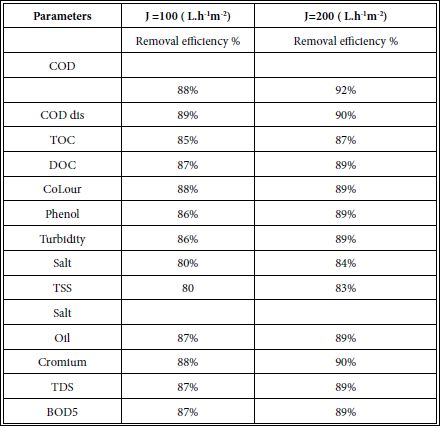
Table 6: Removal efficiencies of pollutants at two water fluxes in the leather industry versus permeate fluxes in FO.
Effect of increasing pressures on permeate fluxes versus time in RO
Thepermeate flux is time independent during continuous operation in RO (Table 7) [44]. This indicates that osmotic pressure is responsible from water flux. Therfore, a pore blocking and a cake filtration was not observed an a fouling system was not obsrved during increasing of pressure from 4 bar to 8 and 16 bar. This result not agree with the studies performed by Ben Abdelmelek et al.,[45,46]. since the pressures used in this study is extremwly high.
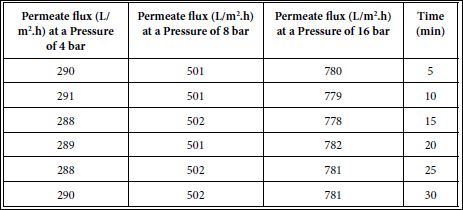
Table 7: Effect of increasing pressures on permeate fluxes versus time in RO.
Effect of time on permeate flux during RO operation study using FO permeate as feed
The permeate flux profile of four FO effluent (permeate) with initial COD concentrations (50,100,200 and 300 mg/l was shown in (Table 8) after 400 min continuous RO operation. The higher COD value of feed in the RO is not resulted in lower permeation flux due to increased feed concentration. As can be shown in this Table an insignificant decline of about 0,6% from an initial flux of 5 L/m2.h for a feed with initial COD value of 50 mg/L at a pressure of 9 bar.

Table 8: Effect of time on permeate flux during RO.
Effect of increasing pressure pressure on permeate flux in RO
Variation of pressure variation between 2 bar and 20 bar showed an increase in flux with increasing pressure (Table 9). The permeate produced was found suitable for reuse in leather tanning operations. The overall water recovery from RO process was about 98,00-99,99% with respect to the feed volume and volume of treated water produced.
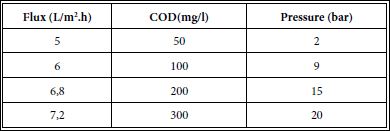
Table 9: Effect of increasing of pressure on permeate flux during RO.
Treatment of Pollutants in FO and RO
Complete removals of phenols, COD, CODdis and the other parameters was successfully achieved during RO experiments (Table 10). The rejection coefficients and removal yields (%) of the pollutants in the permeate are given in this Table. Due to the organic content of the solutes concentration in the bulk was low a concentration gradient increase was not detected across the membrane. No reduced hydrodynamic shear was detected in the vicinity of the valley regions of the membrane used in RO. The suspended and colloidal organic matter adsorption and deposition was not detected. As a result, a resistance did not occured aganist to the fouling. This was not hinder the transport of pollutants on the pore of the membranes.
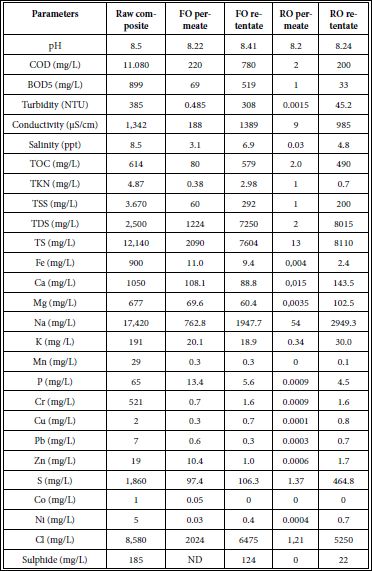
Table 10: Limits for water irrigation (Food and Agriculture Organization of the United Nations (FAO) 1985) [47].
Cost Analysis
The FO–RO sequential membrane process yielded 150 l/m2 h clean water by removing allthe pollutants with yıelds varying between 99% and 99.99% from the leather wastewater. The clean permeate water produced per day is 25 L/m2 day. The cost estimation was based on the annualized capital and operational cost with Equatons 6 and 7 [46].
Annualized investment x Annualized capital cost=Total capital ($) ×Cost recovery factor Water flux per year (m3) (6)
Cost recovery factor is calculated by the Equation xxx
Cost recovery factor = ni (1 + i) n / n(1 + i) n −1 (7)
Where n is the plant life (13 years) and i is the interest rate (12%).
Annualized cost can be computed with Equation 8.
Annualized operational cost=Total operational cost per year ($) Water flux per year (m3) (8)
The overall annualized cost as calculated by summing up the annualized investment cost and annualized operational stands at 1.01 USD per m3 of clean reusable water.
Reuse of Treated Water in Leather Processing
Although wastewater reclamation and reuse may be prohibitive for some small scale plants, it cannot be denied that this practice could relieve water stress, conserving significant amounts of fresh water which can be used in order to remedy seasonal water scarcity In this study the permeate of RO wastewater was a good quality water and it is reusable according to the limits and recommended water quality criteria given in Tables 7 and 8 for irrigation [47,48]. This effiuents should protect the surface water bodies from the onslaught of hazardous wastewater discharge.
Gelatine, Collagene and Cromium Recoveries from the Retentate of RO
Chrome recoveries from the RO retentates re chemically depicted as collagen-chromium complex. Hydrolysis of this waste involves the breakdown of bonds responsible for its stability [49]. The bonds are responsible for collagen stability as the collagen-chromium bond. Other covalent bonds have linkage between the complex chromium ion and the ionized carboxyl groups on collagen. There, the RO penetrate was subjected with an alkali for denaturation and degrading the protein fraction. These studies were performed 70°C temperature and at a pH of 10 according to procedure gıven by Dang et al., (2019) [50]. The alkaline condition was achieved by the utilization of sodium carbonate. The collagen was broken down to large molecular weight peptides into aqueous solution while the chromium was converted to an insoluble condition under alkaline conditions. The chemical characteristics of the hydrolysate was as follows: The peptides passed into the aqueous solution as collagen hydrolysates whose concentration is expressed as % Total Nitrogen. The hydrolysis yield was 78% for Total nitrogen. The production of low molecular weight degradative products showed the reduction in the dry matter content of the collagen hydrolysate The Composition of hydrolysate were inorganic ash 1 % TKN, 47% Chromium, 21% collagen and 19% gelatine according to a dried compounds.
Conclusions
Sequential FO/RO process proved the feasibility of treat the leather industry pollutants and of reusing the treated wastewater. The FO/ RO process can be used as alternative method to treat effectively the pollutants (TSS, TDS, BOD, COD, Cl-1, Na+1, SO -2 , NO -1,Cr+3) from the leather industry wastewater and to recovery of gelatine, collagen and chromiumas economical merits organic compounds which thus reduced the treatment cost.
References
- Ayoub GM, Hamzeh A, Al-Hindi M (2013) The impact of process sequences on pollutant removal efficiencies in tannery wastewater Water Air& Soil Pollution 224: 1-13.
- Ramteke PW, Awasthi S, Srinath T, Joseph B (2010) Efficiency assessment of common effluent treatment plant (CETP) treating tannery effluents. Environmental Monitoring and Assessment 169: 125-131.
- Mandal T, Maity S, Dasgupta D, Datta S (2010) Advanced oxidation process and biotreatment: Their roles in combined industrial waste- water treatment. Desalination 250: 87-94.
- Purkait MK, Bhattacharya PK, De S (2005) Membrane filtration of leather plant effluent: Flux decline mechanism. Journal of Mem- brane Science 258: 85-96.
- Gisi SD, Galasso M, Feo GD (2009) Treatment of tannery waste- water through the combination of a conventional activated sludge process and reverse osmosis with a plane Desalination 241: 337-342.
- Fababuj-Roger M, Mendoza-Roca JA, Galiana-Aleixandre MV, Bes- Pia A, Cuartas-Uribe B, et (2007) Reuse of tannery wastewaters by combination of ultrafiltration and reverse osmosis after a con- ventional physical-chemical treatment. Desalination 204: 219-226.
- Suthanthararajan R, Ravindranath E, Chits K, Umamaheswari B, Ramesh T, et (2004) Membrane application for recovery and re- use of water from treated tannery wastewater. Desalination 164: 151-156.
- Ciora RJ, Liu PRK (2003) Ceramic membranes for environmental related applications. Fluid/Particle Separation Journal 15: 51-60.
- Shaffer DL, Werber JE, Jaramillo H, Lin S, Elimelech M (2015) For- ward osmosis: Where are we now?. Desalination 356: 271-284.
- Shim SM, Kim WS (2013) A numerical study on the performance prediction of forward osmosis process. J Mech Sci Technol 27: 1179-1189.
- An X, Hu Y, Wang N, Zhou Z, Liu Z (2019) Continuous juice concen- tration by integrating forward osmosis with membrane distillation using potassium sorbate preservative as a draw solute. J Memb Sci 573: 192-199.
- Ang WL, Mohammad AW, Johnson D, Hilal N (2019) Forward os- mosis research trends in desalination and wastewater treatment: A review of research trends over the past decade. J Water Process Eng 31: 100886.
- Awad AM, Jalab R, Minier-Matar J, Adham S, Nasser MS, et al. (2019) The status of forward osmosis technology implementation. Desalination 461: 10-21.
- Aydiner C, Sen U, Topcu S, Sesli D, Ekinci D, et al. (2014) Tech- no-economic investigation of water recovery and whey powder pro- duction from whey using UF/RO and FO/RO integrated membrane Desalin and Water Treat 52: 123-133.
- Bamaga OA, Yokochi A, Zabara B, Babaqi AS (2011) Hybrid FO/ RO desalination system: preliminary assessment of osmotic energy recovery and designs of new FO membrane module Desalination 268: 163-169.
- Bell EA, Poynor TE, Newhart KB, Regnery J, Coday BD, et al. (2017) Produced water treatment using forward osmosis mem- branes: Evaluation of extended-time performance and fouling. J MembSci 525: 77-88.
- Blandin G, Verliefde ARD, Tang CY, Le-clech P (2015) Opportunities to reach economic sustainability in forward osmosis-reverse osmo- sis hybrids for seawater desalination. Desalination 363: 26-36.
- Scholz W, Lucas M (2003) Techno-economic evaluation of mem- brane filtration for the recovery and re-use of tanning chemicals. Water Research 37: 1859-1867.
- Ning RY, Shen PTL (1998) Observations from analysis of reverse osmosis membrane Foulants. Ultrapure Water: 37-40.
- Ning RY (2004) Sustaining the productivity of reverse osmosis plants. The Analyst Fall 1-5.
- Scholz WG, Rouge P, Bodalo A, Leitz U (2005) Desalination of mixed tannery effluent with membrane bioreactor and reverse os- mosis treatment. Environmental Science & Technology 39: 8505-8511.
- Greenberg AE, Eaton AD, Clesceri LS, Rice EW (2005) Standard Methods for the Examination of Water and Wastewater.
- Ranganathan K, Kabadgi S (2011) Studies on feasibility of reverse osmosis (membrane) technology for treatment of tannery wastewa- Journal of Environmental Protection 2: 37-46.
- Krishnamoorthi S, Saravanan K (2011) Tannery industry effluent treatment by combinations of activated sludge process and com- bined reverse osmosis Journal of Environmental Research and Development 5: 978-988.
- QinJJ, OoMH, Lee H, Kolkman R(2004) Dead-end ultrafiltration for pretreatment of RO in reclamation of municipal wastewater J Membr Sci 243: 107-113.
- Abdel-Jawad M, Al-Shammari S, Al-Sulaimi J (2002) Non-conven- tional treatment of treated municipal wastewater for reverse osmo- Desalination 142: 11-18.
- Van Hege K, Verhaege M, Verstraete W (2002)Indirect electro- chemical oxidation of reverse osmosis membrane concentrates at boron-doped diamond electrodes. Electrochem. Commun 4: 296-300.
- Water Practice and Technology (2010) Managing the reverse osmo- sis concentrate from the Western Corridor recycled water scheme 5: 1-8.
- Westerhoff P, Moon H, Minakata D, Crittenden J (2009) Oxidation of organics in retentates from reverse osmosis wastewater reuse Water Res 43:3992-3998.
- Dialynas E, Mantzavinos D, Diamadopoulos E (2008) Advanced treatment of the reverse osmosis concentrate produced during rec- lamation of municipal wastewater. Water Res 42: 4603-4608.
- (2018) Standard Methods, American Water Works Association (AWWA) USA.
- Walker JM (2016) The Protein Protocols Humana press 545.
- Eslah S, Shokrollahzadeh S, Jazani OM, Samimi A (2018) Forward osmosis water desalination: fabrication of graphene oxide-polyam- ide/polysulfone thin-film nanocomposite membrane with high water flux and low reverse salt diffusion. Sci Technol 53: 573-583.
- Wu S, Zou S, Liang G, Qian G, He Z (2018) Enhancing recovery of magnesium as struvite from landfill leachate by pretreatment of calcium with simultaneous reduction of liquid volume via forward Sci. Total Environ 610-611: 137-146.
- Kim JE, Phuntsho S, Ali SM, Choi JY, Shon Hk (2018) Forward osmosis membrane modular configurations for osmotic dilution of seawater by forward osmosis and reverse osmosis hybrid system. Water Res. 128: 183-192.
- Cath TY, Childress AE, Elimelech M (2006) Forward osmosis: Prin- ciples, applications, and recent developments. J Membr Sci 281: 70-87.
- Choi Y, Choi J, Oh H, Lee S, Yang DR, et (2009) Toward a com- bined system of forward osmosis and reverse osmosis for seawater desalination. Desalination 247: 239-246.
- Coday BD, Heil DM, Xu P, Cath TY (2013) Effects of transmem- brane hydraulic pressure on performance of forward osmosis mem- Environ Sci Technol 47:2386-2393.
- Jin X, Shan J, Wang C, Wei J, Tang CY (2012) Rejection of phar- maceuticals by forward osmosis membranes. J Hazard Mater 227: 55-61.
- Sant’Anna V, Marczak LDF, Tessaro IC (2012) Membrane concen- tration of liquid foods by forward osmosis: Process and quality J Food Eng 111: 483-489.
- Tang W, Ng HY (2008) Concentration of brine by forward osmosis: Performance and influence of membrane structure. Desalination 224: 143-153.
- Xie M, Price WE, Nghiem LD (2012) Rejection of pharmaceutical- ly active compounds by forward osmosis: Role of solution pH and membrane orientation. Sep Purif Technol 93: 107-114.
- Zhao S, Zou L (2011) Effects of working temperature on separation performance, membrane scaling and cleaning in forward osmosis desalination. Desalination 278: 157-164.
- Jin X, Jawor A, Kim S, Hoek E (2009) Effects of feed water tem- perature on separation performance and organic fouling of brackish water RO membranes. Desalination 239: 346-359.
- Abdelmelek SB, Greaves J, Ishida KP, Cooper WJ, Song W (2011) Removal of Pharmaceutical and Personal Care Products from Re- verse Osmosis Retentate Using Advanced Oxidation Processes. Environ SciTechnol 45: 3665-3671.
- Jae-Wook L, Tae-Ouk K, Il-Shik M (2006) Performance of polyam- ide reverse osmosis membranes for steel wastewater reuse. De- salination 189: 309-322.
- Foods and Agriculture Organization of the United Nations (FAO). 1985. FAO Irrigation and Drainage Paper, 29 1. Food and Agri- culture Organization of the United Nations: Rome, Italy.
- Tchobanoglous G, Leverenz HL, Nellor MH, Crook J (2011) Direct Potable Reuse: The Path WateReuse Research Founda- tion and Water Reuse California. Washington, D.C.
- Bixio D, Thoeye C, Koning JD, Joksimovic D, Savic D, et (2006) Wastewater reuse in Europe. Desalination 187: 89-101.
- Dang X, Yang M, Wang Y (2019) Recovery and utilization of colla- gen protein powder extracted from chromium leather scrap. Waste Environmental Science and Pollution Research 18: 201-209.
Citation: Sponza DT (2021) Treatment of leather Industry Wastewater with Sequential Forward Osmosis (FO) and Reverse Osmosis (RO) Hibrid Prosesses and Recoveries of Economical Merit Materials. J Nanosci Nanomed Nanobio 4: 008.
Copyright: © 2021 Sponza DT. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and re- production in any medium, provided the original author and source are credited.


