*Corresponding Author:
Owais Dar,
Department of Cardiology, Royal Brompton & Harefield Hospitals Charity, London, UK
Tel: +44 01895 828 975
E-mail: o.dar@rbht.nhs.uk
Abstract
At any given moment, a small proportion of heart failure patients have advanced heart failure that may also benefit from either heart transplantation or mechanical circulatory support. Worldwide there is a donor organ shortage therefore left ventricular assist devices are often used as an alternative treatment for long term support of advanced heart failure patients. Short term survival and quality of life is comparable between left ventricular assist devices and heart transplantation however longer-term outcomes are better with heart transplantation. This article will give the reader an overview of LVAD’s and their current role in the management of advanced heart failure patients.
Keywords
Advanced heart failure; Left ventricular assist devices; Mechanical circulatory support
Introduction
It wasn’t until the 1980’s after the introduction of cyclosporine as an immunosuppressant agent that Heart Transplantation (HTx) became widely accepted. Prior to that outcomes were poor and HTx was only carried out by a handful of centres. In Europe and North America around 5000 HTx are carried out annually [1]. Despite this every year many patients die waiting for an HTx. The main reason for this is limited donor availability.
In 1994 the Texas Heart Institute published the world’s first ever case of an advanced heart failure patient who was discharged from hospital to lead a near normal life without a heart transplant [2]. Patient was supported at home by a pioneering battery powered artificial heart pump known as a Left Ventricular Assist Device (LVAD). The patient, a 33 year old engineer, was admitted with decompensation of known dilated cardiomyopathy. He was inotrope and intra-aortic balloon pump dependent. Weighing in greater than 90kg and blood group O meant he had little chance of getting a HTx. Finally, patient went on to die after 16 months of LVAD support, without ever getting an HTX. Currently over 5000Mechanical Circulatory Support (MCS) or Left Ventricular Assist Devices (LVAD) are implanted annually. A lack of donor availability has meant that LVADs have satisfied the great need for an alternative treatment option for patients in need of a heart transplant.
In order to provide a broader understanding of the clinical context of LVAD therapy a guide to the diagnosis of advanced heart failure will be provided along with brief comparison to heart transplantation.
What is the difference between heart failure and advanced heart failure?
Heart failure is defined as a syndrome with typical symptoms and signs caused by an abnormality of the heart which leads to a reduced cardiac output and/or raised cardiac filling pressures [3]. This definition covers a wide range of patient phenotypes, from mildly symptomatic patients with a good quality of life and prognosis to severely symptomatic patients with a poor quality and limited life expectancy. Dividing a heart failure cohort into those with and without advanced heart failure helps to identify patients who may benefit from palliative care, cardiac transplantation or mechanical circulatory support. In clinical practice the term advanced heart failure is often also referred to as “end stage” heart failure. An estimated 1-10% of the heart failure population have advanced heart failure [4-6]. Parameters which should alert you to a diagnosis of advanced heart failure and trigger a referral for heart transplant or mechanical circulatory support include:
- Inotrope dependence
- Presence of symptoms at rest or with minimal exertion and inability to perform many activities of daily living
- Cardio pulmonary exercise test peak oxygen uptake (pMVO2)< 14mls/kg/min
- Six-Minute Walk Distance (6MWTD) < 300m
- Cardiac cachexia, low serum sodium (<130mmol/L)
- Raisedserum Brain Natriuretic Peptide (BNP)>400 pg/ml or NT-proBNP>1600pg/L, or rising despite treatment
- Failing or intolerant of conventional heart failure therapy
- Rising diuretic requirements
- Uncontrolled arrythmia, frequent appropriate defibrillator (ICD) shocks
- Worsening renal function due to cardio renal syndrome
- Worsening liver function due to right heart failure despite optimal medical therapy
- Repeated hospitalization for heart failure requiring intravenous diuretics.
- Rising pulmonary pressures (pulmonary artery systolic pressure > 50mmHg)
- Very low Left Ventricular Ejection Fraction (LVEF) <30%
- Cardiac index <2.0 L/min/m2
- Estimated 1-year mortality by the Seattle Heart Failure Model (SHFM) of >20%
Who should I refer for transplant/ Mechanical Circulatory Support (MCS) versus palliative care?
Timing of heart transplantation or MCS is crucial. Operating too soon, means the patient takes an unnecessary risk of an operation and is exposed to the long-term complications of transplantation and MCS. Operating too late (i.e., renal failure, liver failure, respiratory failure), substantially reduces the chances of surviving surgery. Timing of advanced heart failure surgery must also consider the duration of time the recipient is expected to wait for a suitably matched donor and the trajectory of the worsening heart failure [7]. Recipients who are blood group O and those who weigh more than 90kg can expect to wait longest. Several contraindications to transplant and mechanical support exist, and such patients should be referred to palliative care or if appropriate have care focused on reversing these contraindications. Some patients have relative contraindications and the decision for advanced heart failure surgery may as a result be unclear. In the United Kingdom (UK) all decisions to list patients for HTx or MCS are done following multidisciplinary team discussion. A list of some contraindications for HTx and MCS include:
- Heart transplant above the age of 70 years of age are seldom carried
- Known cancer
- BMI >30kg/m2
- Diabetes with – Proliferative retinopathy, nephropathy, neuropathy, HbAbA1C>7.5%
- Severe peripheral vascular disease
- Irreversible renal failure creatinine clearance <50ml/min, eGFR <40ml/min
- Active smoker
- Substance misuse (alcohol, recreational drugs)
- Cognitive impairment
- History of non-compliance
- Inadequate social support (housing, finance, family etc)
- Irreversible poor lung function
- Irreversible liver dysfunction
- Recent pulmonary embolus
- Sepsis or uncontrolled infection
- Other conditions which impact quality of life and prognosis and reduce the ability of the recipient to benefit from HTx or MCS (e.g., severe arthritis and multi system auto immune diseases)
Survival and complications post Heart Transplant
Around a 170 adult heart transplants are carried out in the UK annually. Median survival post heart transplant is 11 years [1]. Those who survive the first year of transplant have a median survival of 14 years [1]. The main limitations to achieving longer median survival is due to the accrual of several transplant related complications.
In the early post op period graft failure, infection and rejection are the commonest cause of death. Beyond 5 years graft failure, transplant coronary artery disease, malignancy and renal failure are the common causes of death. Nearly a quarter of patients will develop severe renal dysfunction by 10 years and nearly half will develop transplant related coronary artery disease by 10 years [1].
What is a Left Ventricular Assist Device (LVAD)?
A LVAD is a mechanical heart pump. Having it requires open heart surgery. Current modern day LVADs are small enough to fit into the palm of your hand. One end of the LVAD pump is attached to the left ventricle of the heart whilst the other end of the LVAD is connected to the aorta. The LVAD works by sucking blood from the left ventricle and then pumping it into the aorta and rest of the body. The LVAD assists the heart while the heart continues to beat. The two work together to provide an adequate circulation for the patient to be able to live longer and live a better quality of life out of hospital. To keep the LVAD working continuously, the LVAD is connected to a cable within the body at one end and to a controller and a power source, outside the body, at the other end. A power source can either be a standard household electrical socket or (if travelling in a car) the cigarette lighter socket in the car. The cable, called a driveline, comes out of the body at the site of the abdomen. The controller monitors the function of the LVAD and the battery power level. The controller will alarm to warn the patient if there is a problem with the LVAD that needs to be fixed. An additional battery is always connected to the controller, so if one battery runs down, there is another to power the LVAD for several more hours. LVAD patients must be anticoagulated with warfarin to prevent device related thrombosis. Use of novel oral anticoagulants instead of warfarin has been associated with worse outcomes (Figure 1).
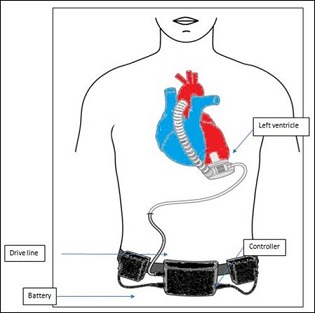
Figure 1: Centrifugal LVAD schematic [8].
Why some patients are selected for LVAD vs. Heart transplant?
There are several possible reasons why patients are recommended a LVAD and not a heart transplant [9].
- The transplant team may feel that the risk of deterioration and dying while waiting for a donor heart is too great when compared to having a LVAD sooner. Unlike with heart transplantation the date for LVAD surgery can be planned.
- Some patients are not able to have a HTx because the risk of heart transplantation is too great. For example patients with pulmonary hypertension (Pulmonary artery systolic pressure >60mmHg, transpulmonary gradient >15, pulmonary vascular resistance >3) or patients with severe kidney disease (eGFR< 40ml/min/1.73m2) or liver failure(cirrhosis, raised bilirubin and transaminase levels).In each case the transplant team may feel that bridging to a transplant by having a LVAD first is less The idea is that the LVAD restores the circulation to normal and will allow the patient to rehabilitate to a fitter state i.e., the kidney failure and pulmonary hypertension resolves in readiness for a HTx later. Having a LVAD first with a view to transplantation later is termed “LVAD as a bridge therapy to transplantation”.
- Some patients may not be suitable for transplant due to the presence of irreversible Such patients may still have the potential to benefit from a LVAD both from a survival and quality of life perspective. LVAD therapy with the intention of living out your life without consideration for transplantation is known as “destination therapy”.
In the UK LVADs are currently only funded by the national health service for use as bridge to transplantation [10]. Although recommended by the National Institute of Clinical Excellence, destination therapy is not funded by the UK NHS because of concerns related to the cost effectiveness of this strategy [11,12].
How has LVAD technology evolved?
First generation LVADs (Heart Mate vented electric device (HM VE), Thoratec, Pleasanton, California)were large and cumbersome which restricted its use to larger patients [13]. It contained multiple moving parts and was prone to malfunction and complications such as bleeding, infection and thrombosis. It was designed to mimic the natural pumping action of the heart and produced pulsatile flow (pulsatile flow LVAD). The HeartMate II LVAD (HM II) and the Heartware LVAD (H-VAD) are 2nd and 3rd generation LVADs respectively [13,14]. Both these devices pump blood continuously during systole and diastole (continuous flow LVADs). They are smaller with a single moving part and are consequently more durable, less noisy and easier to implant. Since 2006 over 13 000 continuous flow LVADs have been implanted worldwide and currently approximately 50% of HTx are transplanted from a LVAD [15].
LVAD trial data and registry evidence
REMATCH, 2001, a Destination therapy trial: advanced HF patients ineligible for HTx were randomised to receive the HeartMate XVE LVAD (n=68) or optimal medical therapy (n=61). Survival at 1 year was significantly better in the LVAD group 52% versus 25% (p=0.002). Ultimately, this trial led to the approval of the Heartmate XVE as DT in 2003 [12].
HM II DT trial, 2009: advanced HF patients ineligible for HTx were randomised in a 2:1 ratio to the continuous flow HMII LVAD (n=134) or the HeartMate XVE LVAD (n=66). Patients with the continuous flow HMII device had improved survival at 2 years (58% versus 24%, p=0.008). The rates of major adverse events were significantly reduced in the group with a continuous-flow left ventricular assist device. In 80% of patients with a continuous-flow left ventricular assist device the functional status also improved to NYHA functional class I or II at 24 months post LVAD surgery. Based on this trial the HM II LVAD was approved for DT in USA in 2010 [13].
ADVANCE-BTT trial, 2012: Compared 140 Heartware-VAD patients (who were eligible for HTx but were believed to be too unwell to survive without a LVAD) to 499 patients pooled from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS registry) who mainly received the HM II LVAD. Survival at 360 days was no different between the two groups (H-VAD 86% versus control group 85%, p=0.61) [ 14].
MOMENTUM III, 2019: Randomised controlled trial comparing 516 HM III patients to 512 HM II patients, from 69 centres across the USA. The actualevent-free survival at 2 years (primary end point) in the intention-to-treat population were 74.7% in the HM III group and 60.6% in the HM II group (p<0.001). Overall survival at 2 years follow-up was similar in the 2 groups HM III 79% versus 76.7% in the HM II group. The stroke (1.4% versus 19.4%, p<0.001), bleeding (43.7% versus 55%, P<0.001) and pump thrombosis (1.4% versus 13.9%, P<0.001) rates were less in the HM III group. The longer term 5-year outcome data is yet to be published [16].
Real life registry data from the International Mechanically Assisted Circulatory Support (IMACS) registry and the mainly North American based Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), suggests the contemporary survival following the implantation of a continuous flow LVADs is 80% at 1 year, 70% at 2 years and 60% at 3 years [17,18]. Although the 2-year survival nowadays is comparable to that of HTx, long-term survival is hampered by frequent device related complications (Figure 2).
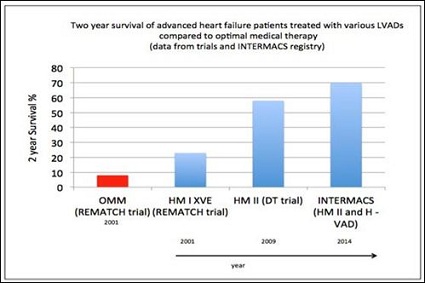
Figure 2: Two year survival of advanced heart failure patients treated with various LVADs compared to optimal medical therapy. (OMM-optimal medical management, HM I XVE-HeartMate I XVE LVAD, HM IIHeartMate II LVAD, INTERMACS-Interagency Registry for Mechanically Assisted Circulatory Support, H-VAD-Heartware LVAD, DT-destination therapy).
LVAD quality of life and complications
When surveyed up to 80% of LVAD patients report a favourable impression of their LVAD during their first 2 years. An improvement in quality of life is seen as early as 3 months post LVAD and is maintained out to at least 2 years post LVAD surgery. Approximately 80% of patients are in NYHA class I or II following implant. On average patients can walk over 200m further on a six-minute walk test following their LVAD [18].
LVAD related complications are not that infrequent. Approximately 20% of patients can expect to have a stroke by 2 years. Pump related infections occur in 20% of patients by 2 years. Just under 90% of patients will be readmitted to hospital within 2 years following the LVAD. 85% will have had a major event (e.g., first occurrence of infection, bleeding, device malfunction, stroke or death) within 2 years of the LVAD. Early post-implant multisystem organ failure, right heart failure, and stroke pose the greatest risks for death. After the first 6 months, stroke remains the major cause of death out to 4years [18].
How is short term MCS different from durable MCS? What is the role of short term MCS?
Short term MCS refers to devices which provide extra hemodynamic support to patients who are deteriorating whilst in cardiogenic shock. They are generally used for a short period of time (usually weeks too few months). Many devices are available e.g., Intra aortic balloon pump (IABP), impella, extracorporeal membrane oxygenation (ECMO), and levotronix LVAD [19]. These devices differ in the level of haemodynamic support they provide (from 1 litre/minute to 5litres/min), the ease with which they can be implanted, the complexity of post implant care, and the complication rates. The type of device used depends on several factors including case specifics, centre experience, and cost. The most striking difference between short term MCS and durable MCS (e.g., LVADs) is that unlike with durable LVADs, patients cannot go home and must be managed either in a critical care or high dependency unit. Short term MCS devices can be used to reverse the low cardiac output related end organ failure and allow time for recovery i.e., “bridge to recovery”. They are also used to provide stability in a deteriorating patient, thereby providing valuable time for the patient to improve sufficiently enough to become eligible for either a HTx or a durable LVAD. If, however despite the short term MCS support the patient continues to deteriorate then treatment withdrawal and palliative care remain the only option. This is often referred to as short term MCS as a “bridge to decision”. These devices are relatively new and there is a paucity of evidence base for their use in cardiogenic shock. Their use is largely limited to a few specialised units often linked with heart transplantation and durable LVAD expertise.
Conclusion
MCS devices have evolved rapidly over the last few decades and have improved the quality of life and survival of many patients living with heart failure. They have helped to deal with the issues of limited donor availability. The 2 year survival and quality of life is comparable to that of heart transplantation. Long term survival and quality of life is hampered by common complications such as stroke, infection and bleeding. Since donor availability remains an ongoing issue, the challenge for the future is to develop durable LVADs with a comparable or better long term survival and quality of life to that of heart transplantation. Developing LVADs with a reduced associated stroke, infection and bleeding risk are probably the key areas to focus on in achieving this goal.
References
- Chambers DC, Cherikh WS, Harhay MO, Hayes Jr D, Hsich E, et (2019) The international thoracic organ transplant registry of the international society for heart and lung transplantation: Thirty-sixth adult heart transplantation report-2019; focus theme: Donor and recipient size match. J Heart Lung Transplant 38: 1056- 1066.
- Frazier OH (1994) First use of an unthethered, vented electric left ventricular assis device for long term support. Circulation 89: 2908-2914.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, et al. (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37: 2129-2200.
- Xanthakis V, Enserro DM, Larson MG, Wollert KC, Januzzi JL, et (2016) Prevalence, neurohormonal correlates, and prognosis of heart failure stages in the community. JACC Heart Fail 4: 808- 815.
- Bjork JB, Alton KK, Georgiopoulou VV, Butler J, Kalogeropoulos Ap, et (2016) Defining advanced heart failure: A systematic review of criteria used in clinical trials. J Card Fail 22: 569-577.
- Fang JC, Ewald GA, Allen LA, Butler J, Canary CAW, et (2015) Advanced (stage D) heart failure: A statement from the heart failure society of America guidelines committee. J Card Fail 21: 519- 534.
- Banner N, Bonser R, Clark A (2011) UK guidelines for referral and assessment of adults for heart
- Heatley G, Poornima Sood P, Goldstein D, Uriel N, Cleveland J, et al. (2016 ) Clinical trial design and rationale of the multicenter study of maglev technology in patients undergoing mechanical circulatory support therapy with heartmate 3 (MOMENTUM 3) investigational device exemption clinical study protocol. J Heart Lung Transplant 35: 528-536.
- Peura J, Colvin-Adams M, Francis G, Grady KL, Hoffman TM, et (2012) Recommendations for the use of mechanical circulatory support: Device strategies and patient selection a scientific statement from the american heart association. Circulation 126: 2648-2667.
- Clarke A, Pulikottil-Jacob R, Connock M, Suri G, Kandal NB, et (2014) Cost-effectiveness of Left Ventricular Assist Devices (LVADs) for patients with advanced heart failure: analysis of the British NHS Bridge To Transplant (BTT) program. Int J Cardiol 171: 338-345.
- National Institute of Health and Care Excellence (2015) Implantation of a left ventricular assist device for destination therapy in people ineligible for heart transplantation. National Institute of Health and Care Excellence, London,
- Rose EA, Gelijns AC, Moskowitz AJ, Stevenson LW, Dembitsky W, et (2001) Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 345: 1435-1443.
- Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, et (2009) Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 361: 2241-2251.
- Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, et (2012) Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 125: 3191-3200.
- Khush KK, Cherikh WS, Chambers DC, Goldfarb S, Hayes Jr D, et (2018) The international thoracic organ transplant registry of the international society for heart and lung transplantation: Thirty-fifth adult heart transplantation report-2018; focus theme: Multiorgan transplantation. J Heart Lung Transplant 37: 1155-1168.
- Mehra M, Goldstein D, Uriel N, Cleveland Jr JC, Yuzefpolskaya M, et al. (2018) Two-year outcomes with a magnetically levitated cardiac pump in heart N Engl J Med 378: 1386-1395.
- Kirklin J, Cantor R, Mohacsi P, Gummert J, De By T, et al. (2016) First annual IMACS report: A global international society for heart and lung transplantation registry for mechanical circulatory support. J Heart Lung Transplant 35: 407-412.
- Kirklin J, Pagani F, Kormos R, Stevenson LW, Blume ED, et al. (2017) Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 36: 1080-1086.
- Werdan K, Gielen S, Ebelt H, Hochman JS ((2014) Mechanical circulatory support in cardiogenic Eur Heart J 35: 156-167.
Citation: Cid-Menendez A, Dar O (2020) Mechanical Circulatory Support Use in Advanced Heart Failure. J Cardio Cardiovasu Med 4: 013.
Copyright: © 2020 Cid-Menendez A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


