*Corresponding Author:
Walid Ben Ali,
Department of Cardiac Surgery, Montreal Heart Institute, University of Montreal, Montreal, Canada
Tel: +1 5145611037
E-mail: dr.walidbenali@gmail.com
Abstract
We report two consecutive cases in a month of transcatheter aortic valve thrombosis, one involving the new Sapien 3 Ultra valve (Edwards Lifesciences, Irvine, CA) treated surgically. These cases highlight the importance of standardization of periprocedural and post procedural anticoagulation protocols to avoid this serious complication.
Keywords
Anticoagulation; Antiplatelet therapy; Edwards Sapien 3 Ultra; TAVR thrombosis
Abbreviations
ACT: Activating Clotting Time
DAPT: Dual Antiplatelet Therapy
MG: Mean Gradient
NOAC: Non-Vitamin K Oral Anticoagulants
PVL: Paravalvular Leak
SAPT: Single Antiplatelet Therapy
TAVR: Transcatheter Aortic Valve Implantation
THV: Transcatheter Heart Valve
TTE: Transthoracic Echocardiography
VKA: Vitamin K Antagonists
Introduction
The 2-year results of PARTNER 3 demonstrated an increased risk (2.6%) of valve thrombosis in low surgical risk patients who underwent TAVR [1]. Moreover, subclinical thromboses were as high as 13% at 30-day in the TAVR arm of the computed tomography substudy [2]. These data shed light on an unmet need of optimal periprocedural and post procedural anticoagulation regimen in the growing TAVR population.
In this case series, we report two consecutive cases of early thrombosis of THV, one involving the new Sapien 3 Ultra valve (Edwards Lifesciences, Irvine, CA).
Case 1
A 78-year-old man was referred for symptomatic severe aortic stenosis. TTE and baseline and procedural characteristics are summarized in (Table 1). He underwent elective transfemoral implantation of a 26-mm Sapien 3 Ultra valve (Edwards Lifesciences, Irvine, CA). Antiplatelet treatment with aspirin was continued peri-procedurally without any interruption. Immediate hemodynamics were favorable with trace paravalvular leak and a MG of 5 mmHg. After a pacemaker implantation for a complete atrioventricular block, a pre-discharge TTE performed on day 7 revealed an asymptomatic increase in aortic valve MG to 54 mmHg. Computed tomography imaging showed a restricted leaflet opening and a 1.5 x 1.1cm mass suspicious for thrombus (Figure 1). A surgical treatment was decided after initiation of intravenous anticoagulation therapy. Aortic valve replacement was successfully performed at day 8 with TAVR explant and implantation of a 23-mm InspirisResilia valve (Edwards Lifesciences, Irvine, CA). Post-operative period was uneventful, and patient was asymptomatic at one-month follow up.
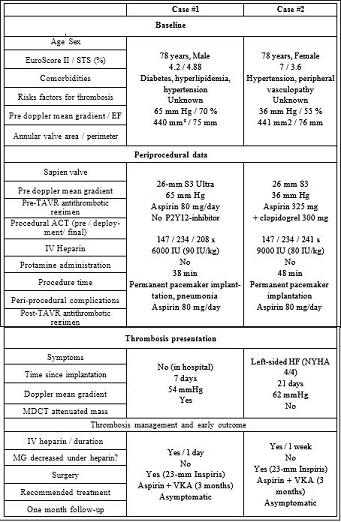
Table 1: Patient characteristics, procedural data and thrombosis management.

Figure 1: Computed tomography imaging and macroscopic appareances of Edwards Sapien Ultra thrombosed leaflets: Panel A: Multidetector computed tomography in transversal axis shows hypoattenuated mass near the stent frame (arrows) that extend to the cusp with decreased leaflet movement, confirmed in long axis by Panel B. Panel C: Explanted transcatheter heart valve demonstrating thrombosis.
Case 2
An intermediate risk (STS Score: 3.6%) 78 years old female underwent a transfemoral TAVR with an Edwards S3 26 mm (Edwards Lifesciences, Irvine, CA) with good immediate hemodynamic results (MG of 4 mmHg and mild PVL) followed by a pacemaker implantation due to a left bundle branch block. TTE and baseline procedural characteristics are summarized in (Table 1). Antiplatelet treatment with aspirin was continued peri-procedurally without any interruption. Three weeks later, she was re-hospitalized for acute left heart failure secondary to a severe THV thrombosis (MG: 62 mmHg), treated surgically with THV explantation and an aortic valve replacement with Inspiris 23mm (Edwards Life sciences, Irvine, CA) bioprosthesisvalve (Figure 2). Patient was discharged day 7 and was asymptomatic at one-month follow-up with no PVL and a MG of 4 mmHg on TTE.
Discussion
To our best knowledge, this is the first reported case of SAPIEN 3 Ultra thrombosis. The SAPIEN 3 Ultra is the latest iteration of balloon-expandable valve by Edwards Life sciences (Irvine, CA, USA) with an improved sealing skirt and a modified delivery system.
Clinical thrombosis is defined as a combination of the THV dysfunction (increased MG ≥ 20 mmHg or at least moderate regurgitation) and an imaging evidence of leaflet thrombosis. Common presentation includes isolated elevated gradients, dyspnea and thromboembolic events [3]. Many factors such obesity, reduced ejection fraction, stent frame under-expansion, patient prothesis mismatch and use of balloon-expandable valve may predispose to THV thrombosis.
Optimal procedural ACT during TAVR and the heparin reversal at the end of the procedure remain controversial. In our routine practice, we target an ACT close to 250 seconds and avoid systematic use of protamin. Al-Kassou et al. recently showed a significantly lower rates of life-threatening and major bleeding complications with protamin administration compared with patients without heparin reversal, without any increasing of clinical thromboembolic event [4]
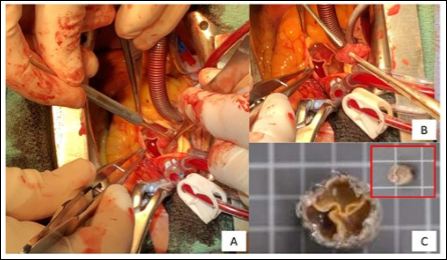
Figure 2: Peroperative and macroscopic appareances of Edwards Sapien S3 thrombosed leaflets: Panel A demonstrates the severely thrombosed three leaflets of the Sapien S3, and a really tickened thrombus with a gelatinous appareance, as shown by the Panel B. Panel C describes the Edwards Sapien S3 piece of resection and a typical thombus without any endocarditis diagnosis.
In the current cases, less than 100 UI/Kg of heparin was administrated, and the ACT was under 250 seconds. This may have favored the THV thrombosis, as both patients had no identified risk factors for device thrombosis except the use of balloon expandable valve.
A literature review of optimal anticoagulation strategy after TAVR over the last five years was performed and summarized in (Table 2). The ARTE trial compared SAPT (aspirin alone) to DAPT (aspirin + clopidogrel) and was prematurely stopped. The composite of death, MI, stroke or transient ischemic attack, or major or life-threatening bleeding tended to occur more frequently in the DAPT group (15.3% vs. 7.2%, p = 0.065). Any difference in valve hemodynamic status at discharge between the 2 groups was observed but 4-D CT data wasn’t available [5]. These results were also confirmed by the POPULAR-TAVI trial [6]. NOAC was also tried as a potential alternative strategy in the GALILEO trial which compared a therapy including rivaroxaban 10mg daily (+ aspirin for 3 months) versus a DAPT therapy. Main results demonstrated a higher rate of major or life-threatening bleeding, death and thromboembolic complications in rivaroxaban group as compared to DAPT strategy [7]. Nonetheless, the GALILEO-4D substudy analyzed 4-D computed tomography imaging at 90 days after randomization and showed a lower rate of subclinical aortic leaflet-motion abnormalities in the NOAC arm [8]. The ATLANTIS trial (NCT 02664649) studied the anticoagulation after TAVR, irrespective of the need of NOAC (using only apixaban) at a dose-regimen labeled for non-valvular atrial fibrillation and tested versus VKA or aspirin. One of the primary endpoints is symptomatic valve thrombosis. Results were recently presented at the ACC.21 congress and demonstrated the non-superiority of apixaban compared to the standard of care despite a numerical reduction of valve leaflet thrombosis when compared with SAPT. Considering these data, our routine practice is tailored based on the 2019 Canadian cardiovascular society who recommends aspirin alone after TAVR if there is no indication of DAPT or anticoagulation [9].
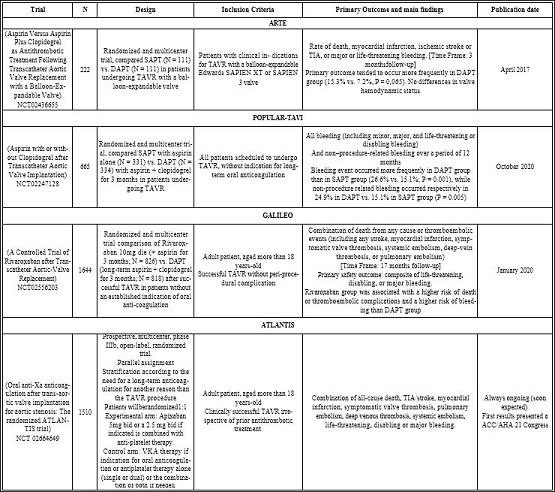
Table 2: Last main and ongoing trials for anticoagulation strategy after TAVR.
Finally, our two cases were treated surgically. The indication was a large size thrombus and high embolic risk in the first case and a failure of first line anticoagulation therapy in the second one. This is supported by the ESC/EACTS guidelines in operable patients although anticoagulation is recommended as first-line therapy (class IC) [10].
Conclusion
In conclusion, these 2 cases of consecutive THV thrombosis highlight the importance of an optimal peri and post-procedural anticoagulation/anti-platelet therapies especially for newer valves with higher sealing fabric feature. ATLANTIS and GALILEO trialsbring a similar information to this anticoagulation dilemma and confirm our routine practice.
Disclosures
WBA has received research grants from Medtronic and Edwards Lifesciences. The other authors have nothing to disclose in relation with this manuscript.
References
- Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, et (202) Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol 77: 1149-1161.
- Makkar RR, Blanke P, Leipsic J, Thourani V, Chakravarty T, et (2020) subclinical leaflet thrombosis in transcatheter and surgical bioprosthetic valves: PARTNER 3 cardiac computed tomography substudy. J Am Coll Cardiol 75: 3003-3015.
- Ng ACT, Holmes DR, Mack MJ, Delgado V, Makkar R, et (2020) Leaflet immobility and thrombosis in transcatheter aortic valve replacement. Eur Heart J 41: 3184-3197.
- Al-Kassou B, Kandt J, Lohde L, Shamekhi J, Sedaghat A, et al. (2020) safety and efficacy of protamine administration for prevention of bleeding complications in patients undergoing JACC Cardiovasc Interv 13: 1471-1480.
- Rodes-Cabau J, Masson JB, Welsh RC, Del Blanco BD, Pelletier M, et (2017) Aspirin versus aspirin plus clopidogrel as antithrombotic treatment following transcatheter aortic valve replacement with a balloon-expandable valve: The ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) randomized clinical trial. JACC Cardiovasc Interv 10: 1357-1365.
- Brouwer J, Nijenhuis VJ, Delewi R, Hermanides RS, Holvoet W, et al. (2020) Aspirin with or without clopidogrel after transcatheter aortic-valve N Engl J Med 383: 1447-1457.
- Dangas GD, Tijssen JGP, Wohrle J, Søndergaard L, Gilard M, et (2020) A Controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N Engl J Med 382: 120-129.
- De Backer O, Dangas GD, Jilaihawi H, Leipsic JA, Terkelsen CJ, et (2020) Reduced leaflet motion after transcatheter aortic-valve replacement. N Engl J Med 382: 130-139.
- Asgar AW, Ouzounian M, Adams C, Afilalo J, Fremes S, et al. (2019) 2019 Canadian cardiovascular society position statement for transcatheter aortic valve implantation. Can J Cardiol 35: 1437-148.
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, et al. (2017) 2017 ESC/EACTS Guidelines for the management of valvular heart Eur Heart J 38: 2739-2791.
Citation: Jamart L, Ohayon P, Durrleman N, Perrin N, Dorval JF, et al. (2021) Les- sons to Learn from Two Cases of TVAR Thrombosis: A Case Series and Literature Overview. J Cardio Cardiovasc Med 5: 027.
Copyright: © 2021 Jamart L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


