*Corresponding Author:
Shabana Shahanavaz,
Department of Pediatrics, Division of Cardiology, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8116-NWT, 1 Children’s Place, St. Louis, MO 63110-1093, USA
Tel: +1 3144546095
E-mail: Shahanavaz_s@kids.wsutl.edu
Abstract
Background: Pediatric heart transplant recipients routinely undergo surveillance cardiac catheterizations requiring various levels of sedation often using Dexmedetomidine. The transplanted heart is removed from direct sympathetic nervous innervations and, as a result, has a blunted chronotropic response to maintain cardiac output in response to hypotension. The hemodynamic effects of Dexmedetomidine in pediatric heart transplant recipients undergoing procedural sedation in the cardiac catheterization laboratory has yet to be studied extensively. We therefore conducted a retrospective evaluation at our center evaluating hemodynamic aspects of Dexmedetomidine in pediatric heart transplant patients. We believe this is a unique dataset with very valuable lessons for multiple centers using this technique for sedation in pediatric heart transplant recipients.
Objective: To determine if Dexmedetomidine is a hemodynamically stable sedative agent in pediatric Orthotropic Heart Transplant Recipients (OHTR).
Design: All OHTR <21 years of age undergoing sedated cardiac catheterizations between 1/2010 and 5/2012 were retrospectively reviewed. Dexmedetomidine was administered as a bolus (0.5-1 mcg/kg over 10 minutes) and/or an infusion (0.5-2 mcg/kg/h). Demographic and hemodynamic data were compared between patients sedated with Dexmedetomidine and those receiving other agents.
Setting: Single center study at a University hospital.
Participants: All OHTR <21 years of age undergoing sedated cardiac catheterizations between 1/2010 and 5/2012.
Intervention: Dexmedetomidine was administered as a bolus (0.5-1 mcg/kg over 10 minutes) and/or an infusion (0.5-2 mcg/kg/h).
Measurements: 158 procedures met inclusion criteria. Dexmedetomidine was used in 64% of OHTR: 59% of whom received other sedatives in addition to Dexmedetomidine. Patients receiving Dexmedetomidine and those who did not were similar in pre procedure blood pressure and shortening fraction. The Dexmedetomidine group had a lower mean age (12.5 yrs vs. 15.2 yrs, p=0.001) and higher mean pre-procedure heart rate (108 vs. 99 bpm, p=0.001). The incidences of bradycardia, hypotension and receiving an intervention for either were similar between groups. A decrease in heart rate greater than 20% from baseline was more likely in patients receiving Dexmedetomidine.
Conclusion: Dexmedetomidine administration in pediatric OHTR was not associated with clinically significant bradycardia or hypotension and is a hemodynamically stable sedative in this population.
Introduction
Dexmedetomidine is a selective alpha2 adrenergic agonist that has sedative, analgesic and anxiolytic properties. Dexmedetomidine does not result in respiratory depression, appears to mimic natural sleep and has centrally mediated anxiolytic effects [1,2]. This quality along with short half life of 6 minutes and terminal half life of 2 hours has led to its increasing use in the cardiac catheterization laboratory as part of a procedural sedation regimen [3-6]. Since the actions of Dexmedetomidine are mediated by α2 adrenoreceptors, Dexmedetomidine has been associated with hypotension and bradycardia [7]. It decreases blood pressure, heart rate and circulating catecholamine’s in a dose dependent manner [7]. Although approved for mechanically ventilated adults for periods less than 24 hours, experience is increasing in using this medication in children.
Pediatric heart transplant recipients routinely undergo surveillance cardiac catheterizations requiring various levels of sedation [8]. The transplanted heart is removed from direct sympathetic nervous innervations and, as a result, has a blunted chronotropic response to maintain cardiac output in response to hypotension [9,10]. The hemodynamic effects of Dexmedetomidine in pediatric heart transplant recipients undergoing procedural sedation in the cardiac catheterization laboratory has yet to be studied extensively.
There is data regarding the use of Dexmedetomidine in one small study of pediatric heart transplant recipients undergoing cardiac catheterization. Dexmedetomidine was administered to twelve patients as a rapid bolus of either 0.25 or 0.5 mcg/kg over 5 minutes [11]. There was a transient increase in systolic blood pressure, diastolic blood pressure, pulmonary artery pressure, pulmonary capillary wedge pressure and systemic vascular resistance at 1 minute, which returned to near baseline after 5 minutes. This study examined bolus dosing but did not comment on whether the hemodynamic changes seen were clinically significant. The hemodynamic effects of rapid bolus dosing of Dexmedetomidine are different compared to the effects seen when it’s administered as an infusion. In adults, Dexmedetomidine administered over 2 minutes, resulting in a biphasic hemodynamic response with an initial increase in blood pressure and reflex bradycardia followed by a stabilization of blood pressure and HR below baseline value [7].
We sought to examine the hemodynamic effects of Dexmedetomidine in pediatric heart transplant recipients when administered as a slow bolus followed by an infusion in the cardiac catheterization laboratory as part of procedural sedation.
The aim of this study was to determine whether Dexmedetomidine is hemodynamically stable drug to administer to pediatric heart transplant recipients. The study was designed to evaluate the risk of hypotension and bradycardia associated with Dexmedetomidine in comparison to other agents in OHTR patients.
Methods
The institutional review board at our institution approved this study (IRB approval number: 201208032). Parental consent was waived due to the retrospective nature of the study with minimal concerns for loss of privacy. All diagnostic catheterizations performed on pediatric heart transplantation recipients from January 2010 until May 2012 was retrospectively reviewed. All patients less than 21 years of age receiving procedural sedation were included. Procedural sedation was defined as all patients not receiving anesthetic gases or artificial airway devices. Procedures where Dexmedetomidine was administered were compared to those where it was not. Either certified nursing staff or an anesthesiologist provided sedation. The choice of sedative agent used was based on practitioner preference. Dexmedetomidine was administered as a bonus of 0.5-1 mcg/kg over 10 minutes and/or an infusion with rates between 0.5-2 mcg/kg/hour. Other agents used in conjunction with Dexmedetomidine included fentanyl, ketamine, midazolam and propofol. The same agents were also used in the patients who did not receive Dexmedetomidine.
Demographic data including sex, age, weight, pre and post procedural heart rate and blood pressure measurements were collected from electronic medical records. Left ventricular shortening fraction results were obtained from the patients’ most recent echocardiogram report prior to the procedure. Maximum and minimum heart rate and blood pressure values recorded at every five to ten minute intervals during the procedure and for the first 30 minutes post procedure were collected from the medical record. Other sedatives administered and doses received were documented. Hypotension and bradycardia were defined by two separate criteria. A 20% decrease from pre-procedural values defined the first criteria. The second criterion was defined according to age appropriate normative values as stated in table
Procedures warranting an intervention for either hypotension or bradycardia were recorded. An intervention was defined as either a decrease in Dexmedetomidine dosing or receiving a rescue medication. The reason for intervention and the intervention performed were both documented.
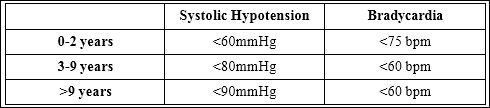
Table 1: Definitions Used for Vitals.
Due to intrapatient variability in response to Dexmedetomidine, each exposure to sedation in the cardiac catheterization laboratory was counted as an individual procedure despite several patients undergoing serial catheterizations during the study period.
Demographic data including gender, age, pre procedural vitals, and pre procedural echocardiogram systolic function were compared between the two groups using a student’s t-test. Chi square testing was used to compare the incidences of bradycardia and hypotension between the two groups. All statistical analysis was performed using SSPS 21.0 (IMB, Armonk, New York). Statistical significance was defined as a P value ≤ 0.05.
Results
The patient records of 271 procedures were reviewed with 158 procedures meeting inclusion criteria. Several patients underwent serial procedures during the study period, resulting in 87 individual patients included. Demographic data is shown in table 2.
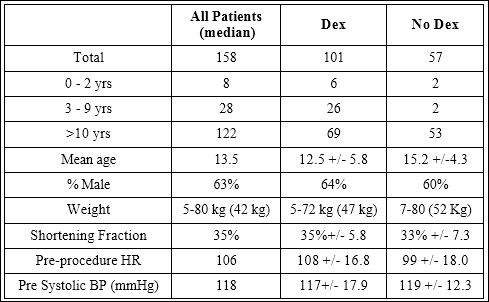
Table 2: Demographic Data
Dexmedetomidine was administered in 101 procedures. In 80 of these, Dexmedetomidine was administered as both a bolus and an infusion. In 19 procedures, it was administered as an infusion alone and twice only a bolus. Other sedative agents were used in addition to Dexmedetomidine in 60 cases (Table 3). Pre procedure blood pressure and shortening fraction were similar in procedures where Dexmede- tomidine was administered compared to those where it was not. In the Dexmedetomidine group, the mean age was lower (12.5 yrs vs. 15.2 yrs, p=0.001) and the mean pre procedure heart rate was higher (108 vs. 99 bpm, p=0.001) (Table 2).
A decrease in heart rate greater than 20% from baseline was more likely when Dexmedetomidine (p=0.001) was administered. The incidence of receiving an intervention (p=0.310), bradycardia for age (p=0.702), hypotension for age (p=0.059) or decrease in blood pressure greater than 20% from baseline (p=0.126) was similar in procedures with Dexmedetomidine compared to those without (Figures 1-5).
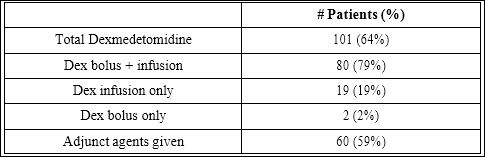
Table 3: Sedation Regimen Used.
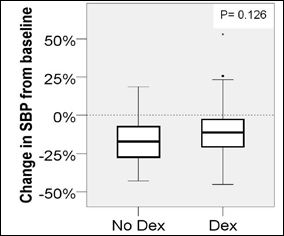
Figure 1: Change in systolic BP from baseline.
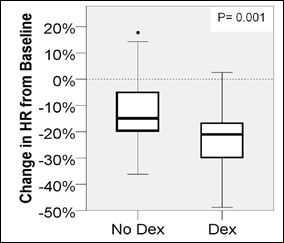
Figure 2: Change in HR from baseline.
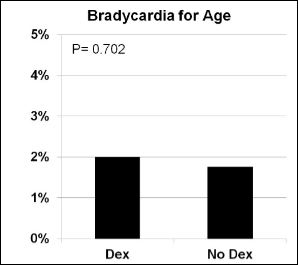
Figure 3: Bradycardia for age.
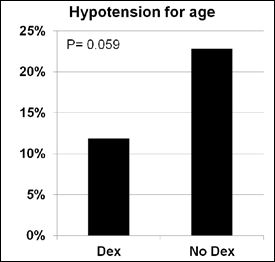
Figure 4: Hypotension for age.
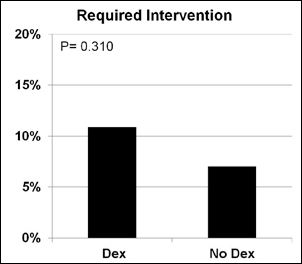
Figure 5: Required intervention.
The Dexmedetomidine dose was decreased during 11 procedures. In two of these (2%) the patients were noted to have respiratory con- cerns (upper airway obstruction or shallow breathing). Rescue medi- cations were administered in six procedures (6%) in the Dexmedeto- midine group compared to four procedures (7%) in the control group. Rescue medications included dopamine, phenylephrine and/or fluid boluses. In four procedures where the Dexmedetomidine dose was decreased, there was no bradycardia or hypotension.
Discussion
Dexmedetomidine is a α2 adrenoreceptors agonist whose sedative and anxiolytic effects are centrally mediated [1]. It is currently approved by the Federal Drug Administration for intensive care and procedural sedation in the adult population and has gained increasing usage in the pediatric anesthesia regimen. The drug has been reported to be used in procedural sedation, including sedation for gastroenterology, otolaryngology, orthopedic and radiologic procedures [12-18]. Our study demonstrates that the drug can be used without significant hemodynamic effects for procedural sedation in OHTR in the cardiac catheterization laboratory.
Dexmedetomidine is currently recognized as an option for sedation of patients with congenital heart disease [19]. Several studies document its usage as a sedative both intraoperative and in the intensive care unit following cardiac surgery, including in single ventricle patients [20-26]. In the cardiac catheterization laboratory, Deutsh et al., evaluated the usage of Dexmedetomidine in children with congenital heart disease [27]. Their study included 9 heart transplant recipients in whom non inferiority of Dexmedetomidine combined with sevoflurane was demonstrated for decreased heart rate, but not for hypotension.
According to the International Society for Heart and Lung Transplantation guidelines, pediatric transplant recipients undergo several surveillance cardiac catheterizations [8]. There is limited data regarding the usage of Dexmedetomidine in pediatric heart transplant recipients. There are documented cases of Dexmedetomidine use, including the prevention of post operative withdrawal symptoms follow cardiac transplantation as well its usage to facilitate the extubation of a transplant recipient recovering from acute pneumonia [28,29]. Jooste et al., reported the results of rapid bolus dosing in 12 pediatric heart transplant recipients undergoing routine diagnostic catheterizations [11]. Their study demonstrated a significant transient increase in systemic blood pressure, which returned to baseline within 5 minutes. However, that study only looked at rapid bolus dosing in a small number of patients. While the study by Deutsh et al., also included transplant recipients, only nine such patients were included [27]. Our study is the largest study to look at Dexmedetomidine dosing in pediatric heart transplant recipients receiving bolus and infusion dosing.
Our study shows that although there was a marginally significant difference was found in blood pressure (0.059) among the patients receiving Dexmetomidine there was no hemodynamically significant bradycardia or hypotension warranting clinical intervention in pediatric heart transplant recipients receiving Dexmedetomidine for procedural sedation. A decrease in heart rate greater than 20% compared to baseline values was seen.
In our study population, patients receiving Dexmedetomidine were older. This is reflective of the sedation practice at the study institution. Patients who received the agent also had a higher baseline heart rate. This could explain the finding that, although patients demonstrated 20% decreases in heart rate from baseline, their heart rate remained within normal age values. Despite this, the rates of hypotension and interventions remained the same between patients who did and did not receive Dexmedetomidine.
This study has limitations inherent with its retrospective nature. Choice of sedative agent and rescue intervention was provider dependent and patients were not randomized. As a retrospective study, we were unable to control for the usages and dosages of other sedative agents. Based on the sedation practices of the study institution, the control arm of this study was small. However, our study does provide support for future prospective studies to further delineate the hemodynamic effects of Dexmedetomidine in this patient population.
Conclusion
Patients receiving Dexmedetomidine for procedural sedation did not demonstrate hemodynamically significant bradycardia or hypotension warranting clinical intervention. However, heart rate decreases greater than 20% compared to baseline values were seen. Our findings conclude that Dexmedetomidine is hemodynamically safe for procedural sedation for pediatric heart transplant patients without significant hemodynamic effects in the cardiac catheterization laboratory.
Disclosures
We have no disclosures from all the authors.
References
- Belleville J, Ward D, Bloor B, Maze M (1992) Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology 77: 1125-1133.
- Shukry M and Miller JA (2010) Update on Dexmedetomidine: use in nonintubated patients requiring sedation for surgical procedures. Ther Clin Risk Manag 6: 111-121.
- Ulgey A, Aksu R, Bicer, C, Akin A, Altuntas R¸ et al. (2012) Is the Addition of Dexmedetomidine to a Ketamine-Propofol Combination in Pediatric Cardiac Catheterization Sedation Useful? Pediatr Cardiol 33: 770-74.
- Munro HM, Tirotta CF, Felix DE, Lagueruela RG, Madril DR, et al. (2007) Initial experience with Dexmedetomidine for diagnostic and interventional cardiac catheterization in Paediatr Anaesth 7: 109-112.
- Chilson K, Easley RB, Brady KM, Tobias JD (2008) Monitored Anesthesia Care with a Combination of Ketamine and Dexmedetomidine during Cardiac Amer J Ther 15: 24-30.
- Gupta P, Tobias JD, Goyal S, Miller MD, De Moor MM, et al. (2009) Preliminary experience with a combination of dexmedetomidine and propofol infusions for diagnostic cardiac catheterization in children. J Pediatr Pharmacol Ther 14: 106-112.
- Su F, Hammer GB (2011) Dexmedetomidine: pediatric pharmacology, clinical uses and Expert Opin Drug Saf 10: 55-66.
- Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, et al. (2010) The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant J Heart Lung Transplant 29: 914-956.
- Cotts WG, Oren RM (1997) Function of the transplanted heart: unique physiology and therapeutic Am J Med Sci 314: 164-172.
- Schure AY and Kussman BD (2011) Pediatric heart transplantation: demographics, outcomes, and anesthetic implications. Paediatr Anaesth 21: 594-603.
- Jooste EH, Muhly WT, Ibinson JW, Suresh T, Damian D, et al. (2010) Acute Hemodynamic Changes After Rapid Intravenous Bolus Dosing of Dexmedetomidine in Pediatric Heart Transplant Patients Undergoing Routine Cardiac Anesth Analg 111: 1490-1496.
- Cheng X, Huang Y, Zhao Q, Gu E (2014) Comparison of the effects of dexmedetomidine-ketamine and sevoflurane-sufentanil anesthesia in children with obstructive sleep apnea after uvulopalatopharyngoplasty: An observational J Anaesthesiol Clin Pharmacol 30: 31-35.
- Jones JS, Cotugno RE, Singhal NR, Soares N, Semenova J, et al. (2014) Evaluation of Dexmedetomidine and Postoperative Pain Management in Patients with Adolescent Idiopathic Scoliosis: Conclusions Based on a Retrospective Study at a Tertiary Pediatric Hospital. Pediatr Crit Care Med 15: 247-252.
- Fagin A, Palmieri T, Greenhalgh D, Sen S (2012) A comparison of dexmedetomidine and midazolam for sedation in severe pediatric burn injury. J Burn Care Res 33: 759-763.
- Siddappa R, Riggins J, Kariyanna S, Calkins P, Rotta AT (2011) High-dose dexmedetomidine sedation for pediatric Paediatr Anaesth 21: 153-158.
- Mason KP, Robinson F, Fontaine P, Prescilla R (2013) Dexmedetomidine Offers an Option for Safe and Effective Sedation for Nuclear Medicine Imaging in Radiology 267: 911-917.
- Olutoye OA, Glover CD, Diefenderfer JW, McGilberry M, Wyatt MM, et al. (2010) The Effect of Intraoperative Dexmedetomidine on Postoperative Analgesia and Sedation in Pediatric Patients Undergoing Tonsillectomy and Anesth Analg 111: 490-495.
- Sethi P, Mohammed S, Bhatia PK and Gupta N (2014) Dexmedetomidine versus midazolam for conscious sedation in endoscopic retrograde cholangiopancreatography: An open-label randomised controlled trial. Indian J of Anaesth 58: 18-24.
- Tobias JD, Gupta P, Naguib A, Yates AR (2011) Dexmedetomidine: Applications for the Pediatric Patient with Congenital Heart Disease. Pediatr Cardio 32: 1075-1087.
- Klamt JG, Vicente WVA, Garcia LV, Ferreira CA (2010) Effects of Dexmedetomidine-Fentanyl Infusion on Blood Pressure and Heart Rate during Cardiac Surgery in Anesthesiol Res Pract. 2010: 869049.
- Garg R, Rao S, John C, Reddy C, Hegde R (2014) Extubation in the Operating Room after Cardiac Surgery in Children: A Prospective Observation Study with Multidisciplinary Coordinated Approach. J Cardiothorac Vasc Anesth 28: 479-487.
- Su F, Nicolson SC, Zuppa AF (2013) A Dose-Response Study of Dexmedetomidine Administered as the Primary Sedative in Infants Following Open Heart Pediatr Crit Care Med 12: 499-507.
- Berkenbosch JW (2010) Dexmedetomidine and pediatric (cardiac) critical care--are we there yet? Pediatr Crit Care Med 11: 148-149.
- Gupta P, Whiteside W, Sabati A, Tesoro TM, Gossett JM, et (2012) Safety and efficacy of prolonged dexmedetomidine use in critically ill children with heart disease*. Pediatr Crit Care Med 13: 660-666.
- Le KN, Moffett BS, Ocampo EC, Zaki J, Mossad EB (2011) Impact of dexmedetomidine on early extubation in pediatric cardiac surgical patients. Intensive Care Med 37: 686-690.
- Tokuhira N, Atagi K, Shimaoka H, Ujiro A, Otsuka Y, et (2009) Dexmedetomidine sedation for pediatric post-Fontan procedure patients. Pediatr Crit Care Med 10: 207-212.
- Deutsch N, Finkel JC, Gold K, Cheng YI, Slack MC (2013) Dexmedetomidine for Patients Undergoing Diagnostic Cardiac Procedures: A Noninferiority Pediatr Cardiol 34: 898-906.
- Vega L, Sanchez-de-Toledo J, Gran F, Ortega J, Pujol M (2014) Prevention of Opioid Withdrawal syndrome after Pediatric Heart Transplantation: Usefulness of Rev Esp Cardiol (Engl Ed) 66: 586-597.
- Chrysostomou C, Zeballos T (2005) Use of Dexmedetomidine in a pediatric heart transplant Pediatr Cardiol 26: 651-654.
Citation: Shahanavaz S, Tucker M, Chilson K, Balzer D (2017) Hemodynamic Effects of Dexmedetomidine in Pediatric Cardiac Transplantation Recipients during Cardiac Catheterization. J Cardio Cardiovasu Med 2: 006.
Copyright: © 2017 Shahanavaz S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


