*Corresponding Author:
Doson Chua,
Department of Pharmacy, St. Paul’s Hospital, Vancouver, BC V6Z 1Y6, Canada
Tel: 6046822344
E-mail: dchua@providencehealth.bc.ca
Abstract
Enoxaparin dosing for treatment of acute coronary syndromes (ACS) in obese patients is controversial and current guidelines do not provide recommendations. This review will systematically search the literature to determine if a maximum dose limit should be applied with weight based enoxaparin dosing in obese ACS patients.
A search of MEDLINE, EMBASE, PUBMED, CENTRAL, CDSR, Google Scholar and Google with the key words “acute coronary syndromes, myocardial infarction, enoxaparin, obese, dosing” was performed. Only articles that were clinical studies with an obese and non obese comparator group were included. Pharmacokinetic studies, studies with laboratory endpoints, review articles, letters and case reports were excluded.
Four clinical studies were retrieved based on the search criteria established. Subgroup analysis from the landmark enoxaparin trials ESSENCE and TIMI 11b in ACS demonstrate that there is no increased rate of bleeding or adverse cardiovascular outcomes in obese patients who receive enoxaparin dosed based on total body weight, without a maximum dose cap, compared to non obese patients. Subgroup analysis from the SYNGERY trial showed that use of enoxaparin without a maximum dose was not an independent predictor of cardiovascular outcomes or bleeding. Other retrospective studies also demonstrate that dosing enoxaparin in obese patients with ACS, without a maximum dose cap, did not lead to higher rates of bleeding. However, analysis of the CRUSADE registry suggested that enoxaparin doses exceeding 150 mg was associated with higher rates of bleeding. Thus, the available evidence consistently suggests that individual enoxaparin doses above 150 mg led to higher rates of bleeding, thus a maximum dose limit of 150 mg of enoxaparin should be applied. We propose a guide that helps clinicians decide if a maximum dose limit should be applied to weight based enoxaparin dosing in obese patients presenting with ACS.
Keywords
Acute coronary syndromes; Dose; Dosing; Enoxaparin; Obese
Abbreviations
ACS : Acute Coronary Syndromes
LMWH : Low Molecular Weight Heparin
NSTEMI : Non-ST Elevation Myocardial Infarction
STEMI : ST Elevation Myocardial Infarction
FDA : Food and Drug Agency
ACC : American College of Cardiology
BMI : Body Mass Index
Introduction
Enoxaparin is a Low Molecular Weight Heparin (LMWH) that is commonly used in the treatment of Acute Coronary Syndromes (ACS). Several pivotal trials have demonstrated the superiority of enoxaparin over unfractionated heparin and enoxaparin is currently recommended over unfractionated heparin for the acute management of ACS [1,2]. The current established dosing of enoxaparin for ACS is 30 mg intravenously (IV), then 1 mg/kg Subcutaneously (SC) twice a day for ST Elevation Myocardial Infarction (STEMI) with fibrinolytic therapy and 1 mg/kg sc twice a day for Non-ST Elevation Myocardial Infarction (NSTEMI) [3,4]. While in the United States, the current Food and Drug Agency (FDA) monograph for enoxaparin does not state a specific dose maximum for weight based enoxaparin for ACS treatment, other countries such as Canada does stipulate a maximum of 100 mg per dose [5,6].
A clinical scenario that commonly arises where obese patients are anticoagulated with enoxaparin in their treatment of ACS and their calculated enoxaparin dose results in doses greater than 100 mg per individual dose. There is debate surrounding the application a maximum dose or a maximum dose limit, or dose cap, with weight-based dosing of enoxaparin for obese patients [7,8,9]. The dilemma surrounding enoxaparin dosing in obesity is the potential of excessive anticoagulationleading to an increased risk of bleeding, if no maximum dose limit is applied, juxtaposed with possible under-dosing that could lead to inadequate anticoagulation.
Pharmacokinetic studies evaluating the accumulation of enoxaparin and monitoring of antiXa levels in obese patients have demonstrated that dosing enoxaparin based on total body weight, without a maximum dose, does not lead to excessive anticoagulation [10]. However these studies are based on laboratory endpoints and may not translate to clinical outcomes. The utility of anti-Xa levels for monitoring efficacy of enoxaparin and LMWHs has been questioned as well [8,11].
Current ACS guidelines do not provide guidance if a maximum dose should be applied to enoxaparin dosing in obese patients. The current American College of Cardiology (ACC) STEMI and NSTEMI guidelines do not provide specific recommendations and largely follow the manufacturer’s recommendations [3,4]. The current CHEST guidelines recommend that there should not be a maximum dose cap applied to enoxaparin dosing in obese patients (to a maximum of 144kg body weight or a Body Mass Index (BMI) of 30 kg/m2), largely based on pharmacokinetic studies [12].
Thus, clinician’s areleft with unclear guidance on whether enoxaparin dosing in obese patients presenting with ACS should have a maximum enoxaparin dose limit applied to them. As current guidelines do not offer any guidance, this review will systematically search the available literature and provide insight for clinicians in how to anticoagulate obese patients presenting with ACS.
Methods
A search of MEDLINE, EMBASE, PUBMED, CENTRAL, CDSR, Google Scholar and Google was performed for clinical studies from May 1990 to May 2016 that compared the use of enoxaparin in obese and non-obese patients for the treatment of ACS. The search was performed using text words and MeSH and EMTREE keywords related to ischemia [acute coronary syndrome, coronary artery disease, myocardial infarct, myocardial infarction, myocardial ischemia, coronary thrombosis, ST segment elevation myocardial infarction, non ST segment elevation myocardial infarction, sudden cardiac death, unstable angina], obesity [obesity, overweight, obes*], enoxaparin [enoxaparin] and dose [dose cap, maximum dose]. Terms within each category were combined with the Boolean operator “OR”, and categories were combined with the operator “AND”. A first search was performed with the combination of the first three categories (ischemia, obesity and enoxaparin) and a second one with the combination of ischemia, enoxaparin and dose. In addition, a hand search of the references of identified articles and of the similar articles suggested by the databases was done.
Only studies with clinical endpoints comparing dose capping to no dose capping in obese patients or dosing in obese versus non-obese patients were included. We excluded studies which reportedanti-Xa levels as the primary outcome, pharmacokinetic endpoints, in vitro or laboratory studies; surveys; expert opinions; reviews; case reports, abstracts, case series and any study not involving obese patients or without comparator arm (Figure 1).
Results
Based on our search parameters, 52 published articles were identified. Four met the inclusion and exclusion criteria and are reviewed below.
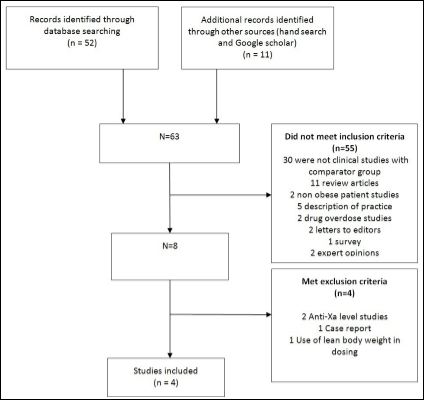
Figure 1: Dosing of enoxaparin in obese patients with acute coronary syndromes literature search strategy and results.
Spinler et al retrospectively analyzed the subgroup of obese patients in the ESSENCE and TIMI IIB study, where no maximum weight based dosing of enoxaparin was stipulated in both studies [13]. The authors compared the clinical outcomes of death, Myocardial Infarction (MI) and urgent revascularization and bleeding in the nonobese patients (n = 2.595) compared to the obese group (n = 921). There was no difference in the rates of death, MI or urgent revascularization in the non-obese group versus the obese group (16.1% versus 14.3%, p = 0.39). As well, there was no difference in the rates of major hemorrhage between the non-obese group compared to the obese group (0.4% versus 1.6%, p = 0.12), although there was a higher rate of any hemorrhage (minor and major) in the obese group. Thus, the obese group (who received enoxaparin based on total body weight without any maximum dose cap) demonstrated similar cardiovascular outcomes and bleeding events compared to non-obese patients. The results from this analysis suggest that enoxaparin should be dosed by total body weight without any maximum dose.
Mahaffey et al analyzed the SYNERGY trial of which 32% of patients were obese (BMI > 30 kg/m2) [14]. Enoxaparin was dosed at 1 mg/kg twice a day without a maximum dose cap. Multivariate logistic regression was used to adjust for independent predictors of bleeding and 30 day rates of death or MI. After adjustment, increased BMI > 30 kg/m2 and use of enoxaparin without a maximum dose were not independent predictors of 30 day rates of death or MI or in-hospital bleeding events (p = 0.42 and p = 0.78 respectively) and no association between BMI and event rates were found. The authors conclude that as no interaction was found between BMI and cardiovascular and bleeding outcomes, enoxaparin should be dosed at 1 mg/kg twice a day regardless of body weight and with no maximum dose (except in patients with extreme obesity).
Spinler et al investigated the CRUSADE national quality improvement initiative database and evaluated the enoxaparin dose administered and bleeding outcomes reported [15]. Of the 19,061 patients with ACS in the CRUSADE registry, 3.899 were defined as obese (greater than 100 kg total body weight). The rates of major bleeding (unrelated to coronary artery bypass graft surgery) was compared among the patients who were non obese (< 100 kg) with those in the weight range of 101-120 kg, 121-150 kg and above 150 kg. All patients in the CRUSADE database received the recommended 1mg/kg twice dailydosing of enoxaparin with no maximum dose limit based on weight. There was no statistical difference in the rates of major bleeding between the non obese group (6.6%), the 101-120kg group (4.6%) and the 121-150 kg group (5.6%). However, the rates of major bleeding increased in the group of patients weighing greater than 150 kg (11.4%) whom received enoxaparin based on weight with no dose limit. The authors conclude that the use of enoxaparin at the standard weight based dosing, with no dose limit in obese patients, was associated with higher bleeding risk in those weighing more than 150 kg.
Hagopian et al conducted a retrospective study in 300 patients whom were treated with enoxaparin for various indications at single doses of at least equal or greater than 0.85 mg/kg [16]. Enoxaparin was used for ACS in 9% of the patients compared to use for deep vein thrombosis (59%) and pulmonary embolism (34%) in this study. One hundred obese patients (BMI >40 kg/m2) in this study were compared to 200 patients whom were non obese (BMI < 40 kg/m2) with the outcome of bleeding; defined as overt bleeding resulting in death, retroperitoneal bleeding, intracranial hemorrhage, decrease in hemoglobin of >20 g/L, decrease in hematocrit of 12% or administration of >2 units of packed red blood cells. Enoxaparin dosing in the obese group was lower than compared to the non obese group (0.98 mg/kg versus 1.04 mg/kg respectively, p < 0.01). However, the rates of any bleeding event was no different in the obese group compared to the non obese group (29% versus 23.5%, p = 0.30). Based on this retrospective data of real life practice, the authors concluded that dosing of enoxaparin in obese patients, with doses capped at a maximum of 150 mg, was not associated with increased bleeding incidence. This study did not specifically study enoxaparin dosing in ACS patients, however ACS was one of the many reasons for anticoagulation in the patient population studied.
Discussion
There are no published studies that prospectively investigated appropriate dosing of enoxaparin in obese patients in treatment of their ACS. Most of the available literature is based on sub-group analysis of obese patients from the major clinical trials of enoxaparin in ACS [13].
Analysis of these obese subgroups of the major enoxaparin trials and retrospective data suggests there is no increased incidence of bleeding in dosing enoxaparin with no maximum dose limit, up to approximately 150 mg per individual dose. While the data is far from robust, the results from these subgroup analysis and retrospective data are consistent.
In total, these four articles included 9943 patients with a BMI of at least 30 kg/m2 that received enoxaparin for ACS. Three of four articles agreed on the definitions of obese being BMI ≥ 30 kg/m2 or morbidly obese BMI ≥ 40 kg/m2 while one article reported the results mainly based on body weight. The heaviest patient weight and BMI reported in the reviewed studies came from Spinler et al., [13] and were 158.6 kg and 62.4 kg/m2 respectively. The subgroup analysis of ESSENCE and TIMI IIB by Spinler showed no difference in efficacy or safety clinical endpoints between non-obese and obese patients treated with enoxaparin (with no maximum dose limit) for ACS, however this subgroup analysis was likely underpowered to detect a potential difference in outcomes [13]. As well, it is plausible that obesity in itself may influence clinical outcomes rather than anticoagulation with enoxaparin. Obesity alone has been identified to be an independent factor for outcomes after an ACS [17].
Other factors should be considered in dosing enoxaparin in obese patients beyond just the total body weight. Enoxaparin is dependent on renal function for elimination and its use is contraindicated in patients with several renal impairment [6]. Patients with moderate to severe renal impairment (e.g. glomerular filtration rate of less than 60 ml/min) would be at risk for enoxaparin accumulation and bleeding. Duration of therapy of enoxaparin should also be a consideration. If a patient is treated with enoxaparin for an extended period of time, there is potential for accumulation of enoxaparin and this would potentially lead to increased bleeding. Presence of such factors would incline a clinician to apply a maximum enoxaparin dose cap or do laboratory monitoring to ensure no excessive accumulation.
We propose a guide to help cliniciansdecidehow to dose enoxaparin in obese patients presenting with ACS (Table 1). The definition of obesity varied amongst the studies, however, the available literature generally establishes a BMI > 30 kg/m2 or total body weight > 100 kg as obese. If the patient is obese and have factors that would increase their risk of bleeding, we suggest use of unfractionated heparin which could be adjusted based on partial thromboplastin time or use of anti Xa level monitoring if enoxaparin use is preferred. While the usefulness of anti Xa level monitoring is debatable, elevated anti Xa levels would suggest excessive anticoagulation [8]. If the patient is obese and the calculated dose of enoxaparin is less than 150 mg but the patient is at a low risk of bleeding, then we would suggest dosing enoxaparin based on total body weight without a maximum dose.
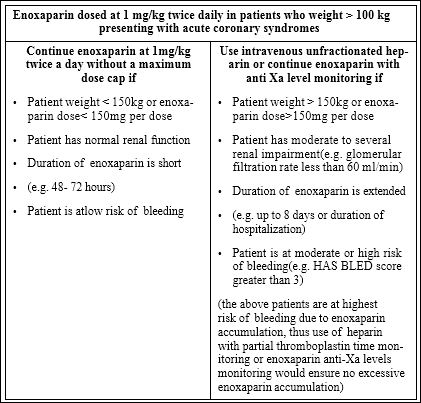
Table 1: Dosing of enoxaparin for treatment of acute coronary syndromes in obese patients.
One exception to our algorithm is patients who receive fibrinolytic therapy for a STEMI. Given the risk of bleeding and intracranial hemorrhage with fibrinolytic therapy, the EXTRACT study limited the maximum dose for the first 2 enoxaparin doses to 100 mg with administration of fibrinolytic therapy [2]. In these circumstances, it would be prudent apply to the maximum of 100mg per enoxaparin dose for the first 2 doses in obese patients.
Conclusion
The dosing of enoxaparin in obese patients is controversial and carries the concerns of over anticoagulation and increase bleedingalong with the potential of under dosing. However, obese subgroups of the landmark enoxaparin ACS trials and other clinical studies suggest that there is no increased risk of bleeding with dosing enoxaparin in obese patients without a maximum dose. However, in patients weighing more than 150 kg or a single dose of enoxaparin greater than 150 mg does have higher risks of bleeding and use of enoxaparin in these patients should be avoided. Other factors should be considered as well, such as renal function and bleeding risk. We provide a summary and recommendations which would help guide clinicians in deciding if their obese patients should be treated with enoxaparin without a maximum dose cap.
References
- Cohen M, Demers C, Gurfinkel EP, Turpie AGG, Fromell GJ, et (1997) A Comparison of Low-Molecular-Weight Heparin with Unfractionated Heparin for Unstable Coronary Artery Disease. New Engl J Med 337: 447-452.
- Antman EM, Morrow DA, McCabe CH, Murphy SA, Ruda M, et al. (2006) Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. New Engl J Med 354: 1477-1488.
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, et al. (2013) 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61: 78-140.
- Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, et al. (2014) 2014 AHA/ACC Guideline for the Management of Patients With Non– ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guide J Am Coll Cardiol 64: 139-228.
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020164s085lbl.pdf
- http://products.sanofi.ca/en/lovenox.pdf
- Nutescu EA, Spinler SA, Wittkowsky A, Dager WE (2009) Low-molecu- lar-weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical Ann Pharmacother 43:1064-1083.
- Thomson P, Brocklebank C, Semchuk W (2009) Treatment Dosing of Low-Molecular-Weight Heparins and the Dose Cap Dilemma: Consider- ations for Patients in Canada. Can J Hosp Pharm 62: 367-374.
- Macie C, Forbes L, Foster GA, Douketis JD (2004) Dosing practices and risk factors for bleeding in patients receiving enoxaparin for the treatment of an acute coronary syndrome. Chest 125: 1616-1621.
- Egan G, Ensom MH (2015) Measuring anti-factor Xa activity to monitor low-molecular weight heparin in obesity: A critical Can J Hosp Pharm 68: 33-47.
- Fraser GL, McKenna JP (2003) Monitoring low molecular weight heparins with antiXa activity: House of cards or firm foundation? Hospital Pharmacy 38: 202-211.
- Garcia DA, Baglin TP, Weitz JI, Samama MM (2012) Parenteral anticoagu- lants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: Amer- ican College of Chest Physicians Evidence-Based Clinical Practice Guide- Chest 141: 24-43.
- Spinler SA, Inverso SM, Cohen M, Goodman SG, Stringer KA, et al. (2003) Safety and efficacy of unfractionated heparin versus enoxaparin in patients who are obese and patients with severe renal impairment: Analysis from the ESSENCE and TIMI 11b studies. Am Heart J 146: 33-41.
- Mahaffey KW, Tonev ST, Spinler SA, Levine GN, Gallo R, et (2010) Obe- sity in patients with non-ST-segment elevation acute coronary syndromes: Results from the SYNERGY trial. Int J Cardiol 139: 123-133.
- Spinler SA, Ou FS, Roe MT, Gibler WB, Ohman EM, et al. (2009) Weight- based dosing of enoxaparin in obese patients with Non-ST-Segment eleva- tion acute coronary syndromes: Results from the CRUSADE Phar- macotherapy 29: 631-638.
- Hagopian JC, Riney JN, Hollands JM, Deal EN (2013) Assessment of bleed- ing events associated with short-duration therapeutic enoxaparin use in the morbidly obese. Ann Pharmacother 47: 1641-1648.
- Ghoorah K, Campbell P, Kent A, Maznyczka A, Kunadian V (2016) Obesity and cardiovascular outcomes: a Eur Heart J Acute Cardiovasc Care 5: 77-85.
Citation: Chua D, Tataru A (2016) Enoxaparin Dosing for Acute Coro- nary Syndromes in Obese Patients-Should There be a Maximum Dose? J Cardio Cardiovasu Med 1: 002.
Copyright: © 2016 Chua D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


